Abstract
Background
To explore trends in the incidence and survival of patients with intrahepatic cholangiocarcinoma (ICC) an unselected population in Western Europe was studied.
Methods
Between 1989 and 2009, all patients newly diagnosed with ICC were selected from the Netherlands Cancer Registry (n = 809). Trends in incidence, treatment and relative survival were calculated according to gender and age. Follow-up for vital status was complete until 1st January 2010.
Results
The incidence rates of ICC increased significantly between 1999 and 2009, especially in the age group 45–59 years [estimated annual percentage change +3.0%, 95% confidence interval (CI) 0.2–5.8]. In the other age groups ICC incidence remained stable. Patients diagnosed with Tumour Lymph Node Metastasis (TNM) stage I mainly underwent surgery (68%), and the majority of the patients with stage II, III and IV received best supportive care (73%). One-year relative survival for patients with ICC increased significantly from 24% in 1989–1994 to 28% in 2005–2009 (P = 0.03), and corresponding 3-year relative survival improved from 4% to 8% (P = 0.02). Three-month and 1-year relative survival for patients with ICC receiving surgery was 91% and 71%, respectively.
Discussion
Between 1999 and 2009, the incidence of ICC rose, especially in the age group 45–59 years, suggesting aetiological influences. Survival rates have improved during the study period.
Introduction
Intrahepatic cholangiocarcinoma (ICC) malignancy arising from the ductal epithelium of the biliary tree has a poor prognosis.1,2 Perhaps because ICC is rare diagnosis, few reports on the epidemiology of ICC are available, and information on survival trends of ICC patients are only available from single institution case series, mostly describing patients who underwent a surgical resection.3–5 Such studies do not reflect the outcome of an unselected group of patients. Because improved computed tomography (CT) and high-resolution magnetic resonance imaging (MRI) of the liver have facilitated the diagnosis of liver malignancies, changes in ICC detection and survival are expected to occur as is reported by two studies from the United States.6,7 Therefore, a population-based study in the Netherlands of all patients diagnosed with ICC between 1989 and 2009 was performed and trends in incidence and survival according to gender, age and treatment were studied.
Methods
Data collection
Incidence and treatment data on ICC (ICD-10 code C22.1) from the period 1989–2009 were provided by the population-based Netherlands Cancer Registry (NCR) which is managed by the Comprehensive Cancer Centres the Netherlands and South.8 The NCR is based on notification of all newly diagnosed malignancies in the Netherlands by the automated pathological archive (PALGA). Additional sources are the national registry of hospital discharges and radiotherapy institutions. Information on patient characteristics, such as gender and date of birth and tumour characteristics, such as date of diagnosis, localization [(International Classification of Diseases for Oncology (ICD-O-3)], histology, stage [Tumour Lymph Node Metastasis (TNM) classification] and primary treatment, are obtained routinely from the medical records.9 The quality of the data is high, as a result of thorough training of the data managers, good access to care and computerized consistency checks. The infrastructure of the Netherlands health care system and the notification procedure used have made it possible to establish a cancer registry in which completeness is estimated to be at least 95% of the country.10,11 In the case of multiple tumours, the same rules were applied as those recommended by the International Association of Cancer Registries.12 The information on vital status was initially obtained from municipal registries and from 1995 onwards from the nationwide population registries network, providing complete coverage of all deceased Dutch residents.
ICC arises from the intrahepatic bile duct epithelium whereas extra-hepatic cholangiocarcinomas and Klatskin tumours involve the biliary tree within the hepatoduodenal ligament.13 To limit potential misclassification bias, patients with a non-classified tumour (histological or clinical), an extra-hepatic cholangiocarcinoma or a Klatskin tumour were excluded (n = 301).
Age was divided into five groups, i.e. <30, 30–44, 45–59, 60–74 and ≥75 years. The study period was divided into four categories, namely 1989–1994, 1995–1999, 2000–2004 and 2005–2009. TNM stage was determined post-operatively and clinical stage was used in case pathological TNM was missing. Treatment was scored as follows: surgery with curative intent including a partial liver resection, chemotherapy (systemic or regional)/ irradiation therapy or no specific anti-cancer therapy, i.e. best supportive care.
Patients younger than 15 years and older than 95 years were excluded from the survival analysis, as well as patients who had the diagnosis made at autopsy.
