Abstract
Objectives
Currently, laparoscopic distal pancreatectomy (LDP) is regarded as a safe and effective surgical approach for lesions in the body and tail of the pancreas. This review compares outcomes of the laparoscopic technique with those of open distal pancreatectomy (ODP) and assesses the efficacy, safety and feasibility of each type of procedure.
Methods
Comparative studies published between January 1996 and April 2012 were included. Studies were selected based on specific inclusion and exclusion criteria. Evaluated endpoints were operative outcomes, postoperative recovery and postoperative complications.
Results
Fifteen non-randomized comparative studies that recruited a total of 1456 patients were analysed. Rates of conversion from LDP to open surgery ranged from 0% to 30%. Patients undergoing LDP had less intraoperative blood loss [weighted mean difference (WMD) −263.36.59 ml, 95% confidence interval (CI) −330.48 to −196.23 ml], fewer blood transfusions [odds ratio (OR) 0.28, 95% CI 0.11–0.76], shorter hospital stay (WMD −4.98 days, 95% CI −7.04 to −2.92 days), a higher rate of splenic preservation (OR 2.98, 95% CI 2.18–3.91), earlier oral intake (WMD −2.63 days, 95% CI −4.23 to 1.03 days) and fewer surgical site infections (OR 0.37, 95% CI 0.18–0.75). However, there were no differences between the two approaches with regard to operation time, time to first flatus and the occurrence of pancreatic fistula and other postoperative complications.
Conclusions
Laparoscopic resection results in improved operative and postoperative outcomes compared with open surgery according to the results of the present meta-analyses. It may be a safe and feasible option for patients with lesions in the body and tail of the pancreas. However, randomized controlled trials should be undertaken to confirm the relevance of these early findings.
Introduction
Laparoscopic surgery is now widely accepted and recognized as a standard technique in many surgical procedures.1,2 Initially, the laparoscopic approach was not commonly used in pancreatic resection; however, increasing experience means laparoscopic distal pancreatectomy (LDP) is now performed more frequently in the surgical management of benign, non-invasive and even malignant lesions in the body and tail of the pancreas.3 Some studies have reported LDP to be associated with decreased intraoperative blood loss, a higher rate of splenic conservation, shorter hospital stay and less morbidity compared with open distal pancreatectomy (ODP).4–6 By contrast, other studies report findings in favour of ODP.7,8 Because these various reports indicate a discrepancy in the published literature, the present authors considered it necessary to summarize and analyse the published data to provide evidence to determine whether the literature supports the use of a laparoscopic approach as an alternative to open surgery in the resection of the distal pancreas.
Materials and methods
Study selection
Major databases including PubMed (MEDLINE), EMBASE, the Science Citation Index Expanded and the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library were searched for studies comparing outcomes in LDP and ODP, published in English from January 1996 to April 2012 (the first LDP was described in 1996). The medical search headings (MeSH) ‘laparoscopy’, ‘pancreatectomy’, ‘comparative study’ and combinations of these were used, as were the keywords ‘laparoscopic’, ‘open distal pancreatic resection’, ‘left pancreatic resection’, ‘pancreatic surgery’, ‘distal pancreatectomy’ and ‘minimally invasive surgery’. The reference lists of articles identified were examined to find relevant studies that had not been identified by the database searches. Only comparative clinical trials with full-text descriptions were included. The final inclusion of articles was determined by consensus between authors TJ and KA; when this failed, a third author (JJX) adjudicated.
Inclusion and exclusion criteria
Two authors (TJ and KA) identified and screened the search findings for potentially eligible studies. Inclusion criteria required the studies to: (i) be written in English and published in peer-reviewed journals; (ii) be human studies; (iii) examine at least one of the predetermined outcomes, and (iv) provide clear documentation of the operative techniques as ‘laparoscopic’ or ‘open’. In contexts in which multiple studies were published from the same institution and/or by the same authors, either the higher-quality study or the most recent publication was included in the analysis.
Exclusion criteria excluded: (i) abstracts, letters, editorials, expert opinions, case reports, reviews and studies lacking control groups; (ii) studies that included only patients undergoing spleen-preserving LDP or ODP, and (iii) studies in which patients converted to open surgery were included in the ODP group.
Outcomes of interest and definitions of complications
The following outcomes were used to compare patients undergoing LDP with those undergoing ODP. Operative outcomes included operative time, intraoperative blood loss, blood transfusion and splenic preservation. Postoperative recovery comprised time to oral intake, time to first flatus and duration of postoperative hospital stay. Postoperative complications included pancreatic fistula, clinically significant fistula [International Study Group on Pancreatic Fistula (ISGPF) grades B and C9], mortality, surgical site infection, intra-abdominal abscess, intra-abdominal fluid collection, postoperative haemorrhage, reoperation and readmission.
Data extraction and quality assessment
Data were extracted by two independent observers (WH and MAJ) using standardized forms. Data recorded included patient and study characteristics, pathological characteristics of resected specimens, and operative and postoperative outcomes. The quality of studies was assessed using the modified Newcastle–Ottawa Scale, modified to reflect the needs of this study.10 The maximum numbers of points awarded in the selection, comparability and outcome categories were three, four and two, respectively. Studies achieving six or more points were considered to be of high quality.11 Only these studies were included in the final analyses. Subgroup analyses included all studies (high and low quality) in order to obtain a cumulative result.
Statistical analysis
Meta-analysis was performed using Review Manager Version 5.0 (Cochrane Collaboration, Oxford, UK). For continuous variables, treatment effects were expressed as the weighted mean difference (WMD) with the corresponding 95% confidence interval (CI). For categorical variables, treatment effects were expressed as the odds ratio (OR) with the corresponding 95% CI. Heterogeneity was evaluated using the chi-squared test and a P-value of <0.1 was considered to indicate statistical significance.12 The fixed-effects model was initially calculated for all outcomes,13 but if the test rejected the assumption of homogeneity of studies, random-effects analysis was performed.14 If data in the included studies were considered to be inappropriate for meta-analysis, some outcomes were presented descriptively. Sensitivity analyses were performed by removing individual studies from the dataset and analysing the effect on the overall results to identify sources of significant heterogeneity. Subgroup analyses were undertaken by including high-quality studies to present cumulative evidence. Funnel plots were constructed to evaluate potential publication bias15 based on the major complication of pancreatic fistula.
Results
Description of trials included in the meta-analysis
The search strategy initially generated 427 relevant clinical reports. Finally, 26 full-text articles4–6,16–38 were identified for further investigation. Of these, two studies16,17 were excluded for various reasons: one study included patients in whom LDP had been converted to open surgery in the ODP group,16 and the other included patients who had undergone only spleen-preserving and splenic vessel-preserving LDP or ODP.17 Three studies18–20 reported from the same institution described overlapping patient populations. According to the inclusion criteria, the highest-quality study18 was included in the present meta-analysis. Similarly, of two studies published by Baker et al.,21,22 the higher-quality study21 was included. Six studies were low-quality studies, which should be excluded. Finally, 15 high-quality studies were identified for inclusion; these included one prospective non-randomized study and 14 retrospective studies. Figure 1 shows the process by which comparative studies were selected for inclusion in the present meta-analysis.
