Abstract
Objectives
Biliary mucinous cystic neoplasms (BMCNs) are recently redefined rare liver tumours in which insufficient recognition frequently leads to an incorrect initial or delayed diagnosis. A concise review of the subtle, sometimes non-specific, clinical, serologic and radiographic features will allow for a heightened awareness and more comprehensive understanding of these entities.
Methods
Literature relating to the presentation, diagnosis, treatment, pathology and outcomes of BMCNs and published prior to March 2012 was reviewed.
Results
Biliary mucinous cystic neoplasms most commonly occur in females (≥60%) in the fifth decade of life. Clinical symptoms, serologic markers and imaging modalities are unreliable for diagnosis of BMCNs, which leads to misdiagnosis in 55–100% of patients. Perioperative cyst aspiration is not recommended as invasive BMCNs can only be differentiated from non-invasive BMCNs by microscopic evaluation for the presence of ovarian-type stroma. Intraoperative biopsy and frozen section(s) are essential to differentiate BMCNs from other cystic liver lesions. The treatment of choice is complete excision and can result in excellent survival with initial correct diagnosis.
Conclusions
A low threshold for considering BMCN in the differential diagnosis of cystic liver lesions and increased attentiveness to its subtle diagnostic characteristics are imperative. The complete surgical resection of BMCNs and the use of appropriate nomenclature are necessary to improve outcomes and accurately define prognosis.
Introduction
Biliary mucinous cystic neoplasms (BMCNs) of the liver are rare entities. Previously, these lesions have been reported under the general terms of ‘biliary cystadenoma’ and ‘biliary cystadenocarcinoma’. This trend continues to pervade the literature. However, this lesion type was redefined and classified by the World Health Organization (WHO) in 2010 and is now defined as ‘a cyst-forming epithelial neoplasm, usually with no communication with the bile ducts, composed of cuboidal to columnar, variably mucin-producing epithelium, associated with ovarian-type subepithelial stroma’ and is subdivided into non-invasive and invasive types.1 Non-invasive mucinous cystic neoplasms (MCNs) are categorized by the highest degree of cytoarchitectural atypia present into three groups: (i) MCNs with low-grade intraepithelial dysplasia; (ii) MCNs with intermediate-grade intraepithelial dysplasia, and (iii) MCNs with high-grade intraepithelial dysplasia.1 If there is an invasive carcinoma component, the lesion is denoted as an MCN with associated invasive carcinoma.1 Based on the current requirement for the presence of ovarian-type stroma, it is likely that many of these neoplasms previously reported as variants without ovarian stroma would now be classified as intraductal papillary neoplasms (IPNs) of the bile ducts with marked cystic changes.1
Invasive BMCNs, along with their slightly more common counterparts, non-invasive BMCNs, arise from the liver, bile duct and, occasionally, gallbladder2 and together are routinely reported to comprise <5% of all liver cysts.3–8 Some authors believe this is an overestimation and assert that BMCNs probably account for <1% of liver cysts.9,10 It has been proposed that BMCNs may represent 5% of all symptomatic hepatic cysts referred for therapy.9,10
As a result of equivocal clinical findings and challenging preoperative radiologic assessment, intrahepatic invasive BMCN is often difficult to distinguish from non-invasive BMCN, hepatic abscess, cholangiocarcinoma and other benign liver cysts.2,8,11 Insufficient recognition as a result of this tumour's low incidence can delay correct diagnosis to the point at which curative management is no longer possible.13,14
The treatment of choice for both non-invasive and invasive BMCN is surgical excision, but resectability is dependent on the anatomic location of the tumour, functional liver reserve and medical comorbidities.2,7,8,15–17 Complete surgical resection is critical in order to reliably identify the tumour's degree of malignancy.2,4 Reported recurrence rates following complete surgical excision vary between 10% and 13%, but can be affected by study sizes that are frequently small as a result of the rarity of these tumours.2,18,19 Unlike other primary hepatic tumours, systemic therapies have not been found to be particularly effective in the treatment of primary invasive BMCN.
A review of the presentation, diagnosis, treatment, pathology and outcomes of BMCNs is presented.
Materials and methods
Searches of MEDLINE and EMBASE were performed to identify case reports, case series and articles pertaining to diagnostic imaging and pathology of MCNs of the liver published prior to March 2012. The search terms included ‘mucinous cystic neoplasm’, ‘biliary cystadenoma’ and ‘biliary cystadenocarcinoma’. Additional articles were obtained by cross-referencing relevant articles. An organized discussion regarding the presentation, diagnosis, pathology, treatment and outcomes of MCNs of the liver was then undertaken. The article selection process is summarized in Fig. 1. The Cochrane Database of Systematic Reviews was then cross-checked to confirm that no similar reviews had been undertaken.
Figure 1.
QUORUM (quality of reporting of meta-analyses) algorithm of review of biliary mucinous cystic neoplasms
Results
Epidemiology and clinical presentation
Intrahepatic non-invasive and invasive BMCNs comprise <5% of all liver cysts.7,8,20 The invasive BMCN (then referred to as a ‘biliary cystadenocarcinoma’) was first described by Willis in 1943 and defined as an entity by Edmondson in 1958 as a multilocular lesion lined by columnar epithelium with an accompanying densely cellular (‘ovarian-like’) stroma.11,12,17 Over the 70 years since its definition, its exact incidence among malignant hepatic epithelial tumours has remained unknown, but has been reported to be as low as 0.41%.7,13,20–22 This may actually be an underestimation as both non-invasive and invasive BMCNs are being discovered with increasing frequency secondary to advances in abdominal imaging and as a result of growing awareness of the cysts themselves.10,15,23,24 Of note, the incidence of simple hepatic cysts has been established by both computed tomography (CT) and autopsies to be 14–24% and to increase with age.20,23
Primary intrahepatic BMCN most commonly presents in the fifth decade of life, occurs more commonly in females (approximately 60%) and appears to occur at even higher rates in women if there is no associated invasive carcinoma.1,11,17,19,24 Clinical manifestations are non-specific and widely variable. Frequent complaints (in approximately 60% of patients) include right upper quadrant or epigastric pain or discomfort, abdominal fullness and a palpable abdominal mass.2,8,15,25,26 Less frequent symptoms include fever, weight loss, jaundice and ascites.2,8 Elevated liver function tests are reported in up to 26% of patients.2 However, it is not uncommon for a patient to be asymptomatic at presentation (30–58%).4,11,27–29 Diagnostic imaging of BMCNs is difficult and can frequently lead to missed or delayed diagnosis.15,16,19 Ultrasound (US), CT and magnetic resonance imaging (MRI) all typically demonstrate non-specific multilocular tumours with septal or mural nodules.2
In a thought-provoking case series by Zhang et al., the incidental finding of invasive BMCN after laparoscopic resection for hepatic cystic lesions is discussed.19 These authors made presumptive diagnoses by US of hepatic cysts in two patients and cystadenomas in three patients.19 All five patients were found to have invasive BMCNs on final pathology, which again emphasizes that invasive BMCNs cannot be reliably diagnosed or distinguished from other liver cysts using preoperative imaging alone.
Differential diagnosis
The critical issue in the workup of a complicated cystic lesion of the liver concerns the distinguishing of primary intrahepatic non-invasive and invasive BMCNs from benign conditions that require only conservative management and observation.30 In addition to primary non-invasive and invasive BMCNs, differential diagnosis should include hepatic abscess, haematoma, haemorrhagic cyst, simple congenital cyst, hydatid (echinococcal) cyst, polycystic disease, cystic hamartoma or post-traumatic cysts, Caroli's disease, other neoplastic lesions such as undifferentiated embryonal sarcoma, cystic primary hepatocellular carcinoma, metastatic pancreatic or ovarian cystadenocarcinoma, intraductal papillary mucinous neoplasm biliary type (IPMN-B) and hepatobiliary mesenchymal tumours (particularly biliary smooth muscle neoplasms), such as biliary leiomyoma, adenomyoma and primary hepatic leiomyosarcoma (Table 1).4,13,20,24,25,31,32
Table 1.
Differential diagnosis of cystic liver lesions
 |
Given the extensive possibilities in differential diagnosis of a complicated cystic hepatic lesion, liver abscess and hydatid cyst are the two entities reported to be the most likely to be confused with non-invasive and invasive BMCNs.31,33 That the incidence of simple hepatic cysts increases with age further complicates the problem as patients with unilocular non-invasive or invasive BMCN are subject to an increased likelihood of misdiagnosis.10 Metastatic lesions with cystic degeneration and cystic cholangiocarcinoma, as well as primary or metastatic necrotic neoplasms, should also be considered.4,13,20,24,25,31,32
Recently, Wang et al. sought to develop an algorithm for the preoperative differentiation of non-invasive and invasive BMCNs in their retrospective review of 20 non-invasive and 30 invasive BMCNs.28 Overall, older age, male gender and shorter symptom duration were associated with a higher possibility of invasive BMCN.28 Arterial blood flow and nodule enhancement tended to be more common in invasive BMCN, but this increase in frequency did not reach statistical significance.28 Additional patients are needed to validate this scoring system, but it may be of potential clinical value in the future.