Statistical analyses
Annual incidence rates for the period 1989–2009 were calculated per 100 000 person-years, using the annual mid-year population size as obtained from Statistics Netherlands. Rates were age standardized to the European standard population [European Standardized Rates (ESR)]. Changes were evaluated by calculating the estimated annual percentage change (EAPC) and the corresponding 95% confidence interval (95% CI). Data regarding mortality trends were not available. Follow-up of vital status of all patients was calculated as the time from diagnosis to death, lost to follow-up or until the 1st of January 2010. The cohort-based method for relative survival analysis was used. Survival trends within 1989–2009 were evaluated using a Poisson regression model.14 SAS software (SAS system 9.2; SAS Institute, Cary, NC, USA) was used to perform the statistical analyses.
Results
Incidence
During the period 1989 to 2009, 809 patients were diagnosed with ICC of whom 785 (97%) were pathologically confirmed. The median age was 68 years (range 23–95) which did not change over time. The age-standardized incidence rate (ESR per 100 000) was similar for males and females. The overall incidence of ICC decreased from 0.22 per 100 000 in 1989 to 0.12 in 1999 (EAPC −5.0%, 95% CI −9.2, −0.7) and thereafter increased to 0.35 per 100 000 in 2009 (EAPC +9.4%, 95% CI 4.6, 14.3). Figure 1 shows the incidence rates by gender.
Figure 1.
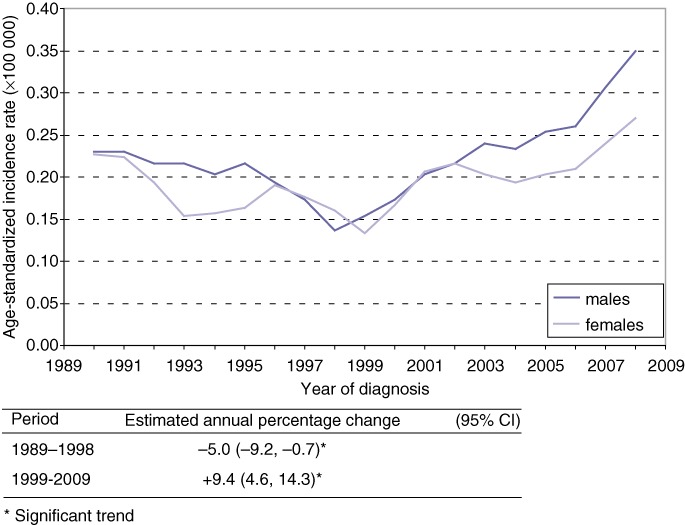
Trends (3-year moving averages) in age-standardized incidence rates (ESR) for intrahepatic cholangiocarcinoma (ICC) according to gender, in the Netherlands, 1989–2009
Figure 2 displays in five age groups the trends of ICC for both genders combined. A significantly increasing trend was observed in the age group 45–59 years (EAPC +3.0%, 95% confidence interval 0.2–5.8). The average annual number of new patients diagnosed with ICC (both sexes) was 0.3 (range 0–1) patients for the age group 0–29 years, 2.9 (range 0–8) patients for 30–44 years, 9.2 (range 3–16) patients for 45–59 years, 16.8 (range 4–31) patients for 60–74 years and 11.4 (range 6–22) patients for age 75 years and over.
Figure 2.
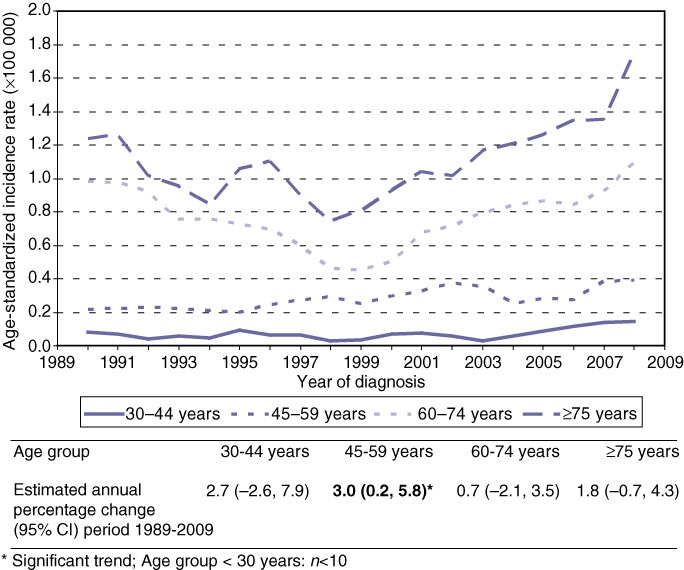
Trends (3-year moving averages) in age-specific incidence rates (ESR) for intrahepatic cholangiocarcinoma (ICC) in the Netherlands, 1989–2009
Treatment
Between 1989 and 2009, data regarding treatment and TNM stage were available in 511 patients and are summarized in Table 1.