Figure 1.
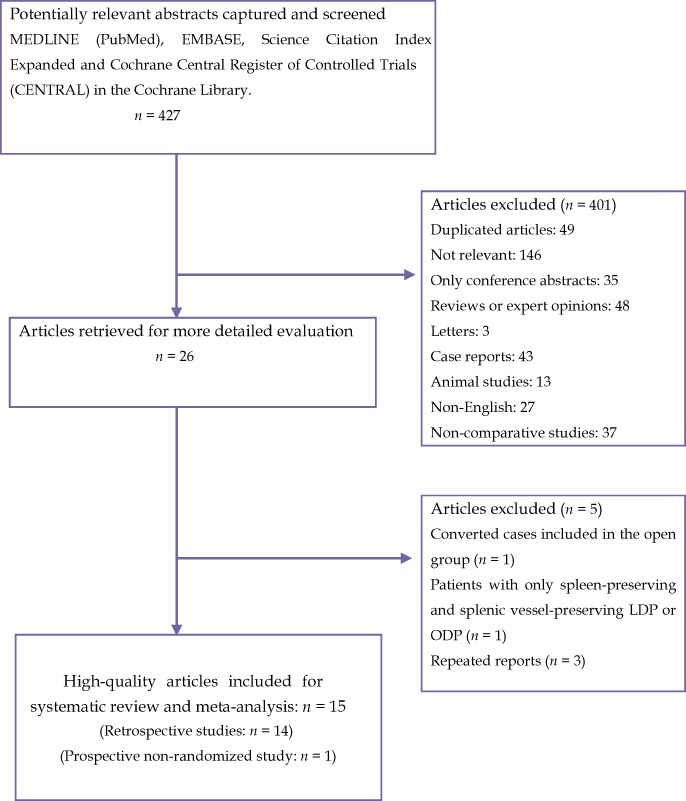
Flow diagram depicting the process of identifying and selecting studies for inclusion. LDP, laparoscopic distal pancreatectomy; OPD, open distal pancreatectomy
Study and patient characteristics
The study characteristics, quality and comparability assessments are shown in Table 1. Details of outcome measures are listed in Table 2. Definitions of pancreatic fistula are shown in Table 3. A total of 1456 patients were included; this number included 561 and 895 patients in the LDP and ODP groups, respectively. Five studies5,6,26,29,32 looked at benign tumours only. The remaining 10 studies4,19,21,27,28,30,31,33,34,38 looked at both benign and malignant lesions. Most of the studies conducted a matched comparative analysis. The results of the analyses are summarized in Table 4.
Table 1.
Characteristics of included studies
| Author(s) | Year | Country | Study design | LDP, n | ODP, n | Inclusion/exclusion criteria | Matching/comparable factorsa | Study qualityb(point scoring scale) |
|---|---|---|---|---|---|---|---|---|
| Velanovich4 | 2006 | USA | Retro | 15 | 15 | Not specified | 2, 4 | ******* |
| Teh et al.5 | 2007 | USA | Retro | 12 | 16 | Benign pancreatic disease (I) | 1, 2, 3, 4 | ****** |
| Matsumoto et al.6 | 2008 | Japan | Retro | 14 | 19 | Benign or borderline malignant pancreatic tumour (I) | 1, 2, 3 | ********* |
| Kim et al.26 | 2008 | Korea | Retro | 93 | 35 | Benign pancreatic disease (I) | 2, 3, 4 | ******* |
| Kooby et al.18 | 2008 | USA | Retro | 23 | 189 | Ductal adenocarcinoma (I) | 1, 2, 3 | ****** |
| Background IPMN/cystadenocarcinoma (E) | ||||||||
| Baker et al.21 | 2009 | USA | PNR | 27 | 85 | Not specified | 1, 3, 4 | ****** |
| Jayaraman et al.27 | 2010 | USA | Retro | 100 | 100 | Additional organ resection (E) | None | ******* |
| DiNorcia et al.28 | 2010 | USA | Retro | 71 | 168 | Laparoscopic-assisted DP (E) | 1 | ****** |
| DP as part of a completion pancreatectomy (E) | ||||||||
| Concomitant portomesenteric venous resection and reconstruction (E) | ||||||||
| DP secondary to debridement for necrotizing pancreatitis (E) | ||||||||
| Non-pancreatic primary neoplasms or pancreatic injury (E) | ||||||||
| Casadei et al.29 | 2010 | Italy | Retro | 22 | 22 | Endocrine and cystic pancreatic tumours (I) | 1, 2 | ******* |
| Ductal adenocarcinoma (E) | ||||||||
| Waters et al.30 | 2010 | USA | Retro | 18 | 22 | Urgent surgery (E) Concurrent major surgery an indication for surgery of acute or chronic pancreatitis (E) | 1, 4 | ****** |
| Mehta et al.31 | 2012 | France | Retro | 30 | 30 | DP for non-pancreatic pathologies (E) | 2, 3, 4 | ****** |
| Resections amounting to less than a DP or total pancreatectomy (E) | ||||||||
| Butturini et al.32 | 2012 | Italy | Retro | 43 | 73 | Benign and borderline neoplasms (I) | 2, 3 | ******** |
| Ductal cancer or other malignant tumours (E) | ||||||||
| Abu et al.38 | 2011 | UK | Retro | 35 | 16 | Additional organ resections (E) | 3, 4 | ***** |
| Fox et al.33 | 2012 | Canada | Retro | 42 | 76 | Additional organ resection (E) | 1, 3, 4 | ***** |
| Limongelli et al.34 | 2012 | Italy | Retro | 16 | 29 | Only tumour enucleation was accomplished (E) | 1, 2, 4 | ****** |
| Additional organ resection (E) | ||||||||
Matching/comparable factors are: 1, American Society of Anesthesiologists (ASA) status; 2, type of pancreatic pathology; 3, mean size of tumour, and 4, presence of chronic pancreatitis.
Based on Newcastle–Ottawa Scale with maximum of **** for selection, ** for comparability, and ** for outcome.
LDP, laparoscopic distal pancreatectomy; ODP, open distal pancreatectomy; Retro, retrospective; PNR, prospective non-randomized; I, inclusion criteria; E, exclusion criteria; IPMN, intraductal papillary mucinous neoplasm; DP, distal pancreatectomy.
Table 2.
