Abstract
AIM: To evaluate the effect of photodynamic therapy (PDT) on metal stent patency in patients with unresectable hilar cholangiocarcinoma (CC).
METHODS: This was a retrospective analysis of patients with hilar CC referred to our institution from December, 1999 to January, 2011. Out of 232 patients, thirty-three patients with unresectable hilar CC were treated. Eighteen patients in the PDT group were treated with uncovered metal stents after one session of PDT. Fifteen patients in the control group were treated with metal stents alone. Porfimer sodium (2 mg/kg) was administered intravenously to PDT patients. Forty-eight hours later, PDT was administered using a diffusing fiber that was advanced across the tumor by either endoscopic retrograde cholangiopancreatography or percutaneous cholangiography. After performance of PDT, uncovered metal stents were inserted to ensure adequate decompression and bile drainage. Patient survival rates and cumulative stent patency were calculated using Kaplan-Meier analysis with the log-rank test.
RESULTS: The PDT and control patients were comparable with respect to age, gender, health status, pre-treatment bilirubin, and hilar CC stage. When compared to control, the PDT group was associated with significantly prolonged stent patency (median 244 ± 66 and 177 ± 45 d, respectively, P = 0.002) and longer patient survival (median 356 ± 213 and 230 ± 73 d, respectively, P = 0.006). Early complication rates were similar between the groups (PDT group 17%, control group 13%) and all patients were treated conservatively. Stent malfunctions occurred in 14 PDT patients (78%) and 12 control patients (80%). Of these 26 patients, twenty-two were treated endoscopically and four were treated with external drainage.
CONCLUSION: Metal stenting after one session of PDT may be safe with acceptable complication rates. The PDT group was associated with a significantly longer stent patency than the control group in patients with unresectable hilar CC.
Keywords: Bile duct cancer, Palliative endoscopic stenting, Photodynamic therapy, Outcome
INTRODUCTION
Hilar cholangiocarcinoma (CC) is a rare tumor that is asymptomatic early on, making it difficult to diagnose, and is associated with a high mortality[1]. Surgical resection with negative histologic margins is the most robust predictor of long-term survival. However, due to the lack of characteristic early symptoms, a definitive diagnosis is often not reached until the tumor is at an advanced stage. As a result, a large proportion of patients are beyond the scope of curative treatment by the time of diagnosis, and only palliative management is possible[2]. Palliative therapies for unresectable CC such as stent, radiotherapy and chemotherapy, have generally been disappointing in prolonging life[3]. Endoscopic or percutaneous biliary drainage alleviates jaundice, but there is no evidence that it prolongs survival.
Although endoscopic biliary metal stenting is the mainstay of palliative treatment in patients with unresectable hilar CC[4], tumor ingrowth or overgrowth is a significant problem in uncovered stents. In particular, the duration of metal stent patency for hilar CCs was shorter than that for distal bile duct cancer because the malignant hilar stricture provides an acute angle that hinders full expansion of the metal stent and promotes biliary sludge formation within. Several clinical trials have reported the therapeutic effect of photodynamic therapy (PDT) for unresectable hilar CC[5,6]. The ability of PDT to destroy cancer and neovascular cells may prolong biliary stent patency. However, the effect of PDT on stent patency has not yet been determined. Therefore, the primary aim of this study was to explore the effect of PDT on stent patency in unresectable hilar CC. We also evaluated overall survival and procedure-related complications after PDT compared with endoscopic biliary drainage alone for unresectable hilar CCs.