Rare presentations
Recurrent jaundice with cholangitis has been reported as a rare presentation. For example, a patient reported by Ishak et al. had three episodes of obstructive jaundice over a 19-month period.8 Initially, the patient underwent excision of a completely occluding common bile duct tumour, but suffered two subsequent recurrences of invasive BMCN.8
Compression of the portal system and inferior vena cava obstruction have also been reported as rare presentations. One such patient, with multiple hepatic tumours but without reported presenting symptoms, was found to have compression of the right main portal vein and hepatofugal flow in the left gastric vein on a post-arterial portogram.7 The patient was reported to have no evidence of disease at 7 months after resection of invasive BMCN.7 Others have reported bilateral lower extremity swelling as a result of vena cava obstruction.8,34 Subclinical fevers have been noted as occasional initial presentations of invasive BMCN.20 Infrequently, presentations with painful haemorrhage, tumour rupture or fever from secondary infection have been described.34,35
Pathophysiology
Biliary mucinous cystic neoplasms are slow-growing, frequently reach a large size, and can progress over a period of years to invasive carcinoma.1 There are several different theories regarding the origin of intrahepatic BMCNs. One of the most predominant theories is malignant epithelial transformation from non-invasive to invasive BMCN, which is believed to occur over a period of several years.8,11,19 In 1970, this transformation was demonstrated in rats fed on an aflatoxin diet.36 The potential for malignant transformation from non-invasive to invasive BMCN has also been documented retrospectively on review of serial CT imaging in a few individuals.15,37–39 This type of invasive BMCN is noted to have a characteristic mesenchymal stroma and a much higher predilection for females, and to follow a relatively indolent course in most patients.2,40
The presence of ovarian stroma in BMCNs suggests a correlation with ovarian MCNs and has led to the hypothesis of an embryonal origin.6 It has been suggested that the close proximity of the liver and gonads during embryonic development is responsible for the migration of gonadal cells into the liver surface and the resultant ovarian stroma in these lesions.6,41 Further, the peritoneal surface epithelium of the embryonic gonads has been found to be lined with bulging cells as opposed to the typical flattened celomic epithelium. The examination of embryos suggests that during the embryonic period, these bulging cells detach and migrate into the surfaces of nearby organs such as the liver.6
Biliary mucinous cystic neoplasms may arise from neoplasia of the normal intrahepatic bile ducts, as a result of malignant transformation of other cystic lesions in the liver or from congenital hepatic or biliary malformations.2,11,19,42–44 These congenital malformations or anomalies may include choledochal cysts, Caroli's disease, congenital hepatic fibrosis, polycystic disease and ectopic remnants of primitive foregut sequestered in the liver.2,8,42,43 The majority of these tumours are slow-growing.2
All types of BMCN can become quite large; they range from 1.2 cm to 40 cm in size28,29,31,45 and are found to be multilocular in up to 84% of patients.2,46 Both non-invasive and invasive BMCNs occur in both the right and left lobes. Some authors have reported that all BMCNs (non-invasive and invasive types) demonstrate a predilection for the right hepatic lobe,5,8,31,34,47 whereas others report a predilection for the left hepatic lobe in non-invasive and/or invasive BMCNs.3,4,15,27,48 Yet other studies have revealed no lobe-specific predilection.21,33,40 The most recent analysis of the clinical characteristics of non-invasive and invasive BMCNs, by Wang et al., found that 70% of tumours were located in the left lobe of the liver (n = 21).28 All invasive BMCNs were noted to affect the left lobe (10/10, 100%), whereas all those in other lobes were benign.28
Invasive BMCN can be differentiated from non-invasive BMCN only by microscopic examination.7,13 Based on experience, frozen-section microscopy can confirm or deny the presence of a BMCN on a consistent basis as it requires only evaluation for the presence or absence of mucinous epithelium and ovarian-type stroma. Thus, it is essential to perform a cyst wall biopsy using frozen sections at the time of operation. Invasive BMCN is more difficult to diagnose as stromal invasion is challenging to identify and may at times be present in only very focal areas (Fig. 2). Therefore, resected cysts should be extensively sampled before a final pathological diagnosis is rendered.
Figure 2.
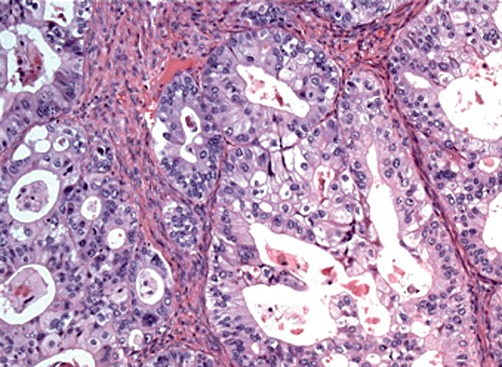
Histopathology showing glands with cribriform architecture infiltrating stroma. (Haematoxylin and eosin stain; original magnification ×40)
Morphologically, invasive BMCN differs from non-invasive BMCN in that cellular pleomorphism, anaplasia and infiltration of the underlying fibrous stroma are present in invasive BMCN, but absent from non-invasive BMCN.8,14,49 The lining cells of the cyst show considerable variation in size and atypia in their nuclei, as well as loss of polarity.8,49 More simply, although extensive infiltration of mucin-producing adenocarcinoma can be found in the walls of the cyst, there can also be occasional patches of lining that are benign and consist of a single layer of cuboid to columnar epithelium.8,50 In the variant in which mesenchymal (‘ovarian-like’) stroma is present, it is visualized between an inner epithelial lining and an outer connective tissue capsule.15,51Figure 3 (a–c) shows illustrative examples. Papillary projections of the epithelial cells are also common.50
Figure 3.