Operative and postoperative outcomes of included comparative studies
| Author | Conversion | Spleen preservation | Operation time, min, mean/median (range) | Hospital stay, days, mean/median (range) | PF/clinically significant PF | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||||||||
| LDP | ODP | LDP | ODP | LDP | ODP | LDP | ODP | LDP | ODP | |||
| Velanovich4 | 3 (20) | NA | NA | NA | NA | 5 (3–9) | 8 (6–23) | 2 (13) | 2 (13) | NA | NA | |
| Teh et al.5 | 2 (16.7) | NA | NA | 212 (60–360) | 278 (180–420) | 6.2 (3–16) | 10.6 (7–19) | 1 (8.3) | 1 (6.2) | 0 | 0 | |
| Matsumoto et al.6 | 1 (7.1) | NA | NA | 290.7 ± 53.2a | 213.8 ± 84.6a | 12.9 ± 4.8a | 23.8 ± 11.8a | 0 | 2 (10.5) | 0 | 0 | |
| Kim et al.26 | NA | 38 (40.8) | 2 (5.7) | 195 (82–453) | 190 (88–482) | 10 (5–36) | 16 (8–65) | 8 (8.6) | 5 (14.3) | 0 | 0 | |
| Kooby et al.18 | 20 (12.6) | 43 (30) | 24 (12) | 230 ± 97a | 216 ± 100a | 5.9 ± 3.8a | 9.0 ± 6.0a | 37 (26)/16 (11) | 64 (32)/36 (18) | 0 | 1 (1) | |
| Baker et al.21 | 1 (3.7) | NA | NA | 236 ± 82a | 253.2 ± 292.3a | 4.0 ± 0.3a | 8.6 ± 0.7a | 6 (22)/4 (14.8) | 12 (14)/12 (14.1) | 0 | 1 (1.2) | |
| Jayaraman et al.27 | 32 (30) | 14 (42.4) | 33 (14.0) | 195 | 160 | 5 | 6 | 8 (10.8) | 13 (5.5) | 0 | 2 (0.8) | |
| DiNorcia et al.28 | 24 (25.3) | 11 (15.5) | 26 (15.8) | 191 (163–214) | 192 (157–236) | 5 (4–6) | 6 (5–8) | 8 (11.3) | 25 (14.9) | 0 | 1 (0.6) | |
| Casadei et al.29 | 0 | 4 (18.2) | 4 (18.2) | 225 ± 83b | 145 ± 49b | 8.0 ± 1.3b | 11.0 ± 3.0b | 2 (9.1) | 4 (18.2) | 0 | 0 | |
| Waters et al.30 | 2 (11) | 5 (28) | 3 (14) | 224 (100–346) | 234 (136–437) | 6 (3–34) | 8 (3–25) | 2 (11.1) | 4 (18.2) | 0 | 0 | |
| Mehta et al.31 | 0 | 21 (70) | 9 (30) | 188 ± 72a | 226 ± 87a | 8.7 ± 4.2a | 12.6 ± 8.7a | 5 (16.7)/5 (16.7) | 4 (13.3)/4 (13.3) | 0 | 1 (3.3) | |
| Butturini et al.32 | 0 | 19 (44.2) | 8 (11) | 180 | 180 | 8 | 9 | 12 (27.9) | 10 (13.7) | 0 | 0 | |
| Abu et al.38 | 0 | 14 (40) | 3 (19) | 200 (120–420) | 225 (120–460) | 7 (3–25) | 11 (5–46) | 10 (29) | 7 (44) | NA | NA | |
| Fox et al.33 | 5 (11.91) | 15 (35.7) | 17 (22.4) | 304 (265–348) | 281 (247–333) | 5 (4–6) | 7 (6–9) | 12 (28.6)/0 (0) | 10 (13.2)/4 (5.3) | NA | NA | |
| Limongelli et al.34 | 1 (6) | 5 (31) | 3 (14) | 204 ± 31a | 160 ± 35a | 6.4 ± 2.3a | 8.6 ± 1.7a | 3 (18)/1 (6) | 6 (20)/5 (17.2) | 0 (0) | 1 (3) | |
Mean ± standard deviation.
Median ± standard deviation.
PF, pancreatic fistula; LDP, laparoscopic distal pancreatectomy; ODP, open distal pancreatectomy; NA, not available.
Table 3.
Definitions of postoperative pancreatic fistula in the included studies
| Author | Definition of postoperative pancreatic fistula |
|---|---|
| Velanovich4 | Amylase-rich fluid after PoD 3 |
| Teh et al.5 | Amylase >1000 after PoD 3 |
| Matsumoto et al.6 | Fluid amylase >5000 U/l after PoD 7 |
| Kim et al.26 | Drainage of >30 ml with amylase level >5-fold more than serum level ≥5 days after surgery |
| Kooby et al.18 | Fluid amylase >350 mg/dl or need for postoperative percutaneous fluid collection |
| Baker et al.21 | ISGPF definition |
| Jayaraman et al.27 | ISGPF definition |
| DiNorcia et al.28 | ISGPF definition |
| Casadei et al.29 | ISGPF definition |
| Waters et al.30 | Not defined |
| Mehta et al.31 | ISGPF (grades B and C) |
| Butturini et al.32 | ISGPF definition |
| Abu et al.38 | ISGPF definition |
| Fox et al.33 | ISGPF definition |
| Limongelli et al.34 | ISGPF definition |
PoD, postoperative day; ISGPF, International Study Group on Pancreatic Fistula.
Table 4.
Results of meta-analysis comparing outcomes in laparoscopic and open distal pancreatectomy in high-quality studies
| Outcome of interest | Studies, n | Patients, n | OR/WMD | 95% CI | P-value* |
|---|---|---|---|---|---|
| Operative outcomes | |||||
| Operation time, min | 5 | 343 | 19.71 | −10.01 to 49.44 | 0.19 |
| Intraoperative blood loss, ml | 5 | 343 | −263.36 | −330.48 to −196.23 | <0.00001 |
| Blood transfused | 5 | 375 | 0.28 | 0.11–0.76 | 0.01 |
| Splenic preservation | 10 | 1148 | 2.98 | 2.18–4.06 | <0.00001 |
| Postoperative recovery | |||||
| Time to oral intake, days | 2 | 161 | −2.63 | −4.23 to −1.03 | 0.001 |
| Time to first flatus, days | 2 | 161 | −1.80 | −2.14 to −1.47 | <0.00001 |
| Hospital stay, days | 5 | 343 | −4.98 | −7.04 to −2.92 | <0.00001 |
| Postoperative complications | |||||
| Clinically significant fistula | 4 | 335 | 0.67 | 0.41–1.09 | 0.11 |
| Postoperative haemorrhage | 4 | 329 | 1.87 | 0.59–5.95 | 0.29 |
| Intra-abdominal abscess | 6 | 331 | 0.78 | 0.25–2.45 | 0.67 |
| Intra-abdominal fluid collections | 3 | 304 | 1.47 | 0.67–3.22 | 0.34 |
| Surgical site infection | 5 | 264 | 0.37 | 0.18–0.75 | 0.006 |
| Mortality | 11 | 1155 | 0.61 | 0.16–2.27 | 0.46 |
| Reoperation | 8 | 683 | 0.90 | 0.43–1.84 | 0.76 |
| Readmission | 5 | 772 | 0.70 | 0.41–1.21 | 0.20 |
WMD, weighted mean difference; OR, odds ratio; 95% CI, 95% confidence interval.