MATERIALS AND METHODS
Patients
This was a non-randomized, retrospective study. From December, 1999 to January, 2011, 232 patients with hilar cholangiocarcinoma were referred to our institution. This study included patients with unresectable hilar CC without chemoradiation who were palliated with only metal stents (control group) or with one session of PDT followed by metal stents (PDT group). Patients were deemed inoperable if they met the following criteria: the presence of type Bismuth-Corlette IV lesions, advanced type III lesions containing T3 tumors, or other types II-III tumors if surgery was contraindicated due to lymph nodes or liver metastases; and age > 80 years or the presence of other co-morbid conditions. Among these patients, metal stents were placed when they met the following conditions: the patient did not want an additional PDT due to the high cost, the patient was referred from a distant location; and there was a change to poor performance status during follow-up. We included patients with lymph node and liver metastasis but excluded those with extrahepatic distant metastasis whose prognoses are expected to be extremely poor.
Using these criteria, 199 patients were excluded because of surgery (n = 38), a plastic stent (n = 72), palliative chemotherapy or radiotherapy (n = 30), more than two sessions of PDT (n = 21), a Karnofsky performance status of less than 60 (n = 10), percutaneous biliary drainage only (n = 11), unavailability for follow-up (n = 4), refusal of endoscopic treatment (n = 12) and sudden death during diagnostic workup (n = 1). After these exclusions, 33 patients with unresectable hilar CC were included in this study. Eighteen patients were treated using uncovered metal stents after one session of PDT (PDT group) and 15 patients were treated by endoscopic metal stenting alone (control group). All patients underwent a standard pretreatment evaluation that included thin-section, contrast-enhanced, multiphase spiral computed tomography and/or magnetic resonance imaging of the abdomen. Tissue diagnosis was obtained by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangioscopy (PTCS) or direct peroral cholangioscopy with biopsy and/or cytology. Patients with no histological confirmation were diagnosed as having malignant disease on the basis of clinical outcome during follow-up for at least 12 mo. All tumors were staged using the American Joint Committee (7th edition) on Cancer Staging criteria for perihilar bile duct cancer[7]. Clinical, laboratory, radiological, endoscopic, and histopathologic data were collected prospectively and analyzed retrospectively. The local institutional review board approved this study.
Photodynamic therapy
PDT was administered by methods described previously[8,9]. Briefly, patients received porfimer sodium (Photofrin II, Axcan Pharma, Quebec, Canada) intravenously at a dose of 2 mg/kg 48-h before ERCP or PTCS. For light distribution, we used flexible cylindrical diffuser probes (BioLitec, Stirling, Scotland) mounted on a 400-μm quartz fiber with an active distal tip length of 2 cm. The light source was a diode laser system (Ceralas PDT 633; CeramOptec, Bonn, Germany) with a maximum power output of 2 W and a wavelength of 633 ± 3 nm. The power emitted by the diffuser tip was calibrated to 400 mW/cm before PDT was conducted using an integrating sphere power meter. The mean irradiation time was 452 s (range: 400-600 s), using a power density of 300-400 mW/cm and energy dose of 180-200 J/cm (of diffuser length). The PDT with PTCS was usually applicable for more advanced Bismuth type-III lesions. To perform the percutaneous PDT procedure, a guide wire was first inserted through the stricture into the common bile duct. A 6-F guiding catheter was then inserted along the guide wire, after which the guide wire was removed. A diffuser fiber was then inserted into the guiding catheter and the stricture site was irradiated from the distal to the proximal region under cholangioscopy and fluoroscopy.
Peroral PDT with ERCP was performed in patients who had coagulopathy or refused percutaneous modality or bismuth II and some of III. To perform the peroral PDT, the preloaded catheter (catheter and PDT fiber) was advanced across the bile duct tumor using a 0.035-inch guide wire. The tip of the catheter was cut just below its metal marker, allowing the fiber to pass. Tumor segments were treated sequentially proximal to distal. In both percutaneous or peroral PDT, PDT was performed in both sides of the intrahepatic duct in Bismuth III and IV strictures. In the case of multiple strictures, as many second branches as possible were treated. After PDT was performed, uncovered metal stents (MI tech, Seoul, South Korea) with diameters of 10 mm and stent lengths of 6 and 8 cm were inserted to ensure adequate decompression and bile drainage. In patients with percutaneous PDT, metal stent was inserted using the Rendezvous technique.