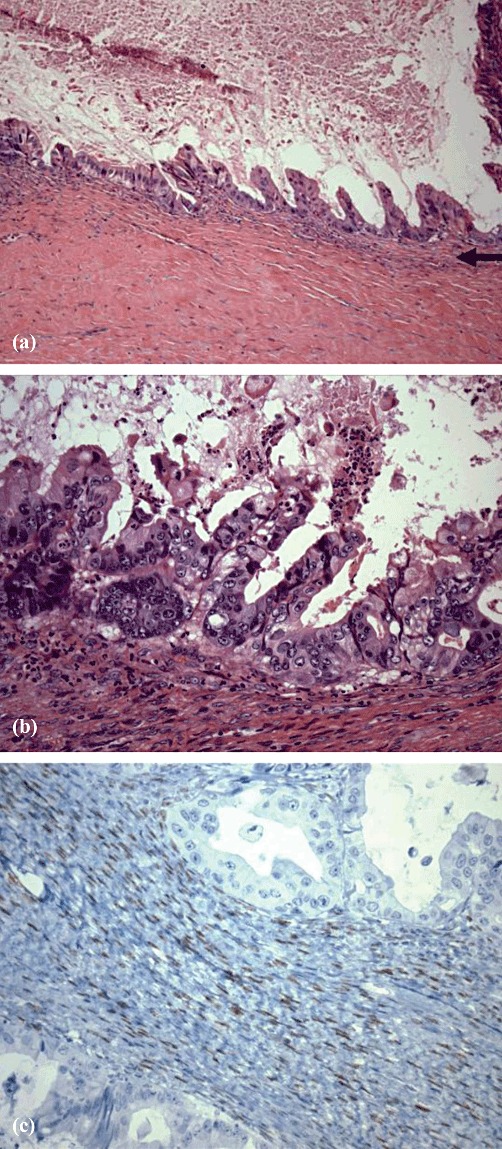
Histopathology showing (a) the cyst lining with papillary projections into the cyst lumen and ovarian-type stroma (arrow) and (b) high-grade dysplasia/carcinoma in situ overlying spindle cell/ovarian stroma. [Haematoxylin and eosin stain; original magnification (a) ×10, (b) ×40.] (c) Immunostain for oestrogen receptor highlighting ovarian-type stroma. (Original magnification ×40)
Invasive BMCN is noted to be strongly reactive for cytokeratins 7, 8, 18 and 19, and epithelial membrane antigen (EMA).1,2,52 Focal expression of carcinoembryonic antigen (CEA) can also be seen.1 In a light microscopic and immunohistochemical study of 70 patients with non-invasive and invasive BMCNs, conducted by Devaney et al., immunohistochemistry did not yield a diagnostic immunoprofile with which to distinguish non-invasive BMCN from invasive BMCN or from other epithelial lesions arising within the abdominal cavity.21
When present, associated invasive carcinoma is usually limited to the primary neoplasm.1 However, in some circumstances the invasive carcinoma may spread to the liver parenchyma or metastasize to regional hepatoduodenal ligament lymph nodes.1 Staging follows the protocol of the tumour–node–metastasis (TNM) classification for intrahepatic cholangiocarcinoma.1
It should be noted that the histologic features of intrahepatic BMCNs parallel those of their pancreatic, ovarian and retroperitoneal counterparts. Notably, all these tumours lack communication with the duct system and contain mucin-producing epithelium.41 Zamboni et al. studied the clinicopathologic features of 56 patients with MCNs of the pancreas and determined that the similarities (i.e. gender, morphology, stromal lutenization) between all four types of MCN suggested a common developmental pathway.41 In comparison with MCNs of the pancreas, intrahepatic BMCNs more commonly have cuboidal, non-mucinous epithelium.1
Intraductal papillary mucinous neoplasm biliary type (sometimes previously referred to as ‘intraductal biliary papilloma’) is one of the more recent tumours added to the differential diagnosis of an MCN in the liver. In 2005, Aoki et al. described a patient with an IPMN-B that was morphologically similar to non-invasive BMCN.53 A preoperative diagnosis of biliary cystadenoma (non-invasive BMCN) was made based on preoperative imaging and resection was performed. On pathologic review, a single layer of normal columnar epithelium and a papillary tumour were found to be growing from the wall of the dilated bile duct,53 but two distinct differences were apparent. Firstly, the interstitium was normal and neither smooth muscle cells nor ovarian-like stroma were detected.53 Secondly, extramural tumour infiltration was not observed and in tumour cells, mucus staining of d-periodic acid Schiff was strongly positive, but immunostaining for CEA and carbohydrate antigen 19-9 (CA 19-9) were negative.53 As with most rare tumours, a definitive diagnosis is usually only made postoperatively. A ‘biliary cystadenoma without ovarian stroma’, as reported in a series of patients by Wheeler and Edmondson,11 may actually be an IPMN-B.53
In earlier classifications, variations of biliary cystadenocarcinoma were described without mesenchymal stroma. These currently would be considered IPMN-Bs, as previously discussed.1,53 It is still important that the differential diagnosis should consider these aggressive lesions, which are not felt to arise from biliary cystadenomas and which are noted to occur more frequently in males (male : female ratio: 2 : 1).2,11,54 When this type does occur in females, it typically presents 30 years earlier than the more indolent form, which also indicates a different point of origin.11
It is imperative to remember that, as previously indicated with reference to the differential diagnosis, a liver abscess can mimic a non-invasive BMCN and even when there is clinical suspicion, aspiration may not yield a correct diagnosis, especially in the chronic phase. Yamamoto et al. reported a patient with a cystic liver lesion in whom negative cytology and cultures grossly appeared to indicate a solid lesion, but whose final diagnosis indicated a chronic liver abscess.55 This also further emphasizes that gross features alone are not sufficiently reliable to enable the definitive diagnosis of a BMCN.
Diagnosis
Laboratory findings
Although there are no specific markers or characteristics that can consistently identify intrahepatic BMCNs, standard liver cancer markers should be considered to rule out other similarly presenting tumours (see Table 1). Liver function tests are generally normal, but elevated levels of bilirubin, alkaline phosphatase and gamma-glutamyl transpeptidase (GGT) have been seen in cases in which intra- or extrahepatic biliary duct compression are present.4,56,57 CA 19-9 may be elevated (particularly if there is an associated invasive carcinoma), but CEA and alpha-fetoprotein (AFP) are usually normal.19,58
Additionally, especially in individuals living in endemic regions or with appropriate travel histories, testing for echinococcal cysts should be carried out (most frequently by serologic tests; eosinophilia may also be present if there is cyst leakage).24,59 Although indirect haemagglutination is a better screening test, the current reference standard is the immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA), which detects IgG antibodies to hydatid cyst fluid-derived native or recombinant antigen B subunits.58–60 However, 10–20% of patients do not produce detectable serum antibodies (IgG), resulting in false negatives.59 Less frequently used, the indirect immunofluorescence assay (IFA) is the most sensitive test (95%), but sensitivity and specificity largely depend on the quality of the utilized antigen.60,61 The sensitivity and specificity of the ELISA are also highly dependent on the antigen preparation method, cross-reactions with other helminthic diseases and non-infectious conditions.59,61 The specificity of the ELISA using hydatid cyst fluid is often unsatisfactory, but high levels of sensitivity (95%) and specificity (100%) can be achieved with pure antibody.58,59,61
A preoperative biopsy is contraindicated in view of the risk for spillage, which may result in the possible seeding of the peritoneum and the subsequent development of peritoneal carcinomatosis, pseudomyxoma or pleural dissemination.2,20,25,32,49,50 Aspiration cytology has been found to have a sensitivity of 66% in distinguishing neoplastic from non-neoplastic liver cysts.16,62,63 Elevated CEA and CA 19-9 levels in the cyst fluid of both BMCNs and simple hepatic cysts have been reported. Koffron et al. compared levels of CEA and CA 19-9 in the cyst fluid of 22 patients with non-invasive BMCNs, four patients with simple cysts and four patients with polycystic liver disease.64 Markedly increased levels of CA 19-9 and mild to marked increases in CEA levels were found in all of the patients with non-invasive BMCNs, whereas no elevated values were found in the eight control patients.64
However, three other studies evaluating the significance of cystic fluid analysis in the differential diagnosis of BMCN found no statistically significant differences in CA 19-9 or CEA levels among patients with, respectively, non-invasive BMCNs, invasive BMCNs and simple cysts.27,29,65
After resection, cyst content analysis can be useful. The content is most frequently viscous and yellowish in colour.66 Dark red and blood-stained aspirate is a commonly described variant and debate continues about its significance and possible prognostic implications, but in general it is considered concerning for malignancy.17,67 Buetow et al. examined the correlation between findings in clinical imaging and pathology results in 27 non-invasive and seven invasive BMCNs, and also evaluated the importance of ovarian stroma.40 A statistically significant correlation was found between non-bilious fluid and the presence of ovarian stroma, but did not indicate whether the tumour was an invasive or a non-invasive BMCN.40
Imaging
Diagnostic imaging of intrahepatic MCNs is difficult and can frequently lead to misdiagnosis.15,16,19,32,40 (See Table 1 for US, CT and MRI findings in various cystic lesions of the liver.) Intrahepatic BMCNs are easily mistaken for simple cysts, hydatid cysts or Caroli's disease.41 In hepatic cysts with intracystic haemorrhage, it may be difficult to distinguish the cyst from a cystic neoplasm.47 It has been reported that correct diagnoses are made in <50% of cases, even when all three imaging modalities are utilized.15,16,68 This claim is supported by reports of previous studies in which prior interventions (e.g. percutaneous drainage, marsupialization, partial resection, internal drainage, sclerosis) were performed as a result of misdiagnosis before eventual resection and appropriate diagnosis in 42–55% of cases and in 100% in one series.9,10,15,33,34
Abdominal US often reveals a well-demarcated, mostly multilocular hypoechogenic mass, which sometimes shows characteristic papillary projections from the cyst wall and septae.1,2,69 Type III hydatid cysts (daughter cysts and/or matrix formation with calcifications) can be differentiated from non-invasive and invasive BMCNs on the basis of oval or round daughter cysts demonstrated on US.70 Computed tomography scanning demonstrates a hypodense cystic lesion consistently in most patients (Fig. 4a, b), but internal papillary projections and intrahepatic bile duct dilatation are both seen in fewer than half of cases.2,15
Figure 4.