Operative outcomes
Five studies6,19,21,31,34 reported operation time. The present analysis showed no statistically significant difference between the two groups (WMD 19.71 min, 95% CI −10.01 to 49.44; P = 0.19). Similarly, findings in five studies6,19,21,31,34 were pooled to provide an estimation of mean blood loss in each of the LDP and ODP groups. Intraoperative blood loss was significantly lower in the LDP group than in the ODP group (WMD −263.36 ml, 95% CI −330.48 to −196.23; P < 0.00001). Additionally, fewer patients required blood transfusions in the LDP group (OR 0.28, 95% CI 0.11–0.76; P = 0.01). The rate of splenic preservation was identified as significantly higher in the LDP group than in the ODP group (OR 2.98, 95% CI 2.18–3.91; P < 0.00001). Forest plots are shown in Fig. 2.
Figure 2.
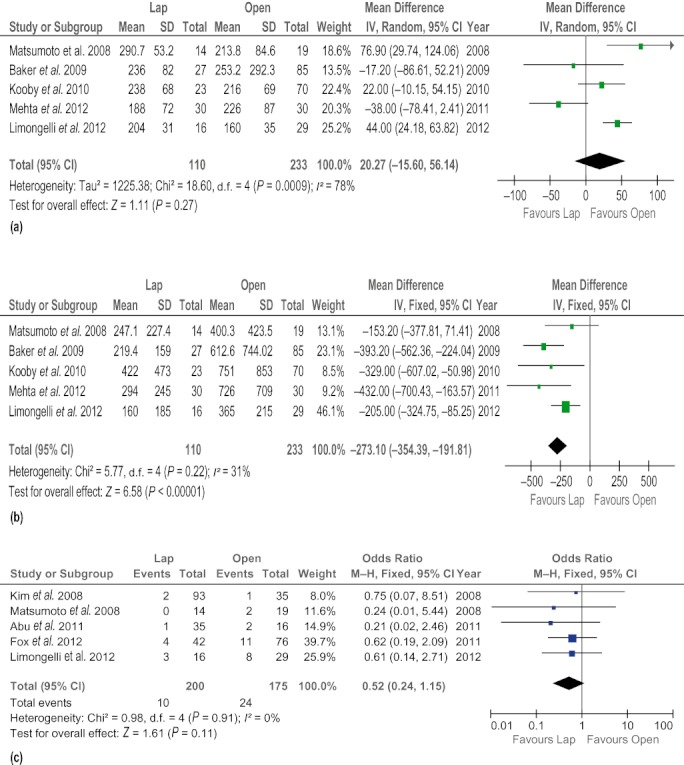
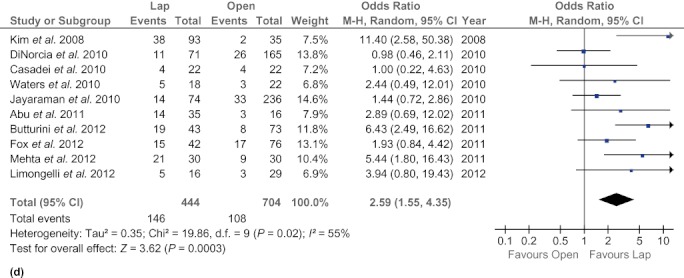
Forest plots illustrating the results of a meta-analysis comparing operative outcomes in laparoscopic and open distal pancreatectomy. Pooled odds ratios (ORs) or weighted mean differences (WMDs) with 95% confidence intervals (CIs) were calculated using the fixed-effects model or the random-effects model. (a) Operation time. (b) Intraoperative blood loss. (c) Blood transfusions. (d) Splenic preservation. SD, standard deviation
Postoperative recovery
Patients in the LDP group had a shorter postoperative hospital stay (WMD −4.98 days, 95% CI −7.04 to −2.92; P < 0.00001) and were able to resume flatus earlier (WMD −1.80 days, 95% CI −2.14 to −1.47; P < 0.000001) than their counterparts in the ODP group (Fig. 3). However, there was no significant difference between the groups in time to oral intake (WMD −2.63 days, 95% CI −4.23 to 1.03; P = 0.001).
Figure 3.
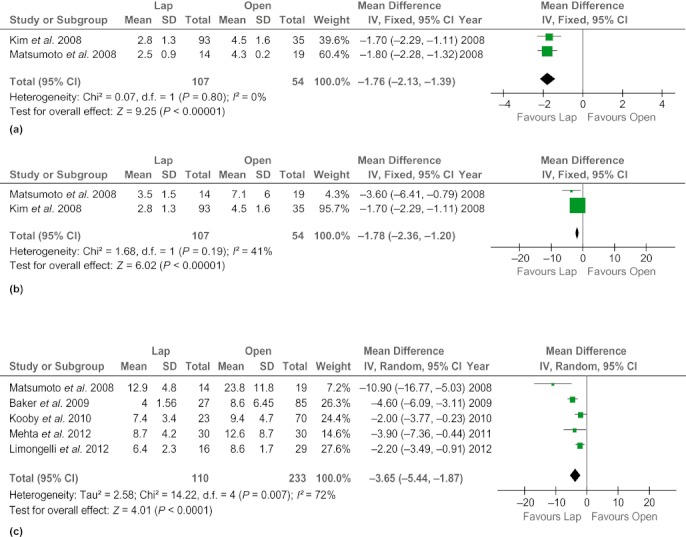
Forest plots illustrating the results of a meta-analysis comparing postoperative recovery outcomes in laparoscopic and open distal pancreatectomy. Pooled weighted mean differences (WMDs) with 95% confidence intervals (CIs) were calculated using the random-effects model. (a) Time to first flatus. (b) Time to oral intake. (c) Length of hospital stay. SD, standard deviation
Postoperative complications
There was no difference in rates of clinically significant fistula (OR 0.67, 95% CI 0.41–1.09; P = 0.11) and mortality (OR 0.61, 95% CI 0.16–2.27; P = 0.46) between the two techniques. The LDP group experienced fewer surgical site infections (OR 0.37, 95% CI 0.18–0.75; P = 0.006), but other postoperative complications, such as intra-abdominal abscesses (OR 0.78, 95% CI 0.25–2.45; P = 0.67), intra-abdominal fluid collections (OR 1.47, 95% CI 0.67–3.22; P = 0.34) and postoperative haemorrhage (OR 1.87, 95% CI 0.59–5.95; P = 0.29), were found to occur at similar frequencies in both groups. In addition, rates of reoperation (OR 0.90, 95% CI 0.43–1.84; P = 0.76) and readmission (OR 0.70, 95% CI 0.41–1.21; P = 0.20) did not differ between the groups (Fig. 4).