Definition of events and follow-up
The clinical outcome was evaluated according to the following parameters: (1) functional success, as measured by a decrease in the bilirubin level to less than 50% of the pretreatment value within 7 d; (2) early complications (occurring within 30 d); (3) late complications including stent malfunction; and (4) revision methods such as endoscopic retrograde biliary drainage or percutaneous transhepatic biliary drainage (PTBD). Stent patency was calculated as the interval between stent insertion and stent occlusion. Stent occlusion was defined as signs of jaundice or cholangitis (e.g., fever, tenderness in the right upper quadrant, and/or > 2-fold elevation of the total serum bilirubin above baseline after stent insertion).
Follow-up of symptoms and biochemical parameters was done at 1 mo intervals. Tumor markers for carbohydrate antigen (CA) 19-9 and abdominal computed tomography scans were done every 3 mo. ERCP was performed to confirm obstruction and to perform biliary decompression if a new episode of cholangitis occurred. Enrolled patients were followed until death. If the patient died with a patent stent, the time interval was recorded as censored data.
Statistical analysis
Statistical analysis were performed using SPSS software (ver. 18.0; SPSS Inc., Chicago, IL). Numerical data are presented as the median with range. Intergroup comparisons were performed using the Mann-Whitney U test. Estimates of probabilities of survival for the follow-up study and cumulative stent patency were calculated using the Kaplan-Meier method with the log-rank test. Data are presented as medians and 95% CI. For survival rates, all deaths related to CC and procedures were included, but deaths unrelated to CC were treated as censored patients. All of the data were analyzed using an intention-to-treat analysis. Intention-to-treat survival was calculated from the day of treatment until death or the last follow-up. P values ≤ 0.05 were deemed to indicate statistical significance.
RESULTS
Thirty-three patients (24 male), mean age 67.9 years (range: 44-89 years) were treated. A total of 18 (55%) patients received PDT (percutaneous PDT = 11, peroral PDT = 7) and metal stenting, whereas 15 (45%) underwent metal stenting alone. Baseline clinical and demographic profiles in the two treatment groups were similar, except for bismuth type IV (Table 1). There were more patients with bismuth type IV in the PDT group than in the control group (10/18 vs 4/15; P = 0.025). There were no significant differences between the groups with regard to age, gender, pre-procedure bilirubin, CA 19-9, stent length, or tumor stage. The diagnosis was confirmed histologically in 14 PDT patients (78%) and 10 control patients (67%). Histological confirmation of malignancy in PDT group was obtained by brush cytology in three ERCP patients and by intraductal biopsy examination in 11 PTCS patients. In the control group, histological confirmation was obtained by brush cytology in nine ERCP patients, and by intraductal biopsy in one direct cholangioscopy (using the mother-baby scope technique). Successful drainage was achieved in 83% and 86% of patients in PDT and control patients, respectively. No significant difference was observed in the degree of decrease of total bilirubin between the two groups (P = 0.2).
Table 1.