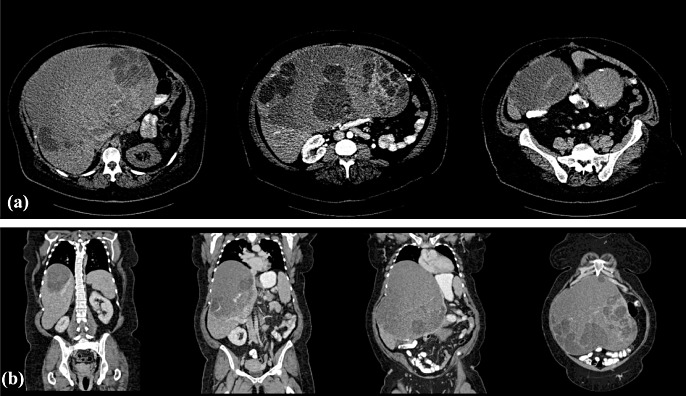
(a) Selected arterial-phase cross-sectional computed tomography images of biliary cystadenocarcinoma. (b) Venous-phase coronal reconstructions demonstrating a patent venous inflow and outflow of segments VI and VII
The presence of a mural nodule was significantly more frequent in BMCNs on multivariate analysis of biliary cystic tumours (non-invasive and invasive BMCNs) and in simple cysts mimicking non-invasive BMCN in US and CT scans.27 In a subset comparison of non-invasive and invasive BMCNs, a mural nodule, calcification, bile duct dilation and intracystic debris were all characteristic of invasive BMCNs.27 Further, several authors have stated that mural nodularity may be strongly indicative of malignancy.5,9,27,33,40,48 Internal septations have been found to be more suggestive of BMCNs (both benign and malignant) than simple cysts in recent series.15,28,29,47 Septations and septal thickening are also significantly more likely to be associated with non-invasive BMCN.29 Calcifications can be seen in both non-invasive and invasive BMCNs and coarse calcifications have been reported to increase the likelihood of invasive BMCN in some studies.29,33,40
With specific reference to a direct comparison between US and CT, one study reported that CT showed decreased sensitivity in demonstrating internal septae (eight of 10 multilocular lesions) in comparison with US (five of five lesions).33 Further CT findings included thick and coarse mural and septal calcifications in two of three invasive BMCNs and mural soft-tissue nodules in the lone case of a unilocular invasive BMCN.33 Ultrasound and CT have been noted to be mutually complementary in the evaluation of BMCNs.33,45
Findings in US or CT of one or more of the following characteristics should be considered highly suspicious for invasive BMCN: multilocular hypodense mass with echogenic internal septations and papillary projections into the cystic space; coarse and thick cyst wall; presence of haemorrhage or necrosis in the cyst; cyst wall enhancement with contrast, and fine septal calcifications in the cyst wall (usually more frequent on CT scan).14–16,19,27
Magnetic resonance imaging of intrahepatic BMCNs may be ordered secondary to the presence of suggestive findings on US or CT scan or it may be the primary imaging method utilized.30,31,63 Intracystic haemorrhage and corresponding low signal intensity on T2 weighted images may suggest malignancy, although, to date, no direct correlation has been found.16
The use of positron emission tomography-CT (PET-CT) with fluorine-18-2-fluro-2-deoxy-D-glucose (FDG) has been reported in only a single case series of four patients with invasive BMCN, all of whom demonstrated intense FDG uptake.71 The authors concluded that it is possible that PET-CT may routinely contribute to the diagnosis of malignant cystic tumours in the liver in the future.71 Two additional reports have described PET scans only and both reported findings positive for malignancy.9,72
Thus, imaging itself cannot completely and reliably differentiate between non-invasive BMCN, invasive BMCN and other hepatic cystic neoplasms, but it is valuable for localizing the lesion and operative planning.19,46,48,63 In the absence of reliable radiologic criteria, diagnosis is often revealed only after the surgical specimen is examined.
Treatment
It is clear that the incomplete excision of primary intrahepatic BMCNs leads to recurrence and thus the mainstay of treatment is complete surgical resection of these tumours.15–17,26,50,51,73 Descriptions of previous experiences with techniques such as aspiration, fenestration, internal drainage, intratumoral sclerosant application and incomplete resection report recurrence rates of 90–100%.25,66,74 Complete resection is important because it is possible to find the synchronous appearance of non-invasive BMCN and the foci of carcinoma in situ, and the latter may be undetected at the borders of the cyst.4,49
In addition to open surgery, laparoscopic surgery for both benign and indeterminate liver lesions including BMCNs has been found to be safe, incurring morbidity rates of 7–10% and no mortality, and to achieve oncologic efficiency.64,75,76 Rates of conversion from laparoscopic to open procedures are low (0–8%).64,75,76 Of two studies that reported follow-up, one study identified no recurrences at a mean of 16 months (range: 4–54 months)64 and the other reported one recurrence at a mean of 55 months (range: 3–115 months).75 Careful patient and tumour selection are imperative to the successful laparoscopic management of BMCNs.64,75,76 There are anecdotal reports of patients with BMCNs managed by orthotopic liver transplant (OLT).77,78 The benign nature and slow growth rate of BMCNs, as well as the ongoing shortage of donor livers, will allow for OLT in only very select patients who are not amenable to other forms of radical surgical therapy.77
Meta-analysis and case series
In 1998, Lauffer et al. reported a meta-analysis of 112 patients with invasive BMCNs already reported in the literature.2 Patients who underwent hepatic lobectomy (n = 24) were found to have 2- and 5-year survival of 65%.2 All of the patients who underwent complete excision of the lesion (n = 16) were alive at 5 years, but two patients had required a second operation for recurrence.2 A 2-year survival rate of 68% was achieved in patients who underwent left or right hemihepatectomy (n = 11) and one patient required an operation for recurrence.2 The partial excision of these tumours (n = 9) yielded survival of 71% at 2 years, but only 36% at 5 years.2 It also led to a 67% recurrence rate in comparison with recurrence rates of 15% and 13% after anatomic resection (n = 6) and complete excision (n = 16), respectively.2 Segmentectomy (n = 6) was reported to yield a 2-year survival rate of 100%, but 50% of patients required reoperation for recurrence.2 The authors of this analysis noted that it was not possible to determine whether the procedures of hepatic lobectomy, hemihepatectomy or segmentectomy resulted in complete or incomplete tumour excision.2 No data on postoperative complications were given.
Since this initial meta-analysis, five recent case series including a total of 89 patients with non-invasive (n = 63) or invasive (n = 26) BMCNs have been published (Table 2).4,6,10,15,72 The average age of these patients was 52 years and 72% (n = 64) were female. In the two series in which gender and age were reported by BMCN subgroup, 88% of patients with non-invasive BMCNs (n = 22) and 39% of patients with invasive BMCNs (n = 7) were female.4,72 Patients with non-invasive BMCNs were noted to be almost 15 years older than those with invasive BMCNs. Right upper quadrant pain was the most common symptom in all BMCNs, regardless of subgroup, occurring in 55% of patients (n = 49).
Table 2.