Figure 4.
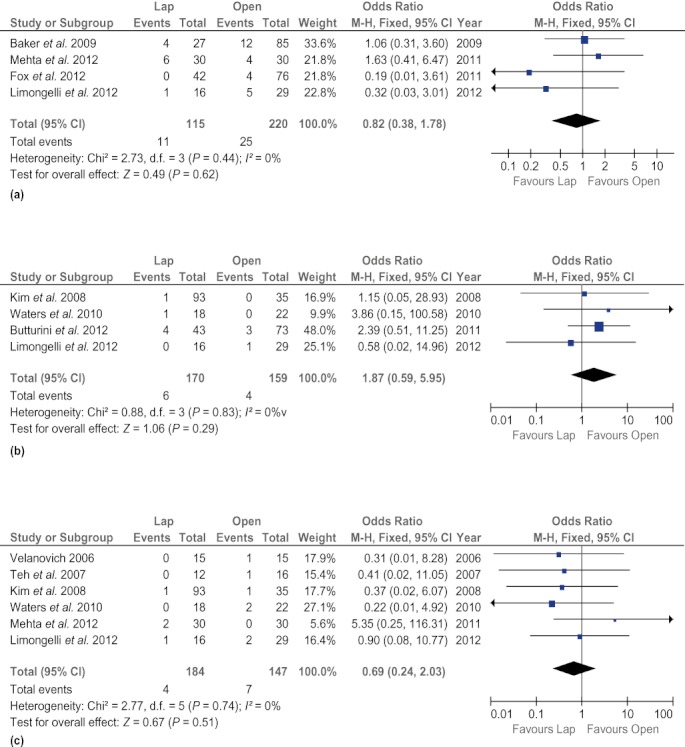
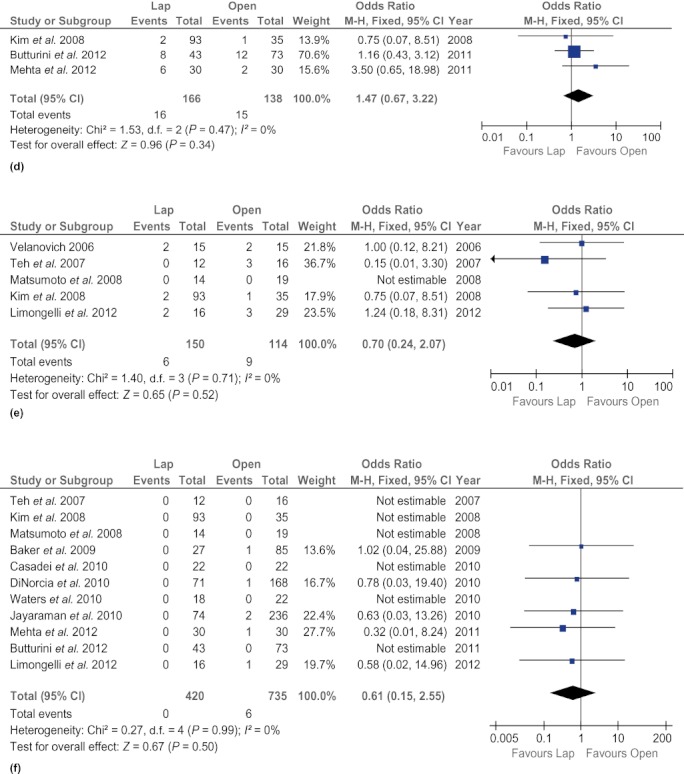
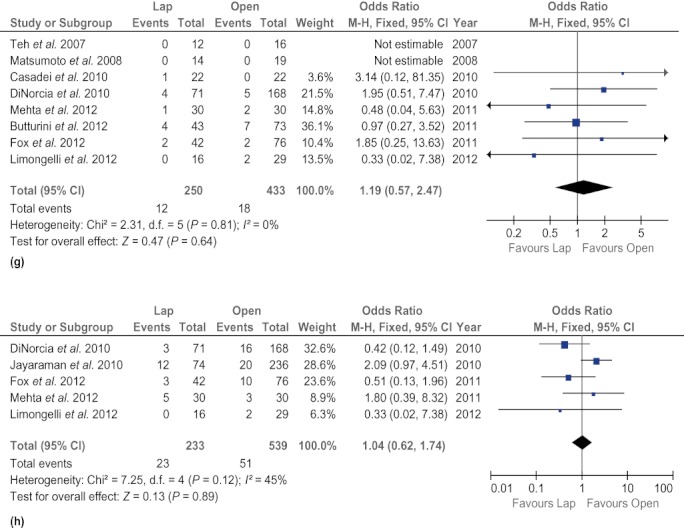
Forest plots illustrating the results of a meta-analysis comparing postoperative complications in laparoscopic and open distal pancreatectomy. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using the fixed-effects model. (a) Clinically significant fistula. (b) Postoperative haemorrhage. (c) Intra-abdominal abscess. (d) Intra-abdominal fluid collections. (e) Surgical site infection. (f) Mortality. (g) Reoperation. (h) Readmission
Sensitivity and subgroup analysis
Sensitivity analyses were carried out by excluding each study from the analysis of each outcome measure. These exclusions did not alter the results obtained in cumulative analyses. In addition, subgroup analyses were undertaken for all outcome measures by including only high-quality studies. Analysis of the high-quality studies showed time to first flatus to be significantly shorter (WMD −1.80 days, 95% CI −2.14 to −1.47; P < 0.00001). However, the analyses for other outcomes did not change in comparison with previous analyses. These are summarized in Table 4.
Publication bias
A funnel plot based on the incidence of pancreatic fistula is shown in Fig. 5. None of the studies lies outside the limits of the 95% CI and hence there is no evidence of publication bias.
Figure 5.