Patient characteristics n (%)
| PDT group (PDT with metal stenting) | Control group (metal stent only) | |
| (n = 18) | (n = 15) | |
| Age, yr (range) | 65.6 (44-89) | 67 (53-82) |
| Male sex | 12 (66) | 12 (80) |
| AJCC stage1 | ||
| II | 3 (17) | 0 (0) |
| IIIA/IIIB | 0 (0)/6(33) | 3 (20)/5 (33) |
| IVA/IVB | 7 (39)/2 (11) | 1 (7)/6 (40) |
| Bismuth type | ||
| II | 3 (17) | 4 (27) |
| III | 5 (28) | 7 (47) |
| IV | 10 (55) | 4 (27) |
| Lymph node metastasis | 13 (72) | 10 (67) |
| Liver metastasis | 2 (11) | 2 (17) |
| CA 19-9 (U/mL) | 229 (0.9-4800) | 480 (1.3-4800) |
| Pre-albumin (g/dL) | 3.5 (2.1-4.5) | 3.2 (2.0-4.3) |
| Bilirubin (mg/dL) | ||
| Pre-treatment | 8.5 (0.6-31.9) | 12.3 (0.9-26.0) |
| Post-treatment | 1.3 (0.5-14.3) | 2.9 (0.3-26.0) |
| Successful drainage2 | 15 (83) | 12 (86) |
| Histologic confirmation | 14 (78) | 10 (67) |
| Unilateral/bilateral stenting | 10 (55)/8 (45) | 11 (73)/4 (27) |
| Early complications of procedure | 3 (17) | 2 (13) |
| Stent malfunctions | 14 (78) | 12 (80) |
| Follow-up period (median, d) | 437 | 230 |
American Joint Committee Cancer (AJCC) staging 7th edition;
Decrease of bilirubin to less than 50% of the pretreatment value within 7 d. Data are expressed as medians (range). PDT: Photodynamic therapy; CA: Carbohydrate antigen.
Stent patency and survival
The metal stent patency duration was longer in the PDT group than in controls (Figure 1A). The median stent patency (range) was 244 ± 66 d (72-570 d) in the PDT group and 177 ± 45 d (70-309 d) in the controls (P = 0.002). Table 2 shows the results of univariate analysis of all variables associated with stent patency in all patients. PDT treatment (P = 0.002) was a significant predictive factor of longer stent patency on univariate analysis (Table 2). Factors including age, gender, bismuth type, pretreatment CA 19-9 and total bilirubin, T stage, number of endoprostheses (unilateral vs bilateral), and PDT method (PTCS vs ERCP) did not significantly affect stent patency according to univariate analysis.
Figure 1.
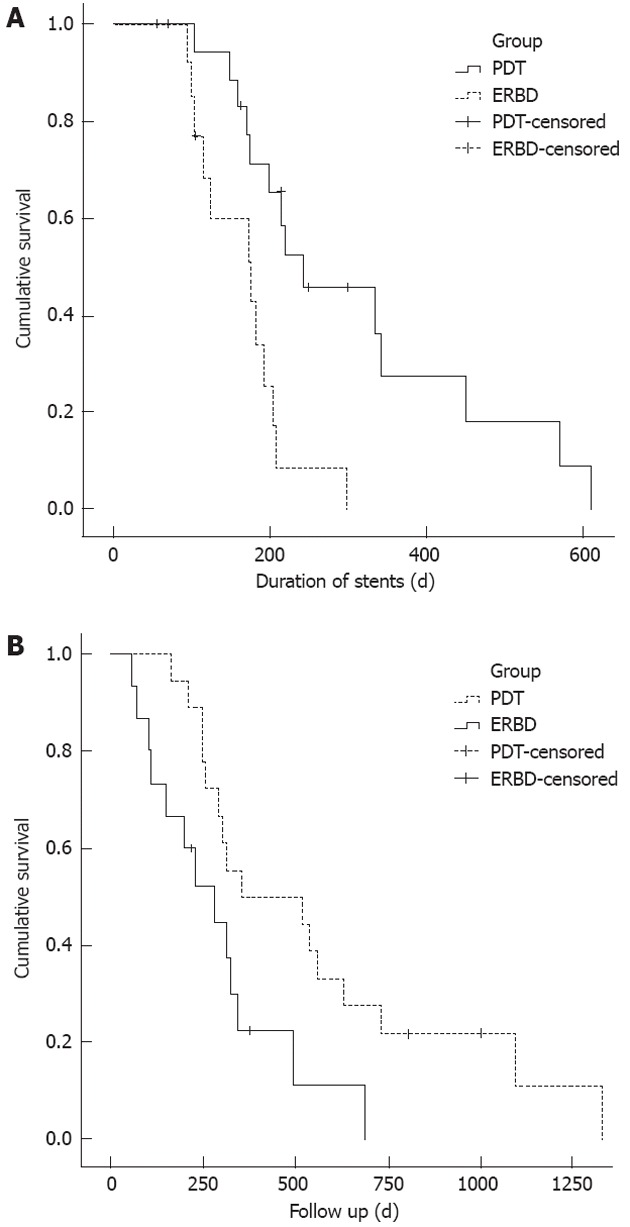
Kaplan-Meier analysis of metal stent patency rates and overall survival in the photodynamic therapy and control groups. A: Kaplan-Meier analysis of metal stent patency rates. The median stent patency was longer in the photodynamic therapy (PDT) group than in the control group (244 d vs 177 d; P = 0.002); B: Kaplan-Meier analysis of overall survival. The median survival in the PDT group was 356 d, vs 230 d in the stent-only group (P = 0.006). ERBD: Endoscopic retrograde biliary drainage.