Recent studies on intrahepatic biliary mucinous cystic neoplasms
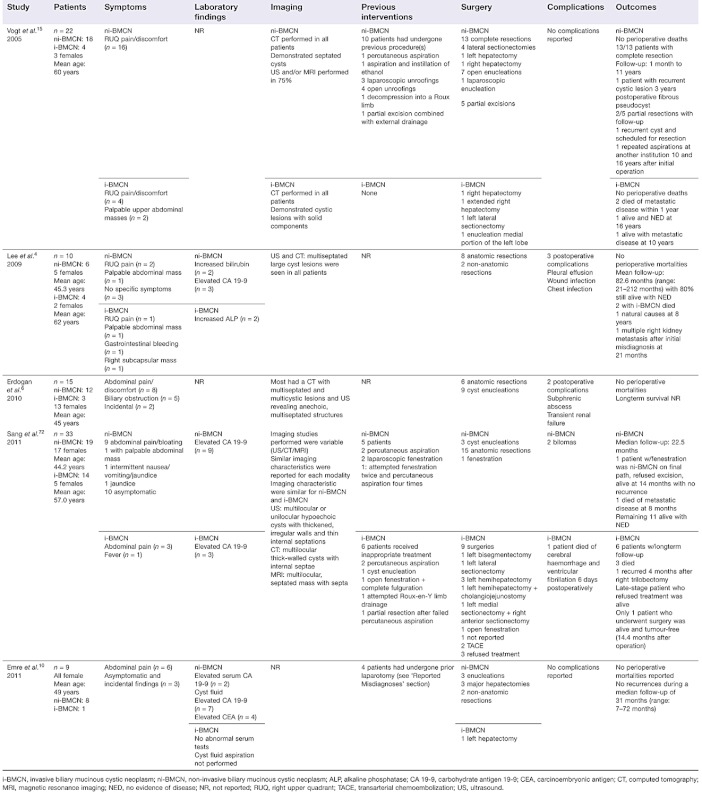 |
Three of the studies reported laboratory findings (n = 52).4,10,72 Levels of CA 19-9 were elevated in 16 patients, 13 of whom had non-invasive BMCNs. Only one study (n = 9) reported cyst fluid tumour markers (aspirations were performed only in non-invasive BMCNs); seven patients were found to have elevated CA 19-9 and four were found to have elevated CEA in their cyst fluid.10 The most common imaging modality was CT scan, but US and/or MRI were also used in all four of the studies that reported imaging results.4,6,15,72 The most prominent findings included multiseptated cysts, sometimes with solid components and thickened irregular walls, as well as internal septations. Previous interventions had been performed in 28% of patients (n = 25) and were widely variable (see Table 2). Two studies did not report on previous interventions.4,6
Surgery was carried out in 94% of patients (n = 84).4,6,10,15,72 Resection was the most common operation and was carried out in 74% of the patients who underwent surgical procedures (n = 62). Enucleation was performed in 16 patients (19%), partial excision in five patients (6%) and cyst fenestration in one patient. Three of the five studies reported complications, resulting in an average complication rate of 17.6% (range: 9.7–30.0%).4,6,72 One perioperative death was reported, but did not appear to be related to the procedure performed.72
One study6 did not report longterm follow-up; two studies4,10 reported only combined follow-up data for all patients, and two studies15,72 provided follow-up data for the non-invasive and invasive BMCN subgroups, respectively. The latter two studies included 37 patients with non-invasive BMCN, of whom 31 underwent complete resection; 30 patients remained alive with no evidence of disease (NED) and one patient died of metastatic disease at 8 months after resection.15,72 One patient who underwent cyst fenestration was alive with no recurrence at 14 months post-procedure (final pathology indicated non-invasive BMCN, but the patient refused resection), and two of five patients with partial disease remained alive with recurrent disease.15,72 Of the 18 patients with invasive BMCNs, 13 patients underwent complete resection and nine underwent longterm follow-up: two remained alive with NED at 14.4 months and 16 years post-procedure, respectively; two remained alive with recurrent disease at 4 months and 10 years post-procedure, respectively, and five died (two with documented metastatic disease).15,72
Reported misdiagnoses
Initial misdiagnosis and the subsequent mistreatment of BMCN remain persistent. Previous interventions, which are widely variable, have been reported in up to 45% of patients and in some cases have ultimately resulted in mortality.10,15,72 It is critical to appreciate these high rates of mismanagement because the goal of investigation is to decrease the occurrence of misdiagnosis and mistreatment in the management of BMCN. The literature includes a report of three patients with preoperative diagnoses of echinococcal cysts who underwent laparotomies secondary to misdiagnosis, which resulted in recurrence or incomplete surgeries, two of whom were misdiagnosed at surgery for a second time as having simple cysts.10 A fourth patient was found to have a suspicious malignant cystic lesion during a cholecystectomy, which was ultimately diagnosed as an invasive BMCN.10 Finally, a fifth patient with an initial misdiagnosis of a retroperitoneal abscess developed multiple right kidney, right rib and right back metastasis and died 21 months after his initial operation.4
Systemic chemotherapy for recurrence after resection
The value of salvage therapy in patients with recurrent metastatic invasive BMCNs after complete resection is unclear. It is particularly difficult to evaluate because distant metastasis is seen to occur very infrequently as local invasion and intrahepatic recurrence after excision tend to be the primary modes of malignant behaviour.4,22 Distant metastases have been reported to occur in up to 20% of patients and up to 13% have been noted to have lymphatic spread.2,57 The most common sites of metastasis are the lungs, pleura, peritoneum, liver, duodenum, stomach and pancreas.1,79 In a few patients, osseous metastases have also been reported.69,79
There is a dearth of literature explicitly reporting the use of chemotherapy as primary treatment, adjuvant therapy or for metastatic recurrence after the complete resection of an invasive BMCN. Lauffer et al. reported three patients in whom chemotherapy and/or radiation (as primary treatment) were administered without surgery and indicated 2- and 5-year survival rates of 33%.2 No details of the therapy were given. Kasai et al. described one patient in whom adjuvant chemotherapy (mitomycin, 5-fluorouracil via the hepatic artery) and radiation were provided and reported survival of 1 year.67 Additionally, at Carolinas Medical Center one patient with an invasive BMCN previously treated by resection via a left hepatic trisegmentectomy with en bloc R0 resection of a stage I cancer (T1N0M0) underwent salvage chemotherapy (gemcitabine, capecitabine, oxaliplatin) initiated for recurrent liver tumour and metastatic disease, but died 6 months later. Given that only a handful of cases in which chemotherapy has been utilized as a primary, adjuvant or salvage therapy have been reported in the literature, no definitive recommendations in this area can be made.
Prognosis
The prognosis in patients with non-invasive BMCNs is excellent if complete resection is possible.1 Several case reports have illustrated longterm survival following complete, uncomplicated surgical excision; this is considered to reflect the slow growth of these tumours.10,51,66,80 Thirty of 31 (97%) recently reported patients with non-invasive BMCN demonstrated significant longterm survival (up to 11 years).4,6,15,72 The one patient in whom metastatic disease developed subsequently is likely to have had a cancer focus in the non-invasive BMCN that was not appreciated on pathology review.
The prognosis in patients with an invasive adenocarcinoma arising in conjunction with BMCN (invasive BMCN) is much harder to predict.1 This is probably multifactorial and secondary to heterogeneity of management (drainage, fenestration, excision, ablation) over a long time period in different institutions, and varying stages and types of tumour (e.g. with and without the presence of ovarian stroma). Additional data are required to define more precisely the prognosis in patients with BMCN with an associated invasive carcinoma using the new definition and classification.
The natural history of invasive BMCN has been observed in a few patients followed for 5–22 years via serial imaging studies which have indicated that the BMCN may take up to 12 years to become malignant.38,39 Patients who receive an initial correct diagnosis and undergo appropriate complete surgical resection would be expected to achieve the best outcomes. However, this is difficult to determine as longterm follow-up results for these patients are often combined with those of patients who have undergone prior procedures or have non-invasive BMCN. There are some data available on patients in whom the correct diagnosis was initially missed or delayed and in whom poorer longterm survival is reported, but, again, survival rates are frequently combined.4 Survival data based on the few patients who proceeded to liver transplant suggest outcomes equivalent to those in patients who undergo transplant for other primary liver tumours.77 The prognosis in recurrent disease with salvage chemotherapy treatment has been detailed for only a few patients.2,67
Patients with lesions that are confined within the cyst wall may have a better prognosis than those with tumours that extend beyond the cystic wall structure.24,26,73 Nakajima et al. performed a clinicopathologic and histochemical evaluation of nine biliary cystadenocarcinomas of the liver.26 Tumour growth was completely confined to the lesion in five patients and extended into the liver parenchyma or adjacent organs in four patients.26 A marked difference between the groups in terms of prognosis was noted (100% survival in patients with confined tumours vs. 0% in patients with non-confined tumours). Although all the patients with invasive tumours died in this series, they survived for an average of 7 months after resection and three remained alive at 1 year post-resection.26
Additionally, other series have suggested that although intrahepatic invasive BMCNs are capable of spreading beyond the liver and metastasizing to distant sites, longterm survival in these patients, even in those with invasive disease, is improved with resection.2,8 This lends credence to the supposition that the biological behaviour of these tumours is widely variable. Too few patients with intrahepatic invasive BMCN have been reported in the literature to support a definitive statement regarding the prognostic value of either tumour invasion or the presence of distinctive mesenchymal stroma.2
At present, it would appear that patients with invasive adenocarcinoma arising in association with BMCN have a better prognosis than those with pure cholangiocarcinoma, which emphasizes the importance of distinguishing between disease types.1 The prognosis of patients with invasive BMCN is better than that of patients with hepatocellular carcinoma (5-year survival of 40%) or cholangiocarcinoma (5-year survival of 22%) if the disease is completely resected.2,10,22,72 This is because primary biliary invasive MCNs appear to have a less invasive nature and a slower growth rate than other malignancies of the liver and invasive MCNs of other sites, such as the pancreas or ovaries.22,79 Overall 5-year survival after resection is 65−71% and can reach 100% in patients in whom histologically negative resection margins are achieved.31,57,79
Conclusions
Preoperative differential workup of a cystic liver tumour should always include BMCN. Presenting symptoms, laboratory values and diagnostic imaging features are unreliable and frequently lead to delayed or incorrect diagnosis and unnecessary procedures that are likely to have a negative effect on survival. Preoperative cyst aspiration is not advocated in order to avert the risk for intraperitoneal seeding, but intraoperative cyst wall biopsy and frozen section(s) are essential to differentiate BMCNs from other cystic liver tumours. Complete excision of a suspected non-invasive or invasive BMCN of the liver is clearly the treatment of choice when the patient is medically fit for surgery and there is no evidence of systemic disease on initial workup. Survival rates and prognosis will become more defined as BMCNs are resected with increased frequency and the appropriate classification is applied.
