Abstract
Objectives
This study describes the management of patients with bilobar colorectal liver metastases (CRLM).
Methods
A retrospective collection of data on all patients with CRLM who were considered for staged resection (n = 85) from January 2003 to January 2011 was performed. Patients who underwent one hepatic resection were considered to have had a failed staged resection (FSR), whereas those who underwent a second or third hepatic resection to produce a cure were considered to have had a successful staged resection (SSR). Survival was calculated from the date of diagnosis of liver metastases. Complete follow-up and dates of death were obtained from the Government of Quebec population database.
Results
Median survival was 46 months (range: 30–62 months) in the SSR group and 22 months (range: 19–29 months) in the FSR group. Rates of 5-year survival were 42% and 4% in the SSR and FSR groups, respectively. Fifteen of the 19 patients who remained alive at the last follow-up date belonged to the SSR group.
Conclusions
In patients in whom staged resection for bilobar CRLM is feasible, surgery would appear to offer benefit.
Introduction
Approximately 142 570 Americans are diagnosed with colorectal cancer annually.1 Of these, 51 370 will die from the disease.1 Metastatic disease is the most common cause of death and occurs in 50% of patients.2,3
Despite improved chemotherapy with oxaliplatin, irinotecan and the addition of biologics such as bevacizumab and cetuximab, patients treated with palliative chemotherapy alone have a median survival of only 18–22 months.4 Liver resection provides the only potential for cure in patients with colorectal liver metastases (CRLM) and 5-year survival rates as high as 60% have been reported.5 These results have increased efforts to convert patients with isolated hepatic disease who are initially deemed unresectable to a technically resectable state by combining preoperative chemotherapy, surgery, ablative technologies, portal vein embolization (PVE) and staged resections.5 Currently, the process of determining the resectability of a patient with CRLM focuses on the quality and amount of liver that will be left after a negative margin (R0) resection – the future liver remnant (FLR) – rather than the amount of liver to be resected. All conversion strategies are aimed at leaving an adequate FLR and depend either on increasing the size of the FLR or on decreasing the magnitude of the hepatic resection so that the FLR will be larger. This paradigm shift in the management of CRLM has resulted in potentially curative resection surgery in patients with complex bilobar liver metastases, who would previously have been deemed unresectable.
A staged hepatectomy occurs when two or more liver resections to be performed at different time-points are required to achieve an R0 resection. The staged hepatectomy strategy takes advantage of the liver's ability to regenerate after injury or resection. This paper reports on a large, single-centre experience of staged liver resection in CRLM.
Materials and methods
Patient selection
Patients with multiple bilobar CRLM that could not be completely resected in one laparotomy were considered for staged hepatectomy (Figs 1–3). Patients underwent computed tomography (CT) of the chest, abdomen and pelvis and an 18F-fluorodeoxyglucose positron emission tomography (18FDG PET) scan, after which they were presented at a multidisciplinary hepatopancreatobiliary (HPB) tumour board meeting. If a patient had liver-only disease (with the exception of resectable lung disease) and good performance status, and all disease could potentially be resected or ablated using a staged approach as determined by the surgeons on the HPB tumour board, he or she was considered for staged resection. Intraoperative ultrasound was used in all patients.
Figure 1.
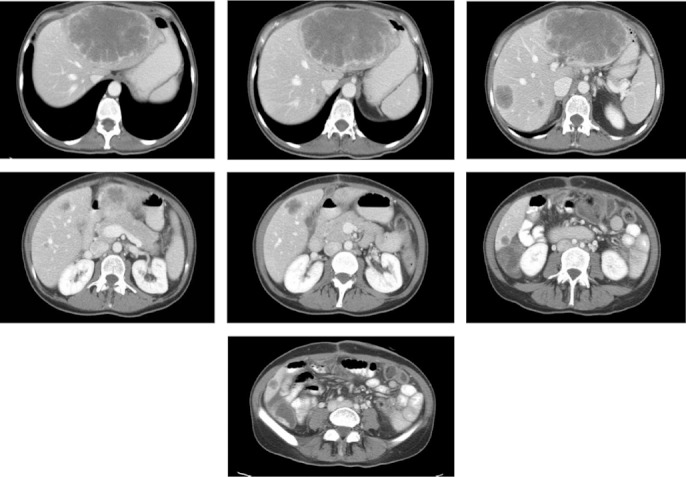
Computed tomography in a typical patient selected for the staged resection pathway at McGill University Health Centre
Figure 3.