Table 2.
Univariate analysis of all prognostic factors associated with stent patency and patient survival in all patients
| Variables | Univariate (Kaplan-Meier and log-rank test) | ||||
| Cases | Median stent patency (d) | P value | Median patient survival (d) | P value | |
| Age (yr) | |||||
| < 65 vs ≥ 65 | 12:21 | 174:206 | 0.524 | 539:290 | 0.103 |
| Gender | |||||
| Male vs female | 24:9 | 193:298 | 0.278 | 322:303 | 0.766 |
| CA 19-9 (U/mL) | |||||
| < 100 vs ≥ 100 | 7:26 | 215:177 | 0.308 | 250:317 | 0.145 |
| Pre-PDT bilirubin (mg/dL) | |||||
| < 3.0 vs ≥ 3.0 | 15:18 | 244:181 | 0.112 | 493:250 | 0.026 |
| T stage1 | |||||
| T1,2 vs T3,4 | 11:22 | 174:207 | 0.388 | 317:283 | 0.527 |
| Number of endoprotheses | |||||
| Unilateral vs bilateral | 21:12 | 206:193 | 0.454 | 317:322 | 0.758 |
| Bismuth type | |||||
| II, III vs IV | 19:14 | 206:193 | 0.644 | 283:356 | 0.551 |
| PDT method | |||||
| PTCS vs ERCP | 11:22 | 200:334 | 0.075 | 322:214 | 0.621 |
| Treatment group | |||||
| PDT vs ERBD | 18:15 | 244:177 | 0.002 | 356:283 | 0.023 |
American Joint Committee Cancer (AJCC) staging, 7th edition. PDT: Photodynamic therapy; PTCS: Percutaneous cholangioscopy; ERBD: Endoscopic retrograde biliary drainage; CA: Carbohydrate antigen; ERCP: Endoscopic retrograde cholangiopancreatography.
Compared with the controls, the PDT group was associated with longer patient survival (Figure 1B). The median survival time (range) was 356 ± 213 d (163-1330 d) in the PDT group and 230 ± 73 d (56-687 d) in the controls (P = 0.006). Table 2 shows the results of the univariate analysis of all variables considered. Statistically significant predictors of longer survival were PDT treatment and a lower total bilirubin level before the procedure.
Complications
Early complications were experienced by two control patients (13%) and three (17%) in the PDT group. In the control group, cholangitis occurred in 2 patients (13%), while in the PDT group, cholangitis and cholecystitis were each observed in one patient (6%). PDT-specific adverse events occurred in one patient in the PDT group, who experienced dermal phototoxicity. No stent migration occurred in either group.
Stent malfunctions occurred in 14 (78%) and 12 (80%) of the control and PDT patients, respectively. Among these 26 patients, 22 (85%) with tumor ingrowth were treated endoscopically with insertion of one or two plastic stents (10F) into unilateral or bilateral ducts through metal stents. In four patients with tumor recurrence, tumor ingrowth and overgrowth occurred in the hilar portion and more proximal intrahepatic duct, resulting in revisionary treatment with PTBD.