Table 1.
Treatment according to Tumour Lymph Node Metastasis (TNM) stage in patients with intrahepatic cholangiocarcinoma in the Netherlands between 1989 and 2009
| TNM stage | N | Treatment n (%) | ||
|---|---|---|---|---|
| Surgery | Chemotherapy and/or irradiationa | No specific anti-cancer therapy | ||
| I | 31 | 21 (68) | 0 (0) | 10 (32) |
| II | 47 | 15 (32) | 4 (8) | 28 (60) |
| III | 121 | 35 (29) | 19 (16) | 67 (55) |
| IV | 312 | 7 (2) | 49 (16) | 256 (82) |
Patients were treated according to hospital protocols, no exact chemotherapy and/or irradiation treatment regimes were available from the registry over time.
Survival
Data regarding survival between 1989 and 2009 were available in 760 patients and missing in 49 patients. The relative survival rates for patients with an ICC between 1989 and 2009 improved (Table 2). The short-term (3, 6 months and 1 year) survival proportions for ICC patients who underwent surgery were 91%, 81% and 71%, respectively. The long-term (3-year and 5-year) survival proportions for ICC patients who underwent surgery were 40% and 34%, respectively. For ICC patients receiving chemotherapy and/or irradiation corresponding short-term numbers were 93%, 74% and 51%, respectively. For ICC patients receiving no anti-cancer therapy the short-term rates were 39%, 23% and 12%, respectively. There were no long-term survivors.
Table 2.
Relative survival of patients with intrahepatic cholangiocarcinoma in the Netherlands, since 1989 according to period of diagnosis
| Period of diagnosis | N | Survival % (SE) | |||
|---|---|---|---|---|---|
| 3 months | 6 months | 1 year | 3 years | ||
| 1989–1994 | 174 | 50 (3.9) | 33 (3.7) | 24 (3.3) | 4 (1.6) |
| 1995–1999 | 137 | 45 (4.3) | 30 (4.0) | 17 (3.2) | 7 (2.3) |
| 2000–2004 | 181 | 55 (3.7) | 37 (3.6) | 24 (3.2) | 11 (2.4) |
| 2005–2009 | 268 | 56 (3.1) | 41 (3.1) | 28 (2.9) | 8 (2.6) |
| P-value for trend | 0.04 | 0.02 | 0.03 | 0.02 | |
SE, standard error.
Discussion
Incidence
This population-based study demonstrates an increase in the incidence of ICC in the Netherlands between 1999–2009 and improvements in survival. The increasing ICC incidence confirms and expands the previous findings on hepatocellular carcinoma (HCC) reported in low endemic areas.6,15 The question is whether the rising incidence rate of ICC is a true increase or a reflection of improved diagnostic tests resulting in more detected tumours [e.g. MRI, multiphase CT, positron emission tomography (PET) scan and endoscopic retrograde cholangiopancreatography] which only became available at larger scale since 2002 in the Netherlands. In the absence of generally used serum markers better imaging techniques might cause an increased detection or reclassification of hepatobiliary tumours. ICC arises from the intrahepatic bile duct epithelium while extra-hepatic cholangiocarcinomas and Klatskin tumours involve the biliary tree within the hepatoduodenal ligament.13 To limit potential misclassification bias, patients with a non-classified tumour (histologically or clinically), an extra-hepatic cholangiocarcinoma or a Klatskin tumour were not included. The number of patients (97%) with ICC histologically confirmed is therefore rather high.
Tumours previously described as unclassified might be classified as ICC today.16 In case of misdiagnosis one would expect the incidence of at least one hepatobiliary tumour to decrease upon the rise of ICC. This has not happened, as the incidence of HCC also increased, and therefore the increasing incidence of ICC seems to be real.15 The increased detection of cancer is usually associated with an increase in the proportion of patients with early-stage cancers in all age groups. Therefore, it would have been interesting to study trends according to disease stage. Unfortunately as a result of changes in TNM-staging classification during the study period, it was not possible to draw any conclusion from trends by disease stage.