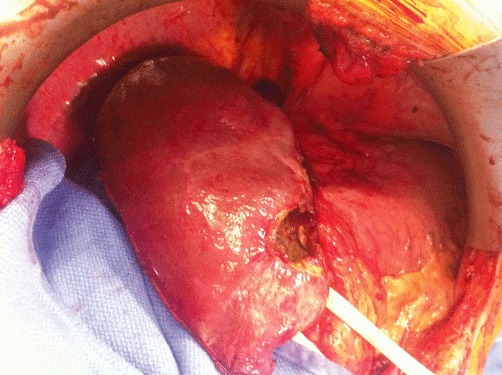
Segment IV at the completion of two-stage hepatectomy in a different patient
Figure 2.

Computed tomography in the patient shown in Fig. 1, with bilateral hepatic metastases, after two-stage hepatectomy, showing hypertrophy of segment IV
Evolution of staged hepatectomy
All patients were administered neoadjuvant chemotherapy with FOLFIRI [folinic acid (leucovorin), fluorouracil (5-FU), irinotecan] or FOLFOX [folinic acid (leucovorin), fluorouracil (5-FU), oxaliplatin]. Bevacizumab was added to the regime from 2006. Response was assessed by CT conducted between the fourth and fifth cycles of chemotherapy. Because the authors consider an initial response to chemotherapy an important prognostic indicator, patients who did not respond to chemotherapy were excluded from further consideration for hepatic resection.6 After the sixth cycle, chemotherapy was discontinued for 6 weeks and the first liver resection was performed.
The approach in managing these patients varied: (i) in some patients, the first resection consisted of a right hepatectomy followed by a second left-sided resection; (ii) other patients were managed with multiple non-anatomical wedge resections to clear one side (the FLR), followed by a second operation to remove the contralateral liver, and (iii) in other patients a left-sided resection was performed first and was followed by a right-sided resection. From the middle of this series, a standard approach was developed that could be applied to most patients. Stage 1 of the staged hepatectomy comprised a left lateral liver resection with or without the caudate and included the clearance of any tumours from segment IV; this was performed laparoscopically if possible. The patient was allowed to recover from stage 1 (2–3 weeks) before a right-side PVE was performed, which was followed 4 weeks later by a CT scan to confirm an adequate FLR. An adequate FLR was defined as a remnant equivalent to 30% in size of the original liver because all patients received neoadjuvant chemotherapy. If any delays occurred, patients would receive one or two cycles of chemotherapy without bevacizumab and then proceed to a right hepatectomy (stage 2) followed by adjuvant chemotherapy to complete a total of 12 cycles. This approach to staged hepatectomy was based on preserving the middle hepatic vein (Fig. 4). This approach ensures that growth between resections will occur in segments IV and I, whereas if a right hepatectomy is carried out first, there is no assurance that the increase in volume will be in segment IV. Follow-up required the patient to attend clinic visits and to undergo CT of the chest and abdomen and measurement of carcinoembryonic antigen (CEA) levels every 3 months for the first 2 years, every 6 months for the subsequent 3 years, and yearly thereafter.
Figure 4.
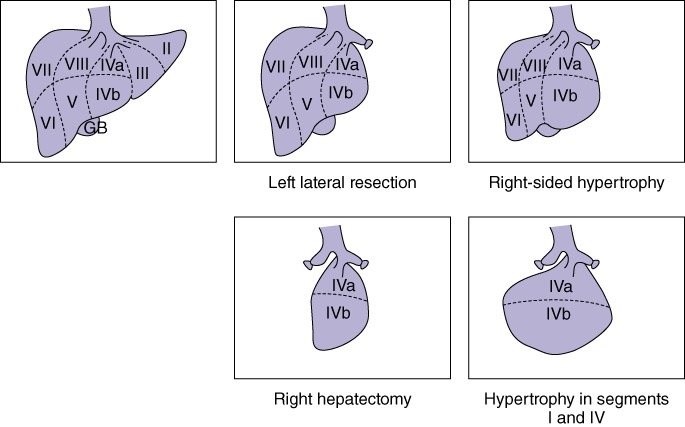
Diagrams showing the staged resection strategy. GB, gallbladder
Portal vein embolization
Portal vein embolization was performed after the first hepatectomy of the two-stage procedure. Embolization was performed via an ipsilateral approach using 90–180-µm polyvinyl alcohol (PVA) and coils to occlude segmental branch origins. In patients undergoing right-sided embolization, the first embolization included both the anterior and posterior sectoral branches of the right portal vein. In general, chemotherapy was discontinued approximately 6 weeks prior to the first hepatic resection and the embolization was performed 2–3 weeks after the resection.