DISCUSSION
Hilar CC has an extremely poor prognosis, with an average five-year survival rate of 5%-10%. Surgery provides the only possibility for a cure, but due to its anatomical location and natural history, the disease is locally advanced in most patients at the time of diagnosis. Therefore, effective palliation to alleviate symptoms associated with jaundice and the prevention of biliary sepsis are the fundamental goals for most patients with hilar CC[5]. Although relief of biliary obstruction by endoscopic placement of metal stents is regarded as an optimal palliative measure in hilar CC, the clinical course after even successful stent insertion is one of disease progression and death from liver failure or cholangitis within 4-9 mo[10]. This clinical course[1] is related to the ability to decompress affected proximal segments[2] and recurrent stent occlusion, because these stents are unable to remodel malignant tissues[11].
PDT is an evolving therapy for treatment of cancers that are resistant to standard oncologic treatment. PDT involves the injection of an intravenous photosensitizing drug followed by endoscopic application of light to the tumor bed. The interaction between light and the photoagent causes death of cancer cells and tumor thrombosis by generating oxygen free radicals. PDT is currently being used for cases of hilar cholangiocarcinoma[12,13]. Even in patients with advanced hilar CC, PDT has been shown to improve survival, quality of life, and to have a performance superior to that of biliary stenting in uncontrolled and randomized controlled trials[6,14-16]. In our study, Kaplan-Meier analysis demonstrated improved survival in the PDT group compared with the stent-only group (356 d vs 230 d, P = 0.006), in accordance with previous reports[11,14,17].
Effective palliation is essential, because biliary drainage and prevention of cholestasis are crucial for prevention of pruritus, cholangitis, and death in patients with hilar CC. The approach to palliative decompression has evolved from surgery and percutaneous to endoscopic management in order to prevent cholestasis and improve mortality. Endoscopy of hilar CCs is generally challenging and complex due to the involvement of multiple bile ducts requiring two or more stents; indeed, patency rates of endobiliary stents are lower than those of distal tumors[16,18,19]. Moreover, the efficacy of endoscopic stenting in a hilar CC is often limited by stent patency, which is related to proximal tumor obstruction, because the stent does not affect tissue remodeling, unlike benign conditions[11,20,21]. To address this issue, multiple studies have investigated the positive effects of the combination of bile duct stenting with PDT on patient survival[6,14,15]. However, a paucity of information exists regarding the effect of PDT on stent patency.
In our study, metal stent patency was longer in the PDT group than in the stent-only group. The median stent patency was 244 d in the PDT group and 177 d in the control group (P = 0.002). The main causes of obstruction of metal stents in bile ducts is tumor ingrowth or overgrowth[22]. PDT offers the possibility of tumor “remodeling”, which can enhance or prolong the decompression effect[23]. Accepting this hypothesis, the ability of PDT to destroy cancer cells and lessen cholestasis may prolong stent patency. In this context, this study is meaningful because the longer stent patency that is achieved by PDT may diminish the need for further procedures, such as stent revision or percutaneous biliary drainage, improving the quality of life of hilar CC patients whose prognosis is poor.
This study was limited because of its retrospective nature and small sample size. Thus it may be not possible to reach statistical significance in terms of differences in overall survival between the groups. Because hilar CC is a rare malignancy and PDT is offered at few tertiary centers in South Korea, inclusion of sufficient patients to complete a well-designed palliative study is problematic. However, the study population was derived from a larger cohort of patients with CC, who were followed until death. Median survival of this cohort was comparable to that of other cohorts reported in the literature, which may decrease the possibility of significant selection bias.
In summary, metal stenting after one session of PDT may be safe with acceptable complication rates. The PDT group was associated with a significantly longer stent patency period and patient survival compared with the control group in patients with unresectable hilar CC. A prospective randomized multicenter study is required to confirm these data.