Conflicts of interest
None declared.
References
- 1.Tsui WMS, Adsay NV, Crawford JM, Hruban R, Kloppel G, Wee A, editors. Mucinous Cystic Neoplasms of the Liver. 4th edn. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 2.Lauffer JM, Baer HU, Maurer CA, Stoupis C, Zimmerman A, Buchler MW. Biliary cystadenocarcinoma of the liver: the need for complete resection. Eur J Cancer. 1998;34:1845–1851. doi: 10.1016/s0959-8049(98)00166-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim JY, Kim SH, Eun HW, Lee MW, Lee JY, Han JK, et al. Differentiation between biliary cystic neoplasms and simple cysts of the liver: accuracy of CT. AJR Am J Roentgenol. 2010;195:1142–1148. doi: 10.2214/AJR.09.4026. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Lee KG, Park HK, Lee KS. Biliary cystadenoma and cystadenocarcinoma of the liver: 10 cases of a single centre experience. Hepatogastroenterology. 2009;56:844–849. [PubMed] [Google Scholar]
- 5.Pojchamarnwiputh S, Na Chiangmai W, Chotirosniramit A, Lertprasertsuke N. Computed tomography of biliary cystadenoma and biliary cystadenocarcinoma. Singapore Med J. 2008;49:392–396. [PubMed] [Google Scholar]
- 6.Erdogan D, Kloek J, Lamers WH, Offerhaus GJA, Busch ORC. Mucinous cystadenomas in liver: management and Origin. Dig Surg. 2010;27:19–23. doi: 10.1159/000268110. [DOI] [PubMed] [Google Scholar]
- 7.Takayasu K, Muramatsu Y, Moriyama N, Yamada T, Hasegawa H, Hirohashi S, et al. Imaging diagnosis of bile duct cystadenocarcinoma. Cancer. 1988;61:941–946. doi: 10.1002/1097-0142(19880301)61:5<941::aid-cncr2820610514>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Ishak KG, Willis GW, Cummins SD, Bullock AA. Biliary cystadenoma and cystadenocarcinoma: report of 14 cases and review of the literature. Cancer. 1977;39:322–338. doi: 10.1002/1097-0142(197701)39:1<322::aid-cncr2820390149>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Thomas KT, Welch D, Trueblood A, Sulur P, Wise P, Gorden DL, et al. Effective treatment of biliary cystadenoma. Ann Surg. 2005;241:769–773. doi: 10.1097/01.sla.0000161982.57360.1b. discussion 773–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emre A, Serin KR, Ozden I, Tekant Y, Bilge O, Alper A, et al. Intrahepatic biliary cystic neoplasms: surgical results of 9 patients and literature review. World J Gastroenterol. 2011;17:361–365. doi: 10.3748/wjg.v17.i3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts. A clinicopathologic study of 17 cases, 4 with malignant change. Cancer. 1985;56:1434–1445. doi: 10.1002/1097-0142(19850915)56:6<1434::aid-cncr2820560635>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Edmondson HA. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1958. Tumors of the liver and intrahepatic bile ducts. National Research Council. Subcommittee on Oncology, Armed Forces Institute of Pathology, Sect VII, Fascicle 25. [Google Scholar]
- 13.Genc V, Cakmak A, Akbari M, Orozakunov E, Ersoz S. Primary biliary cystadenocarcinoma mimicking a complicated hydatid cyst. Chirurgia (Bucur) 2010;105:249–251. [PubMed] [Google Scholar]
- 14.Yu Q, Chen T, Wan YL, Min J, Cheng Y, Guo H. Intrahepatic biliary cystadenocarcinoma: clinical analysis of 4 cases. Hepatobiliary Pancreat Dis Int. 2009;8:71–74. [PubMed] [Google Scholar]
- 15.Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadenocarcinoma of the liver: a single centre experience. J Am Coll Surg. 2005;200:727–733. doi: 10.1016/j.jamcollsurg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Del Poggio P, Jamoletti C, Forloni B, De Benedictis R, Mattiello M, Corti D, et al. Malignant transformation of biliary cystadenoma: a difficult diagnosis. Dig Liver Dis. 2000;32:733–736. doi: 10.1016/s1590-8658(00)80339-4. [DOI] [PubMed] [Google Scholar]
- 17.Willis R. Carcinoma arising in congenital cysts of the liver. J Pathol Bacteriol. 1943;55:492–495. [Google Scholar]
- 18.Gomez-Martin C, Rodriguez A, Malon D, Cortes-Funes H. Biliary cystadenocarcinoma with mesenchymal stroma. Clin Transl Oncol. 2010;12:234–237. doi: 10.1007/s12094-010-0495-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Yu J, Yan S, Zheng SS. Cystadenocarcinoma of the liver: a case report. Hepatobiliary Pancreat Dis Int. 2005;4:464–467. [PubMed] [Google Scholar]
- 20.Bacher H, Cerwenka H, Werkgartner G, El-Shabrawi A, Hoss G, Preidler K, et al. Primary biliary cystadenocarcinoma perforating the duodenum and left intrahepatic biliary tree – mimicking a hydatid cyst. Liver. 1999;19:39–41. doi: 10.1111/j.1478-3231.1999.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 21.Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078–1091. [PubMed] [Google Scholar]
- 22.Gourley WK, Kumar D, Bouton MS, Fish JC, Nealon W. Cystadenoma and cystadenocarcinoma with mesenchymal stroma of the liver. Immunohistochemical analysis. Arch Pathol Lab Med. 1992;116:1047–1050. [PubMed] [Google Scholar]
- 23.Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58:626–629. doi: 10.1016/s0009-9260(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita H, Tanimura H, Onishi H, Kasano Y, Uchiyama K, Yamaue H. Clinical features and imaging diagnosis of biliary cystadenocarcinoma of the liver. Hepatogastroenterology. 2001;48:250–252. [PubMed] [Google Scholar]
- 25.Manouras A, Markogiannakis H, Lagoudianakis E, Katergiannakis V. Biliary cystadenoma with mesenchymal stroma: report of a case and review of the literature. World J Gastroenterol. 2006;12:6062–6069. doi: 10.3748/wjg.v12.i37.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima T, Sugano I, Matsuzaki O, Nagao K, Kondo Y, Miyazaki M, et al. Biliary cystadenocarcinoma of the liver. A clinicopathologic and histochemical evaluation of nine cases. Cancer. 1992;69:2426–2432. doi: 10.1002/1097-0142(19920515)69:10<2426::aid-cncr2820691007>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Seo JK, Kim SH, Lee SH, Park JK, Woo SM, Jeong JB, et al. Appropriate diagnosis of biliary cystic tumours: comparison with atypical hepatic simple cysts. Eur J Gastroenterol Hepatol. 2010;22:989–996. doi: 10.1097/MEG.0b013e328337c971. [DOI] [PubMed] [Google Scholar]
- 28.Wang CMR, Liu H, Du X, Liu L, Lu X, Zhao H. Intrahepatic biliary cystadenoma and cystadenocarcinoma: an experience of 30 cases. Dig Liver Dis. 2012;44:426–431. doi: 10.1016/j.dld.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Choi HK, Lee JK, Lee KH, Lee KT, Rhee JC, Kim KH, et al. Differential diagnosis for intrahepatic biliary cystadenoma and hepatic simple cyst: significance of cystic fluid analysis and radiologic findings. J Clin Gastroenterol. 2010;44:289–293. doi: 10.1097/MCG.0b013e3181b5c789. [DOI] [PubMed] [Google Scholar]
- 30.Kamyab A, Curtiss W, Mittal V, Jacobs M. Primary intrahepatic biliary cystadenocarcinoma. Am Surg. 2011;77:117–118. [PubMed] [Google Scholar]
- 31.Williams DM, Vitellas KM, Sheafor D. Biliary cystadenocarcinoma: seven-year follow-up and the role of MRI and MRCP. Magn Reson Imaging. 2001;19:1203–1208. doi: 10.1016/s0730-725x(01)00453-2. [DOI] [PubMed] [Google Scholar]
- 32.Wu JM, Wu YM, Ho MC, Hu RH, Lee PH. Surgical treatment of biliary cystadenomas. Int Surg. 2008;93:373–376. [PubMed] [Google Scholar]