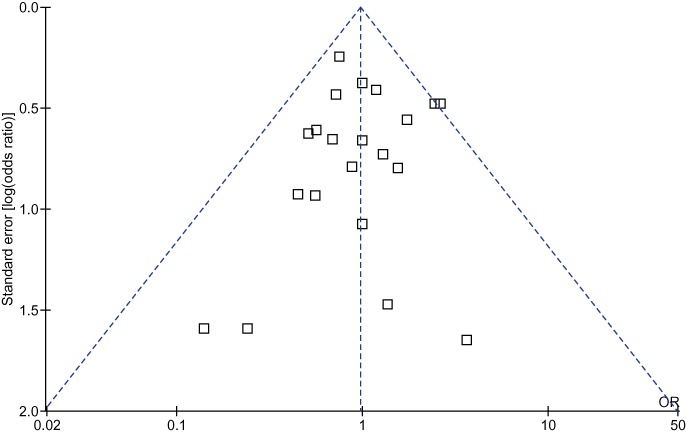
Funnel plot based on incidences of pancreatic fistula. The funnel plot revealed no publication bias
Discussion
Gagner and Pomp39 are considered to have pioneered the introduction of laparoscopy in pancreatic surgery for chronic pancreatitis and published the first description of an LDP. Initially, laparoscopy was mainly used for diagnostic purposes in pancreatology. However, laparoscopic pancreatic resections are gaining in popularity as a result of improvements in technology and increasing laparoscopic surgical experience. Distal pancreatectomy, which involves the resection of the pancreas to the left of the superior mesenteric vessels, is regarded as the standard procedure in chronic pancreatitis or benign and malignant tumours in the body or tail of the pancreas.40 The increased use of LDP represents a paradigm shift from the practice of ODP and the former is now recognized as providing feasible, safe and effective treatment for some conditions of the pancreas.3
The results of the present meta-analysis favour the laparoscopic approach with regard to intraoperative blood loss and blood transfusion rate. Interestingly, there was no difference in operating time between the laparoscopic and open interventions, although the pooled estimate tends to favour the ODP group. This may reflect the fact that the current data were not stratified according to surgical experience, although stage on the learning curve has been shown to affect intraoperative blood loss, operation time and other intraoperative parameters.4 Moreover, patient-specific factors, such as localized fibrosis, inflammatory changes caused by the tumour and tumour infiltration can also prolong the duration of surgery.5
En bloc splenectomy is usually performed during conventional ODP. Some studies have advocated splenic preservation whenever possible as it has been found to be associated with a reduced incidence of perioperative infectious complications and severe complications, and a shorter hospital stay.41–44 The present results illustrate that LDP favours splenic preservation. These findings are consistent with the higher rates of splenic preservation, of up to 85% in some series, reported after LDP,45 which may be attributable to the improved vision afforded by the laparoscopic approach, which, in turn, facilitates the more accurate dissection of the pancreas from the splenic vessels and the splenic hilum.31,32
Margin-negative resection is the only way of curing pancreatic cancer.46 The success of any oncological operation is determined by the achievement of tumour-free margins and lymph node yield. Some studies in the past have suggested that pancreatic adenocarcinoma is a contraindication to laparoscopic resection47,48 because the role and oncologic safety of laparoscopic resection in pancreatic cancer remain unknown. Recent studies49,50 have shown that the laparoscopic approach to malignant pancreatic tumours is feasible and results in similar rates of morbidity and mortality as it does in benign tumours. Of the studies included in the present analyses, only a few studies21,30 with small sample sizes reported this outcome and thus the available data are not sufficient to make a cumulative analysis. Furthermore, none of the studies provide data on longterm oncologic follow-up and therefore additional well-designed studies are needed to provide convincing evidence.
Laparoscopic surgery has the advantage of requiring smaller incisions and less bowel manipulation than does open surgery and thereby reduces pain and analgesic requirements, and facilitates the earlier recovery of bowel function and ambulation.5,6 The results of this present meta-analysis were found to be consistent with published data in terms of indicating a shorter time to oral intake and shorter hospital stay in LDP patients than in ODP patients. When only high-quality studies were included in the analysis, time to first flatus was found to be greatly reduced in the LDP group. Additionally, a low rate of conversion to open surgery was observed, which highlights the feasibility of the laparoscopic approach.
The present analysis found that surgical site infections occurred less frequently in the LDP group compared with the ODP group. This may reflect the association between laparoscopic surgery and reduced surgical trauma, which results in a less acute phase response compared with open surgery, and the fact that local (peritoneal) immune function is affected by carbon dioxide as a result of pneumoperitoneum.51 The present analyses failed to demonstrate any differences between the LDP and ODP groups with regard to postoperative mortality, haemorrhage, reoperation rate, readmission rate, intra-abdominal fluid collections and intra-abdominal abscesses. It is of note that the sample sizes for reporting these complications were small; studies with larger sample sizes are needed to facilitate an accurate summary. It was not possible to undertake cumulative analyses to assess overall morbidity, as reported in many studies, because the criteria used to define such complications varied among the studies. Criteria for assessing morbidity included the DeOliveira scoring system, the Martin scoring system, the National Cancer Institute's common toxicity criteria and the Clavien classification system; it is inappropriate to pool results obtained using such varied systems in order to make a cumulative analysis.
Pancreatic fistula remains the most challenging complication in pancreatic surgery as it can lead to intra-abdominal abscess, delayed gastric emptying, haemorrhage, sepsis and electrolyte imbalances.52 Reported rates of pancreatic fistula in distal pancreatectomy vary between 23% and 26%.53,54 In the present analysis, the pooled results show no significant difference in the rate of pancreatic fistula between the LDP (17%) and ODP (18%) groups and no difference in the rate of clinically significant fistula. It is important to note that although the majority of the reports included in the present analysis used the ISGPF definition of pancreatic fistula, variation exists in this regard and therefore, in order to achieve homogeneity and increase the reliability of the present results, only studies that used the ISGPF definition were included in the analysis.
This meta-analysis of non-randomized studies may have several limitations, which must be taken into account when considering the results. Firstly, all of the studies included were non-randomized in nature and therefore the results provide only a possible estimate. This remains the biggest limitation of this study and results in a weak level of evidence. However, the present study made a strong attempt to select the best evidence available by selecting high-quality studies and pooling their findings. This does not resolve the problem, but it does improve the quality of the synthesized data and adds reliability to the results. It also highlights the need for better designed randomized studies that are able to resolve all of the relevant questions. It is hoped that this will also provide guidelines for the design of future trials on the subject. Secondly, it is important to note that the results were not stratified according to whether the underlying pathology was benign or malignant in nature as the studies included patients with different types of pancreatic disease. This may have an effect on the outcome measures. Thirdly, significant heterogeneity was seen among the included studies for some outcome measures. This may well reflect differences in adjuvant treatment measures and medical insurance systems. However, investigation of this heterogeneity through meta-regression was not possible because of the small number of studies and the unavailability of relevant data. Additionally, it will be of crucial importance to ascertain the financial implications of these procedures in order to make recommendations for their specific indications.
Conclusions
In conclusion, this is the most comprehensive review to date to compare outcomes of LDP and ODP. The present results indicate that LDP is a safe and feasible technique in comparison with ODP. The current findings are reliable and the pooled estimates enable the resolution of some of the discrepancies in data among individual studies in the literature. However, these results need to be validated in large, well-designed randomized controlled trials.
Acknowledgments
The authors would like to thank Professor Robert Sutton, Liverpool National Institute of Health Research (NIHR) Pancreas Biomedical Research Unit, for his support of this work and acknowledge the NIHR for a Biomedical Research Unit award.
Conflicts of interest
None declared.