COMMENTS
Background
Although endoscopic biliary metal stenting is the mainstay of palliative treatment in patients with unresectable hilar cholangiocarcinoma (CC), tumor ingrowth or overgrowth in uncovered stents is a significant problem. The duration of metal stent patency for hilar CCs was shorter than that for the distal bile duct. Photodynamic therapy (PDT) is an evolving therapy for treatment of hilar CCs that are resistant to standard oncologic treatment.
Research frontiers
The authors observed that the one session of PDT with metal stenting was associated with a significantly longer stent patency period and patient survival compared with the metal stent only group in patients with unresectable hilar CC.
Innovations and breakthroughs
Metal stent patency was longer in the PDT group than in the stent-only group. The median stent patency was 244 d in the PDT group and 177 d in the control group (P = 0.002). The ability of PDT to destroy cancer cells and lessen cholestasis may prolong stent patency.
Applications
Just one session of PDT with endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangioscopy (PTCS) before metal stent placement is promising because it prolongs stent patency in unresectable hilar CC. A prospective randomized multicenter study is required to assess this technique.
Terminology
PDT is an emerging palliative strategy for unresectable hilar CC based on the intravenous administration of photosensitizing agents that preferentially accumulate in malignant cells. PDT is performed to the targeted area of bile ducts using ERCP or PTCS.
Peer review
PDT in patients with CC has been reported as effective palliative treatment providing improvement in cholestasis and quality of life, and prolonging survival. The authors conclude that metallic stenting after PDT may be a safe, acceptable modality associated with longer stent patency in patients with unresectable hilar CC.
Footnotes
Peer reviewer: Jong H Moon, MD, PhD, Professor of Medicine, Digestive Disease Center, Soon Chun Hyang University Bucheon Hospital, No. 1174 Jung-Dong, Wonmi-Ku, Bucheon 420-767, South Korea
S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 2.Hejna M, Pruckmayer M, Raderer M. The role of chemotherapy and radiation in the management of biliary cancer: a review of the literature. Eur J Cancer. 1998;34:977–986. doi: 10.1016/s0959-8049(97)10166-6. [DOI] [PubMed] [Google Scholar]
- 3.Gao F, Bai Y, Ma SR, Liu F, Li ZS. Systematic review: photodynamic therapy for unresectable cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2010;17:125–131. doi: 10.1007/s00534-009-0109-3. [DOI] [PubMed] [Google Scholar]
- 4.Fogel EL, McHenry L, Sherman S, Watkins JL, Lehman GA. Therapeutic biliary endoscopy. Endoscopy. 2005;37:139–145. doi: 10.1055/s-2004-826146. [DOI] [PubMed] [Google Scholar]
- 5.Quyn AJ, Ziyaie D, Polignano FM, Tait IS. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB (Oxford) 2009;11:570–577. doi: 10.1111/j.1477-2574.2009.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Compton CC, Fritz AG, Greene FL, Trotti A, editors . AJCC cancer staging handbook from the AJCC cancer staging manual. 7th ed. New York: NY: Springer; 2010. [Google Scholar]
- 8.Shim CS, Cheon YK, Cha SW, Bhandari S, Moon JH, Cho YD, Kim YS, Lee LS, Lee MS, Kim BS. Prospective study of the effectiveness of percutaneous transhepatic photodynamic therapy for advanced bile duct cancer and the role of intraductal ultrasonography in response assessment. Endoscopy. 2005;37:425–433. doi: 10.1055/s-2005-861294. [DOI] [PubMed] [Google Scholar]