If the increase in ICC is a true reflection of an increased frequency, as can be suggested, the reason for this increase is unknown.6,17 Several medical conditions are potentially related to ICC, including biliary cirrhosis, cholecystitis, alcoholic liver disease, liver cirrhosis, type II diabetes mellitus, chronic pancreatitis, hepatitis C viral infection (HCV), hepatitis B viral infection (HBV), obesity, non-alcohol fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC) and inflammatory bowel diseases including ulcerative colitis.18 One possible explanation, for the observed increases, supported by recent studies, might be an increase in HCV- and/or HBV-related ICC.19 However, details on presence of HCV and/or HBV in this population were not available, preventing us from further investigating these hypotheses. Chronic inflammation, cholestasis and chronic liver damage are associated with malignant transformation of the biliary epithelium.18 Perhaps the increasing incidence of ICC might be influenced by an increasing prevalence of PSC.6,16,17 PSC-related cancers are reported to peak after the 4th or 5th decade of life.18,20 In this study, the rising incidence occurred predominantly in patients aged from 45 to 59 years which is possibly explained by an increasing prevalence of PSC.7 Although data regarding changes in prevalence of risk factors for ICC were not analysed, the increasing incidence of ICC is likely to be a true phenomenon rather than a reflection of improved diagnosis or a detection bias.11 Other explanations of the observed trends may be misclassification and changes in registration and/or diagnostic practices over time, therefore these data need to be interpreted with caution. The distinction between intrahepatic and extrahepatic cholangiocarcinoma is somewhat arbitrary, especially if it concerns a hilar cholangiocarcinoma. But, as extrahepatic cholangiocarcinoma in the Netherlands is five to six times more common than ICC, misclassification of only a small proportion of the extrahepatic tumours as intrahepatic has a relatively large influence on the incidence of ICC.
Treatment and survival
Tumour resection is the only potential cure for ICC, mostly patients in TNM stage I are able to receive this therapy. Pre-operative evaluation includes an assessment of patients' fitness for surgery, evaluation of the presence of metastatic disease and analysing the possibility to create a resection margin free from cancer.21 If any of these conditions are not fulfilled, surgical therapy is not indicated and palliative modalities are recommended.
Aljiffry et al. reviewed the literature according to outcome after surgical resection for ICC and found 5-year survival rates ranging from 15% to 40%, similar to the 5-year survival rate in this study of 34%.13 Because only a few patients received surgery, trends in survival over time could not be calculated for surgery.
After a complete surgical resection, a strategy aimed at optimizing local control with post-operative radiation alone or in combination with chemotherapy may theoretically provide a benefit.22 However, the available literature consists mainly of an uncontrolled small series, and many reports consist of a mix of bile duct cancers, gallbladder cancer, ampullary cancer, and either pancreatic or hepatocellular cancers; as a result, the benefit of any type of adjuvant therapy remains uncertain.5,23 In general, no single drug or combination has consistently led to objective tumour shrinkage or an increased median survival beyond the expected 8 to 15 months.5,23 It is interesting to see that patients who received chemotherapy/irradiation had a similar short-term survival rate, especially at 3 and 6 months (93% and 74%) compared with patients who underwent surgery (91% and 81%). Studies on palliative chemotherapy on patients with ICC have reported a median survival time between 6 and 15 months.13 The survival benefit for patients able to receive surgery was increased with duration of follow-up, as the long-term survival in patients receiving surgery is much better compared with patients receiving chemotherapy/irradiation.
As the benefit of any type of adjuvant therapy remains doubtful, the improved survival can be attributed to improved palliative treatments (stenting and drainage), improved overall supportive medical care, or both.
The improved survival could indicate lead-time bias related to early detection as patients with associated factors are controlled more often. For instance patients with PSC have a lifetime risk of ICC which ranges from 8% to 20% and therefore these data need to be interpreted with caution.16
In conclusion, this population-based analysis appears to demonstrate an increasing incidence of ICC since 1999. Overall survival of patients with ICC appears to be improved, suggesting possible influences of improved imaging techniques, a better patient selection for surgery or improved surgical techniques. As a result of limitations both these statements should be interpreted with caution. This study demonstrates that although improvements in ICC survival were achieved over time, patients with these tumours continue to have a very poor prognosis. In spite of several advances over the last decades, an ample opportunity for improvement still remains.