References
- 1.Sain AH. Laparoscopic cholecystectomy is the current ‘gold standard’ for the treatment of gallstone disease. Ann Surg. 1996;224:689–690. doi: 10.1097/00000658-199611000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith CD, Weber CJ, Amerson JR. Laparoscopic adrenalectomy: new gold standard. World J Surg. 1999;23:389–396. doi: 10.1007/pl00012314. [DOI] [PubMed] [Google Scholar]
- 3.Song KB, Kim SC, Park JB, Kim YH, Jung YS, Kim MH, et al. Single-centre experience of laparoscopic left pancreatic resection in 359 consecutive patients: changing the surgical paradigm of left pancreatic resection. Surg Endosc. 2011;25:3364–3372. doi: 10.1007/s00464-011-1727-9. [DOI] [PubMed] [Google Scholar]
- 4.Velanovich V. Case–control comparison of laparoscopic versus open distal pancreatectomy. J Gastrointest Surg. 2006;10:95–98. doi: 10.1016/j.gassur.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Teh SH, Tseng D, Sheppard BC. Laparoscopic and open distal pancreatic resection for benign pancreatic disease. J Gastrointest Surg. 2007;11:1120–1125. doi: 10.1007/s11605-007-0222-z. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto T, Shibata K, Ohta M, Iwaki K, Uchida H, Yada K, et al. Laparoscopic distal pancreatectomy and open distal pancreatectomy: a non-randomized comparative study. Surg Laparosc Endosc Percutan Tech. 2008;18:340–343. doi: 10.1097/SLE.0b013e3181705d23. [DOI] [PubMed] [Google Scholar]
- 7.Stutchfield BM, Joseph S, Duckworth AD, Garden OJ, Parks RW. Distal pancreatectomy: what is the standard for laparoscopic surgery? HPB. 2009;11:210–214. doi: 10.1111/j.1477-2574.2009.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng WH, Canter RJ, Bold RJ. Perioperative outcomes for open distal pancreatectomy: current benchmarks for comparison. J Gastrointest Surg. 2011;15:2053–2058. doi: 10.1007/s11605-011-1677-5. [DOI] [PubMed] [Google Scholar]
- 9.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Athanasiou T, Al-Ruzzeh S, Kumar P, Crossman MC, Amrani M, Pepper JR, et al. Off-pump myocardial revascularization is associated with less incidence of stroke in elderly patients. Ann Thorac Surg. 2004;77:745–753. doi: 10.1016/j.athoracsur.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms – a meta-analysis. Surgery. 2007;141:203–211. doi: 10.1016/j.surg.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6:341–350. doi: 10.1002/sim.4780060325. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finan KR, Cannon EE, Kim EJ, Wesley MM, Arnoletti PJ, Heslin MJ, et al. Laparoscopic and open distal pancreatectomy: a comparison of outcomes. Am Surg. 2009;75:671–679. [PubMed] [Google Scholar]
- 17.Bruzoni M, Sasson AR. Open and laparoscopic spleen-preserving, splenic vessel-preserving distal pancreatectomy: indications and outcomes. J Gastrointest Surg. 2008;12:1202–1206. doi: 10.1007/s11605-008-0512-0. [DOI] [PubMed] [Google Scholar]
- 18.Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, et al. Left-sided pancreatectomy: a multicentre comparison of laparoscopic and open approaches. Ann Surg. 2008;248:438–446. doi: 10.1097/SLA.0b013e318185a990. [DOI] [PubMed] [Google Scholar]
- 19.Kooby DA, Hawkins WG, Schmidt CM, Weber SM, Bentrem DJ, Gillespie TW, et al. A multicentre analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg. 2010;210:779–785. doi: 10.1016/j.jamcollsurg.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Cho CS, Kooby DA, Schmidt CM, Nakeeb A, Bentrem DJ, Merchant NB, et al. Laparoscopic versus open left pancreatectomy: can preoperative factors indicate the safer technique? Ann Surg. 2011;253:975–980. doi: 10.1097/SLA.0b013e3182128869. [DOI] [PubMed] [Google Scholar]
- 21.Baker MS, Bentrem DJ, Ujiki MB, Stocker S, Talamonti MS. A prospective single institution comparison of perioperative outcomes for laparoscopic and open distal pancreatectomy. Surgery. 2009;146:635–643. doi: 10.1016/j.surg.2009.06.045. discussion 643–645. [DOI] [PubMed] [Google Scholar]
- 22.Baker MS, Bentrem DJ, Ujiki MB, Stocker S, Talamonti MS. Adding days spent in readmission to the initial postoperative length of stay limits the perceived benefit of laparoscopic distal pancreatectomy when compared with open distal pancreatectomy. Am J Surg. 2011;201:295–299. doi: 10.1016/j.amjsurg.2010.09.014. discussion 299–300. [DOI] [PubMed] [Google Scholar]
- 23.Tang CN, Tsui KK, Ha JP, Wong DC, Li MK. Laparoscopic distal pancreatectomy: a comparative study. Hepatogastroenterology. 2007;54:265–271. [PubMed] [Google Scholar]
- 24.Nakamura Y, Uchida E, Aimoto T, Matsumoto S, Yoshida H, Tajiri T. Clinical outcome of laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Surg. 2009;16:35–41. doi: 10.1007/s00534-008-0007-0. [DOI] [PubMed] [Google Scholar]
- 25.Shimura T, Suehiro T, Mochida Y, Hashimoto S, Okada K, Asao T, et al. Laparoscopy-assisted distal pancreatectomy with mobilization of the distal pancreas and the spleen outside the abdominal cavity. Surg Laparosc Endosc Percutan Tech. 2006;16:387–389. doi: 10.1097/01.sle.0000213731.65085.61. [DOI] [PubMed] [Google Scholar]
- 26.Kim SC, Park KT, Hwang JW, Shin HC, Lee SS, Seo DW, et al. Comparative analysis of clinical outcomes for laparoscopic distal pancreatic resection and open distal pancreatic resection at a single institution. Surg Endosc. 2008;22:2261–2268. doi: 10.1007/s00464-008-9973-1. [DOI] [PubMed] [Google Scholar]
- 27.Jayaraman S, Gonen M, Brennan MF, D'Angelica MI, DeMatteo RP, Fong Y, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg. 2010;211:503–509. doi: 10.1016/j.jamcollsurg.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 28.DiNorcia J, Schrope BA, Lee MK, Reavey PL, Rosen SJ, Lee JA, et al. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. J Gastrointest Surg. 2010;14:1804–1812. doi: 10.1007/s11605-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casadei R, Ricci C, D'Ambra M, Marrano N, Alagna V, Rega D, et al. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: a case–control study. Updat Surg. 2010;62:171–174. doi: 10.1007/s13304-010-0027-6. [DOI] [PubMed] [Google Scholar]