- 9.Cheon YK, Cho YD, Moon JH, Jang JY, Kim YS, Kim YS, Lee MS, Lee JS, Shim CS. Diagnostic utility of interleukin-6 (IL-6) for primary bile duct cancer and changes in serum IL-6 levels following photodynamic therapy. Am J Gastroenterol. 2007;102:2164–2170. doi: 10.1111/j.1572-0241.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 10.Prat F, Chapat O, Ducot B, Ponchon T, Pelletier G, Fritsch J, Choury AD, Buffet C. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1–7. doi: 10.1016/s0016-5107(98)70291-3. [DOI] [PubMed] [Google Scholar]
- 11.Kahaleh M, Mishra R, Shami VM, Northup PG, Berg CL, Bashlor P, Jones P, Ellen K, Weiss GR, Brenin CM, et al. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol. 2008;6:290–297. doi: 10.1016/j.cgh.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Cheon YK, Lee TY, Lee SM, Yoon JY, Shim CS. Longterm outcome of photodynamic therapy compared with biliary stenting alone in patients with advanced hilar cholangiocarcinoma. HPB (Oxford) 2012;14:185–193. doi: 10.1111/j.1477-2574.2011.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheon YK. The role of photodynamic therapy for hilar cholangiocarcinoma. Korean J Intern Med. 2010;25:345–352. doi: 10.3904/kjim.2010.25.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berr F, Wiedmann M, Tannapfel A, Halm U, Kohlhaw KR, Schmidt F, Wittekind C, Hauss J, Mössner J. Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival. Hepatology. 2000;31:291–298. doi: 10.1002/hep.510310205. [DOI] [PubMed] [Google Scholar]
- 15.Cheon YK, Cho YD, Baek SH, Cha SW, Moon JH, Kim YS, Lee JS, Lee MS, Shim CS, Kim BS. Comparison of survival of advanced hilar cholangiocarcinoma after biliary drainage alone versus photodynamic therapy with external drainage. Korean J Gastroenterol. 2004;44:280–287. [PubMed] [Google Scholar]
- 16.Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426–2430. doi: 10.1111/j.1572-0241.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 17.Dumoulin FL, Gerhardt T, Fuchs S, Scheurlen C, Neubrand M, Layer G, Sauerbruch T. Phase II study of photodynamic therapy and metal stent as palliative treatment for nonresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2003;57:860–867. doi: 10.1016/s0016-5107(03)70021-2. [DOI] [PubMed] [Google Scholar]
- 18.Becker CD, Glättli A, Maibach R, Baer HU. Percutaneous palliation of malignant obstructive jaundice with the Wallstent endoprosthesis: follow-up and reintervention in patients with hilar and non-hilar obstruction. J Vasc Interv Radiol. 1993;4:597–604. doi: 10.1016/s1051-0443(93)71930-2. [DOI] [PubMed] [Google Scholar]
- 19.Ducreux M, Liguory C, Lefebvre JF, Ink O, Choury A, Fritsch J, Bonnel D, Derhy S, Etienne JP. Management of malignant hilar biliary obstruction by endoscopy. Results and prognostic factors. Dig Dis Sci. 1992;37:778–783. doi: 10.1007/BF01296439. [DOI] [PubMed] [Google Scholar]
- 20.Polydorou AA, Cairns SR, Dowsett JF, Hatfield AR, Salmon PR, Cotton PB, Russell RC. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32:685–689. doi: 10.1136/gut.32.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deviere J, Baize M, de Toeuf J, Cremer M. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc. 1988;34:95–101. doi: 10.1016/s0016-5107(88)71271-7. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien S, Hatfield AR, Craig PI, Williams SP. A three year follow up of self expanding metal stents in the endoscopic palliation of longterm survivors with malignant biliary obstruction. Gut. 1995;36:618–621. doi: 10.1136/gut.36.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berr F, Tannapfel A, Lamesch P, Pahernik S, Wiedmann M, Halm U, Goetz AE, Mössner J, Hauss J. Neoadjuvant photodynamic therapy before curative resection of proximal bile duct carcinoma. J Hepatol. 2000;32:352–357. doi: 10.1016/s0168-8278(00)80083-5. [DOI] [PubMed] [Google Scholar]