- 33.Korobkin M, Stephens DH, Lee JKT, Stanley RJ, Fishman EK, Francis I, et al. Biliary cystadenoma and cystadenocarcinoma: CT and sonographic findings. AJR Am J Roentgenol. 1989;153:507–511. doi: 10.2214/ajr.153.3.507. [DOI] [PubMed] [Google Scholar]
- 34.Lewis WD, Jenkins RL, Rossi RL, Munson L, ReMine SG, Cady B, et al. Surgical treatment of biliary cystadenoma. A report of 15 cases. Arch Surg. 1988;123:563–568. doi: 10.1001/archsurg.1988.01400290045007. [DOI] [PubMed] [Google Scholar]
- 35.Lempinen M, Halme L, Numminen K, Arola J, Nordin A, Makisalo H. Spontaneous rupture of a hepatic cystadenoma and cystadenocarcinoma: report of two cases. J Hepatobiliary Pancreat Surg. 2005;12:409–414. doi: 10.1007/s00534-005-0998-8. [DOI] [PubMed] [Google Scholar]
- 36.Cruickshank AH, Sparshott SM. Malignancy in natural and experimental hepatic cysts: experiments with aflatoxin in rats and the malignant transformation of cysts in human livers. J Pathol. 1971;104:185–190. doi: 10.1002/path.1711040305. [DOI] [PubMed] [Google Scholar]
- 37.Lei S, Howard JM. Biliary cystadenocarcinoma arising from benign cystadenoma. Arch Surg. 1992;127:1478. doi: 10.1001/archsurg.1992.01420120112019. [DOI] [PubMed] [Google Scholar]
- 38.Kubota E, Katsumi K, Iida M, Kishimoto A, Ban Y, Nakata K, et al. Biliary cystadenocarcinoma followed up as benign cystadenoma for 10 years. J Gastroenterol. 2003;38:278–282. doi: 10.1007/s005350300048. [DOI] [PubMed] [Google Scholar]
- 39.Ajiki T, Fujimori T, Nakamura T, Maeda S, Saito Y, Suehiro I, et al. A case of biliary cystadenocarcinoma followed up from five years. Dig Endosc. 1995;7:93–97. [Google Scholar]
- 40.Buetow PC, Buck JL, Pantongrag-Brown L, Ros PR, Devaney K, Goodman ZD, et al. Biliary cystadenoma and cystadenocarcinoma: clinical-imaging–pathologic correlations with emphasis on the importance of ovarian stroma. Radiology. 1995;196:805–810. doi: 10.1148/radiology.196.3.7644647. [DOI] [PubMed] [Google Scholar]
- 41.Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, et al. Mucinous cystic tumours of the pancreas: clinico-pathological features, prognosis, and relationship to other mucinous cystic tumours. Am J Surg Pathol. 1999;23:410–422. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Richmond HG. Carcinoma arising in congenital cysts of the liver. J Pathol Bacteriol. 1956;72:681–683. [Google Scholar]
- 43.Gallagher PJ, Millis RR, Mitchinson MJ. Congenital dilatation of the intrahepatic bile ducts with cholangiocarcinoma. J Clin Pathol. 1972;25:804–808. doi: 10.1136/jcp.25.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ucci A, Devinne P. Biliary cystadenocarcinoma arising in a congenital cyst. Hum Pathol. 1985;16:92–94. doi: 10.1016/s0046-8177(85)80219-7. [DOI] [PubMed] [Google Scholar]
- 45.Teoh AY, Ng SS, Lee KF, Lai PB. Biliary cystadenoma and other complicated cystic lesions of the liver: diagnostic and therapeutic challenges. World J Surg. 2006;30:1560–1566. doi: 10.1007/s00268-005-0461-7. [DOI] [PubMed] [Google Scholar]
- 46.Ren XL, Yan RL, Yu XH, Zheng Y, Liu JE, Hou XB, et al. Biliary cystadenocarcinoma diagnosed with real-time contrast-enhanced ultrasonography: report of a case with diagnostic features. World J Gastroenterol. 2010;16:131–135. doi: 10.3748/wjg.v16.i1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mortele KJ, Peters HE. Multimodality imaging of common and uncommon cystic focal liver lesions. Semin Ultrasound CT MR. 2009;30:368–386. doi: 10.1053/j.sult.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Lewin M, Mourra N, Honigman I, Flejou JF, Parc R, Arrive L, et al. Assessment of MRI and MRCP in diagnosis of biliary cystadenoma and cystadenocarcinoma. Eur Radiol. 2006;16:407–413. doi: 10.1007/s00330-005-2822-x. [DOI] [PubMed] [Google Scholar]
- 49.Delis SG, Touloumis Z, Bakoyiannis A, Tassopoulos N, Paraskeva K, Athanassiou K, et al. Intrahepatic biliary cystadenoma: a need for radical resection. Eur J Gastroenterol Hepatol. 2008;20:10–14. doi: 10.1097/MEG.0b013e3282f16a76. [DOI] [PubMed] [Google Scholar]
- 50.Iemoto Y, Kondo Y, Fukamachi S. Biliary cystadenocarcinoma with peritoneal carcinomatosis. Cancer. 1981;48:1664–1667. doi: 10.1002/1097-0142(19811001)48:7<1664::aid-cncr2820480731>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 51.Siren J, Karkkainen P, Luukkonen P, Kiviluoto T, Kivilaakso E. A case report of biliary cystadenoma and cystadenocarcinoma. Hepatogastroenterology. 1998;45:83–89. [PubMed] [Google Scholar]
- 52.Hytiroglou P, Zurac S, Popescu I, Popovici D, Tanasescu C, Saxena R, et al. Biliary cystadenocarcinoma with two types of tumour cells. Virchows Arch. 2000;437:555–559. doi: 10.1007/s004280000272. [DOI] [PubMed] [Google Scholar]
- 53.Aoki S, Okayama Y, Kitajima Y, Hayashi K, Imai H, Okamoto T, et al. Intrahepatic biliary papilloma morphologically similar to biliary cystadenoma. J Gastroenterol Hepatol. 2005;20:321–324. doi: 10.1111/j.1440-1746.2005.03242.x. [DOI] [PubMed] [Google Scholar]
- 54.Ishibashi Y, Ojima H, Hiraoka N, Sano T, Kosuge T, Kanai Y. Invasive biliary cystic tumour without ovarian-like stroma. Pathol Int. 2007;57:794–798. doi: 10.1111/j.1440-1827.2007.02176.x. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto T, Fukumoto N, Ichikawa T, Hai S, Ogawa M, Tanaka S, et al. Huge liver abscess radiologically mimicking cystadenocarcinoma. Osaka City Med. 2009;55:53–59. [PubMed] [Google Scholar]
- 56.Kanamori H, Kawahara H, Oh S, Mine T, Osawa H, Murakami T, et al. A case of biliary cystadenocarcinoma with recurrent jaundice. Diagnostic evaluation of computed tomography. Cancer. 1985;55:2722–2724. doi: 10.1002/1097-0142(19850601)55:11<2722::aid-cncr2820551133>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 57.Chamberlain RS, Blumgart LH. Mucobilia in association with a biliary cystadenocarcinoma of the caudate duct: a rare cause of malignant biliary obstruction. HPB Surg. 2000;11:345–351. doi: 10.1155/2000/48426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biava MF, Dao A, Fortier B. Laboratory diagnosis of cystic hydatic disease. World J Surg. 2001;25:10–14. doi: 10.1007/s002680020002. [DOI] [PubMed] [Google Scholar]
- 59.Scherer K, Gupta N, Caine WP, Panda M. Differential diagnosis and management of a recurrent hepatic cyst: a case report and review of literature. J Gen Intern Med. 2009;24:1161–1165. doi: 10.1007/s11606-009-1062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pawlowski ZS, Eckert J, Vuitton DA, Ammann RW, Kern P, Craig PS, et al. Echinococcosis in Humans: Clinical Aspects, Diagnosis and Treatment. Paris: World Organization for Animal Health; 2001. [Google Scholar]
- 61.Shaw JM, Bornman PC, Krige JE. Hydatid disease of the liver. S Afr J Surg. 2006;44:70–72. 74–77. [PubMed] [Google Scholar]