Acknowledgments
The work on this research project was performed within the framework of the project ‘Progress against cancer in the Netherlands since the 1970s?’. The authors thank the NCR for providing data from the cancer registry and the registration clerks for the dedicated data collection.
Conflict of interest
None declared.
References
- 1.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109–114. doi: 10.1055/s-2007-1007302. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J. Epidemiology of liver cancer in Europe. Can J Gastroenterol. 2000;14:621–630. doi: 10.1155/2000/815454. [DOI] [PubMed] [Google Scholar]
- 3.Tan JCC, Coburn NG, Baxter NN, Kiss A, Law CHL. Surgical management of intrahepatic cholangiocarcinoma – A population-based study. Ann Surg Oncol. 2007;15:600–608. doi: 10.1245/s10434-007-9627-x. [DOI] [PubMed] [Google Scholar]
- 4.Tamandl D, Herberger B, Gruenberger B, Puhalla H, Klinger M, Gruenberger T. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2008;15:2787–2794. doi: 10.1245/s10434-008-0081-1. [DOI] [PubMed] [Google Scholar]
- 5.Puhalla H, Schuell B, Pokorny H, Kornek GV, Scheithauer W, Gruenberger T. Treatment and outcome of intrahepatic cholangiocellular carcinoma. Am J Surg. 2005;189:173–177. doi: 10.1016/j.amjsurg.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 7.Shaib YH, Davila JA, McGlynn K, El-serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Sanden van der GAC, Coebergh JWW, Schouten LJ, Visser O, van Leeuwen FE. Cancer incidence in the Netherlands in 1989 and 1990: first results of the nation-wide Netherlands Cancer Registry. Eur J Cancer. 1995;31A:1822–1829. doi: 10.1016/0959-8049(95)00355-m. [DOI] [PubMed] [Google Scholar]
- 9.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology (ICD-O) 3rd edn. Geneva: World Health Organization; 2000. [Google Scholar]
- 10.Schouten LJ, Hoppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, the Netherlands. Int J Epidemiol. 1993;22:369–376. doi: 10.1093/ije/22.3.369. [DOI] [PubMed] [Google Scholar]
- 11.Berkel J. General practitioners and completeness of cancer registry. J Epidemiol Community Health. 1990;44:121–124. doi: 10.1136/jech.44.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IARC/IACR. Multiple Primaries. IARC Internal Report No. 94/ 003. Lyon: International Agency for Research on Cancer; 1994. [Google Scholar]
- 13.Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol. 2009;15:4240–4262. doi: 10.3748/wjg.15.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 15.Witjes CD, Karim-Kos HE, Visser O, van den Akker SAW, de Vries E, Ijzermans JNM, et al. Hepatocellular carcinoma in a low endemic area: rising incidence and improved survival. Eur J Gastroenterol Hepatol. 2012;24:450–457. doi: 10.1097/MEG.0b013e32835030ce. [DOI] [PubMed] [Google Scholar]
- 16.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 17.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma rising frequency, improved survival, and determinants of outcome after resection. Ann Surg Oncol. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 18.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvaro D, Crocetti E, Ferretti S, Bragazzi MC, Capocaccia R AISF. Cholangiocarcinoma committee. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010;42:490–495. doi: 10.1016/j.dld.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 20.de Valle MB, Bjornsson E, Lindkvist B. Mortality and cancer risk related to primary sclerosing cholangitis in a Swedish population-based cohort. Liver Int. 2012;32:441–448. doi: 10.1111/j.1478-3231.2011.02614.x. [DOI] [PubMed] [Google Scholar]
- 21.Gatto M, Bragazzi MC, Semeraro R, Napoli C, Gentile R, Torrice A, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253–260. doi: 10.1016/j.dld.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 23.Park I, Lee JL, Ryu MH, Kim TW, Sook Lee S, Hyun Park D, et al. Prognostic factors and predictive model in patients with advanced biliary tract adenocarcinoma receiving first-line palliative chemotherapy. Cancer. 2009;115:4148–4155. doi: 10.1002/cncr.24472. [DOI] [PubMed] [Google Scholar]