- 30.Waters JA, Canal DF, Wiebke EA, Dumas RP, Beane JD, Aguilar-Saavedra JR, et al. Robotic distal pancreatectomy: cost-effective? Surgery. 2010;148:814–823. doi: 10.1016/j.surg.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 31.Mehta SS, Doumane G, Mura T, Nocca D, Fabre JM. Laparoscopic versus open distal pancreatectomy: a single-institution case–control study. Surg Endosc. 2012;26:402–407. doi: 10.1007/s00464-011-1887-7. [DOI] [PubMed] [Google Scholar]
- 32.Butturini G, Inama M, Malleo G, Manfredi R, Melotti GL, Piccoli M, et al. Perioperative and longterm results of laparoscopic spleen-preserving distal pancreatectomy with or without splenic vessels conservation: a retrospective analysis. J Surg Oncol. 2012;105:387–392. doi: 10.1002/jso.22117. [DOI] [PubMed] [Google Scholar]
- 33.Fox AM, Pitzul K, Bhojani F, Kaplan M, Moulton CA, Wei AC, et al. Comparison of outcomes and costs between laparoscopic distal pancreatectomy and open resection at a single centre. Surg Endosc. 2012;26:1220–1230. doi: 10.1007/s00464-011-2061-y. [DOI] [PubMed] [Google Scholar]
- 34.Limongelli P, Belli A, Russo G, Cioffi L, D'Agostino A, Fantini C, et al. Laparoscopic and open surgical treatment of left-sided pancreatic lesions: clinical outcomes and cost-effectiveness analysis. Surg Endosc. 2012;26:1830–1836. doi: 10.1007/s00464-011-2141-z. [DOI] [PubMed] [Google Scholar]
- 35.Eom BW, Jang JY, Lee SE, Han HS, Yoon YS, Kim SW. Clinical outcomes compared between laparoscopic and open distal pancreatectomy. Surg Endosc. 2008;22:1334–1338. doi: 10.1007/s00464-007-9660-7. [DOI] [PubMed] [Google Scholar]
- 36.Aly MY, Tsutsumi K, Nakamura M, Sato N, Takahata S, Ueda J, et al. Comparative study of laparoscopic and open distal pancreatectomy. J Laparoendosc Adv Surg Tech A. 2010;20:435–440. doi: 10.1089/lap.2009.0412. [DOI] [PubMed] [Google Scholar]
- 37.Vijan SS, Ahmed KA, Harmsen WS, Que FG, Reid-Lombardo KM, Nagorney DM, et al. Laparoscopic vs. open distal pancreatectomy: a single-institution comparative study. Arch Surg. 2010;145:616–621. doi: 10.1001/archsurg.2010.120. [DOI] [PubMed] [Google Scholar]
- 38.Abu Hilal M, Hamdan M, Di Fabio F, Pearce NW, Johnson CD. Laparoscopic versus open distal pancreatectomy: a clinical and cost-effectiveness study. Surg Endosc. 2011;26:1670–1674. doi: 10.1007/s00464-011-2090-6. [DOI] [PubMed] [Google Scholar]
- 39.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408–410. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- 40.Andren-Sandberg A, Wagner M, Tihanyi T, Lofgren P, Friess H. Technical aspects of left-sided pancreatic resection for cancer. Dig Surg. 1999;16:305–312. doi: 10.1159/000018740. [DOI] [PubMed] [Google Scholar]
- 41.Shoup M, Brennan MF, McWhite K, Leung DH, Klimstra D, Conlon KC. The value of splenic preservation with distal pancreatectomy. Arch Surg. 2002;137:164–168. doi: 10.1001/archsurg.137.2.164. [DOI] [PubMed] [Google Scholar]
- 42.Mekeel KL, Moss AA, Reddy KS, Mulligan DC, Harold KL. Laparoscopic distal pancreatectomy: does splenic preservation affect outcomes? Surg Laparosc Endosc Percutan Tech. 2011;21:362–365. doi: 10.1097/SLE.0b013e31822e0ea8. [DOI] [PubMed] [Google Scholar]
- 43.Aldridge MC, Williamson RC. Distal pancreatectomy with and without splenectomy. Br J Surg. 1991;78:976–979. doi: 10.1002/bjs.1800780827. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Cruz L, Blanco L, Cosa R, Rendon H. Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumours? World J Surg. 2008;32:904–917. doi: 10.1007/s00268-008-9467-2. [DOI] [PubMed] [Google Scholar]
- 45.Taylor C, O'Rourke N, Nathanson L, Martin I, Hopkins G, Layani L, et al. Laparoscopic distal pancreatectomy: the Brisbane experience of forty-six cases. HPB. 2008;10:38–42. doi: 10.1080/13651820701802312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang DK, Johns AL, Merrett ND, Gill AJ, Colvin EK, Scarlett CJ, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27:2855–2862. doi: 10.1200/JCO.2008.20.5104. [DOI] [PubMed] [Google Scholar]
- 47.Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc. 2010;24:1533–1541. doi: 10.1007/s00464-009-0806-7. [DOI] [PubMed] [Google Scholar]
- 48.Patterson EJ, Gagner M, Salky B, Inabnet WB, Brower S, Edye M, et al. Laparoscopic pancreatic resection: single-institution experience of 19 patients. J Am Coll Surg. 2001;193:281–287. doi: 10.1016/s1072-7515(01)01018-3. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Cruz L, Cosa R, Blanco L, Levi S, Lopez-Boado MA, Navarro S. Curative laparoscopic resection for pancreatic neoplasms: a critical analysis from a single institution. J Gastrointest Surg. 2007;11:1607–1621. doi: 10.1007/s11605-007-0266-0. discussion 1621–1622. [DOI] [PubMed] [Google Scholar]
- 50.Gumbs AA, Chouillard EK. Laparoscopic distal pancreatectomy and splenectomy for malignant tumours. J Gastrointest Cancer. 2012;43:83–86. doi: 10.1007/s12029-011-9347-0. [DOI] [PubMed] [Google Scholar]
- 51.Targarona EM, Balague C, Knook MM, Trias M. Laparoscopic surgery and surgical infection. Br J Surg. 2000;87:536–544. doi: 10.1046/j.1365-2168.2000.01429.x. [DOI] [PubMed] [Google Scholar]
- 52.Knaebel HP, Diener MK, Wente MN, Buchler MW, Seiler CM. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92:539–546. doi: 10.1002/bjs.5000. [DOI] [PubMed] [Google Scholar]
- 53.Fahy BN, Frey CF, Ho HS, Beckett L, Bold RJ. Morbidity, mortality, and technical factors of distal pancreatectomy. Am J Surg. 2002;183:237–241. doi: 10.1016/s0002-9610(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 54.Pannegeon V, Pessaux P, Sauvanet A, Vullierme MP, Kianmanesh R, Belghiti J. Pancreatic fistula after distal pancreatectomy: predictive risk factors and value of conservative treatment. Arch Surg. 2006;141:1071–1076. doi: 10.1001/archsurg.141.11.1071. [DOI] [PubMed] [Google Scholar]