- 62.Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, 3rd, Dorfman GS, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 63.Del Poggio P, Buonocore M. Cystic tumours of the liver: a practical approach. World J Gastroenterol. 2008;14:3616–3620. doi: 10.3748/wjg.14.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koffron A, Rao S, Ferrario M, Abecassis M. Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery. 2004;136:926–936. doi: 10.1016/j.surg.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 65.Waanders E, van Keimpema L, Brouwer JT, van Oijen MG, Aerts R, Sweep FC, et al. Carbohydrate antigen 19-9 is extremely elevated in polycystic liver disease. Liver Int. 2009;29:1389–1395. doi: 10.1111/j.1478-3231.2009.02055.x. [DOI] [PubMed] [Google Scholar]
- 66.Tsiftsis D, Christodoulakis M, de Bree E, Sanidas E. Primary intrahepatic biliary cystadenomatous tumours. J Surg Oncol. 1997;64:341–346. doi: 10.1002/(sici)1096-9098(199704)64:4<341::aid-jso17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 67.Kasai Y, Sasaki E, Tamaki A, Koshino I, Kawanishi N. Carcinoma arising in the cyst of the liver – report of three cases. Jpn J Surg. 1977;7:65–72. doi: 10.1007/BF02469388. [DOI] [PubMed] [Google Scholar]
- 68.Wang R, Liu C, Shi J, Wu Q, Wang B, Liu X, et al. Intrahepatic biliary cystadenocarcinoma: a case report with literature review. J Chin Clin Med. 2009;4:655–657. [Google Scholar]
- 69.Cheung YK, Chan FL, Leong LL, Collins RJ, Cheung A. Biliary cystadenoma and cystadenocarcinoma: some unusual features. Clin Radiol. 1991;43:183–185. doi: 10.1016/s0009-9260(05)80476-3. [DOI] [PubMed] [Google Scholar]
- 70.Koroglu M, Akhan O, Akpinar E, Oto A, Gumus B. Biliary cystadenoma and cystadenocarcinoma: two rare cystic liver lesions. JBR-BTR. 2006;89:261–263. [PubMed] [Google Scholar]
- 71.Takanami K, Kaneta T, Yamada S, Takahashi S. F-18 FDG PET/CT scan in biliary cystadenocarcinoma. Clin Nucl Med. 2009;34:470–472. doi: 10.1097/RLU.0b013e3181a7d16b. [DOI] [PubMed] [Google Scholar]
- 72.Sang X, Sun Y, Mao Y, Yang Z, Lu X, Yang H, et al. Hepatobiliary cystadenomas and cystadenocarcinomas: a report of 33 cases. Liver Int. 2011;31:1337–1344. doi: 10.1111/j.1478-3231.2011.02560.x. [DOI] [PubMed] [Google Scholar]
- 73.Wang ZX, Jia QB, Yan LN, Wang WT, Zhou LX, Jiao ZY, et al. [Diagnosis and treatment of intrahepatic biliary cystadenocarcinoma – a report of 11 cases.] Ai Zheng. 2007;26:524–527. [PubMed] [Google Scholar]
- 74.Fairchild R, Reese J, Solomon H, Garvin P, Esterl R. Biliary cystadenoma: a case report and review of the literature. Mo Med. 1993;90:656–657. [PubMed] [Google Scholar]
- 75.Ardito F, Tayar C, Laurent A, Karoui M, Loriau J, Cherqui D. Laparoscopic liver resection for benign disease. Arch Surg. 2007;142:1188–1193. doi: 10.1001/archsurg.142.12.1188. discussion 1193. [DOI] [PubMed] [Google Scholar]
- 76.Abu Hilal M, Di Fabio F, Teng MJ, Godfrey DA, Primrose JN, Pearce NW. Surgical management of benign and indeterminate hepatic lesions in the era of laparoscopic liver surgery. Dig Surg. 2011;28:232–236. doi: 10.1159/000321891. [DOI] [PubMed] [Google Scholar]
- 77.Romagnoli R, Patrono D, Paraluppi G, David E, Tandoi F, Strignano P, et al. Liver transplantation for symptomatic centrohepatic biliary cystadenoma. Clin Res Hepatol Gastroenterol. 2011;35:408–413. doi: 10.1016/j.clinre.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 78.O'Grady JG. Treatment options for other hepatic malignancies. Liver Transpl. 2000;6 (Suppl.):23–29. doi: 10.1053/jlts.2000.18687. [DOI] [PubMed] [Google Scholar]
- 79.Berjian RA, Nime F, Douglass HO, Jr, Nava H. Biliary cystadenocarcinoma: report of a case presenting with osseous metastasis and a review of the literature. J Surg Oncol. 1981;18:305–316. doi: 10.1002/jso.2930180312. [DOI] [PubMed] [Google Scholar]
- 80.Matsumato S, Miyake H, Mori H. Case report: biliary cystadenoma with mucin-secretion mimicking a simple hepatic cyst. Clin Radiol. 1997;52:316–318. doi: 10.1016/s0009-9260(97)80065-7. [DOI] [PubMed] [Google Scholar]
- 81.Alobaidi M, Shirkhoda A. Malignant cystic and necrotic liver lesions: a pattern approach to discrimination. Curr Probl Diagn Radiol. 2004;33:254–268. doi: 10.1067/j.cpradiol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Ralls PW, Henley DS, Colletti PM, Benson R, Raval JK, Radin DR, et al. Amoebic liver abscess: MR imaging. Radiology. 1987;165:801–804. doi: 10.1148/radiology.165.3.3317504. [DOI] [PubMed] [Google Scholar]
- 83.Li D, Hann LE. A practical approach to analysing focal lesions in the liver. Ultrasound Q. 2005;21:187–200. doi: 10.1097/01.ruq.0000174752.34633.41. [DOI] [PubMed] [Google Scholar]
- 84.Heirwegh G, Claikens B. Type I hydatid cyst of the liver: typical MRI features. JBR-BTR. 2005;88:136–137. [PubMed] [Google Scholar]
- 85.Venkatanarasimha N, Thomas R, Armstrong EM, Shirley JF, Fox BM, Jackson SA. Imaging features of ductal plate malformations in adults. Clin Radiol. 2011;66:1086–1093. doi: 10.1016/j.crad.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 86.Yang L, Chen LB, Xiao J, Han P. Clinical features and spiral computed tomography analysis of undifferentiated embryonic liver sarcoma in adults. J Dig Dis. 2009;10:305–309. doi: 10.1111/j.1751-2980.2009.00400.x. [DOI] [PubMed] [Google Scholar]
- 87.Yu F, Chen J, Yang K, Wu C, Chou Y. Hepatobiliary cystadenocarcinoma: report of two cases. J Gastrointestin Liver Dis. 2008;17:203–206. [PubMed] [Google Scholar]
- 88.Moon WK, Kim WS, Kim IO, Yeon KM, Yu IK, Choi BI, et al. Undifferentiated embryonal sarcoma of the liver: US and CT findings. Pediatr Radiol. 1994;24:500–503. doi: 10.1007/BF02015012. [DOI] [PubMed] [Google Scholar]
- 89.Sato Y, Ren XS, Nakanuma Y. Caroli's disease: current knowledge of its biliary pathogenesis obtained from an orthologous rat model. Int J Hepatol. 2012;2012:1–10. doi: 10.1155/2012/107945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee JH, Kim KS, Chung CW, Park YN, Kim BR. Hepatic resection of metastatic tumour from serous cystadenocarcinoma of the ovary. J Korean Med Sci. 2002;17:415–418. doi: 10.3346/jkms.2002.17.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franko J, Cole K, Pezzi CM, Montone KT, Redmond J. Serous cystadenocarcinoma of the pancreas with metachronous hepatic metastasis. Am J Clin Oncol. 2008;31:624–625. doi: 10.1097/01.coc.0000227529.77138.01. [DOI] [PubMed] [Google Scholar]
- 92.Hoang TV, Bluemke DA. Biliary papillomatosis: CT and MR findings. J Comput Assist Tomogr. 1998;22:671–672. doi: 10.1097/00004728-199807000-00031. [DOI] [PubMed] [Google Scholar]
- 93.Braeye L, Vanheste R. Biliary papillomatosis. Hepatology. 2010;52:1512–1514. doi: 10.1002/hep.23914. [DOI] [PubMed] [Google Scholar]
- 94.Shamseddine A, Faraj W, Mukherji D, El Majzoub N, Khalife M, Soubra A. Unusually young age distribution of primary hepatic leiomyosarcoma: case series and review of the adult literature. World J Surg Oncol. 2010;8:56. doi: 10.1186/1477-7819-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shivathirthan N, Kita J, Iso Y, Hachiya H, Kyunghwa P, Sawada T, et al. Primary hepatic leiomyosarcoma: case report and literature review. World J Gastrointest Oncol. 2011;3:148–152. doi: 10.4251/wjgo.v3.i10.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalaitzakis E, Sturgess R. Biliary leiomyoma diagnosed by Spyglass cholangioscopy (with video) Gastrointest Endosc. 2011;74:409–410. doi: 10.1016/j.gie.2011.03.1123. [DOI] [PubMed] [Google Scholar]