Data collection and statistical analysis
Data on the colorectal primary pathology, date of diagnosis of liver metastases, size of metastases, number and distribution of metastases, operative technique and findings, and tumour board discussion were collected using the McGill University Health Centre (MUHC) prospective tumour registry database and by chart review. Clinic charts and follow-up CT scans were utilized to collect data on tumour recurrence. Morbidity was defined according to the Clavien grade.7
Continuous variables are expressed as the median [interquartile range (IQR)]. The date of death was confirmed in all instances of patient death by accessing the Government of Quebec registry. Complete follow-up to March 2012 was obtained in all patients. Fisher's exact test, the chi-squared test and Mann–Whitney U-test were used as appropriate. Survival was calculated from the date of diagnosis of liver metastasis using the Kaplan–Meier method; differences were examined with the log-rank test. A multivariable logistic regression analysis was used to calculate odds ratios (ORs) with 95% confidence intervals (CIs). A P-value of <0.05 was considered to indicate statistical significance. All statistical calculations were performed using R Version 2.8.1 (R Project for Statistical Computing; http://www.r-project.org).
Results
From January 2003 to January 2011, 85 patients with bilobar CRLM were placed on a staged resection pathway at the Royal Victoria Hospital, MUHC. The baseline characteristics of the patients are illustrated in Table 1. Table 2 shows morbidity and mortality in the cohort.
Table 1.
Clinical and pathologic features of the study population (n = 85)
| Variable | Value |
|---|---|
| Age, years, median (IQR) | 57 (48–65) |
| Gender, male/female, n (%) | 56 (66%)/29 (34%) |
| Node-positive primary disease, n (%) | 65 (76%) |
| Disease-free interval of <12 months, n (%) | 21 (25%) |
| CEA of >200 ng/ml, n (%) | 13 (15%) |
| More than one lesion, n (%) | 85 (100%) |
| Lesion measuring >5 cm, n (%) | 15 (18%) |
| Synchronous lesion at 6 months, n (%) | 63 (74%) |
| Number of lesions, median (IQR) | 6 (2–14) |
| Size of largest lesion, cm, median (IQR) | 3.4 (1–14) |
| Extrahepatic metastases, n (%) | 11 (13%) |
| First-stage hepatectomy | |
| Right-side hepatectomy, n (%) | 27 (32%) |
| Left-side hepatectomy, n (%) | 47 (55%) |
| Interval between surgeries, days, median (IQR) | 158 (126–204) |
| Portal vein embolization, n (%) | 48 (56%) |
IQR, interquartile range; CEA, carcinoembryonic antigen.
Table 2.
Morbidity according to the Clavien classification of complications
| Clavien grade | Morbidity after first hepatectomy, n | Morbidity after second or third hepatectomy, n |
|---|---|---|
| I | 3 | 2 |
| II | 11 | 11 |
| IIIa | 5 | 4 |
| IIIb | 2 | 4 |
| IVa | 1 | 0 |
| IVb | 0 | 0 |
| V | 0 | 0 |
Two-stage hepatectomy
All patients preoperatively received a traditional chemotherapy regimen based on 5-fluorouracil (FOLFIRI or FOLFOX); 44 patients (52%) also received bevacizumab.
Outcomes in the 85 patients considered for staged hepatectomy following neoadjuvant chemotherapy are shown in Fig. 5. All patients scheduled for first-stage hepatectomy underwent a major resection (three or more segments).
Figure 5.
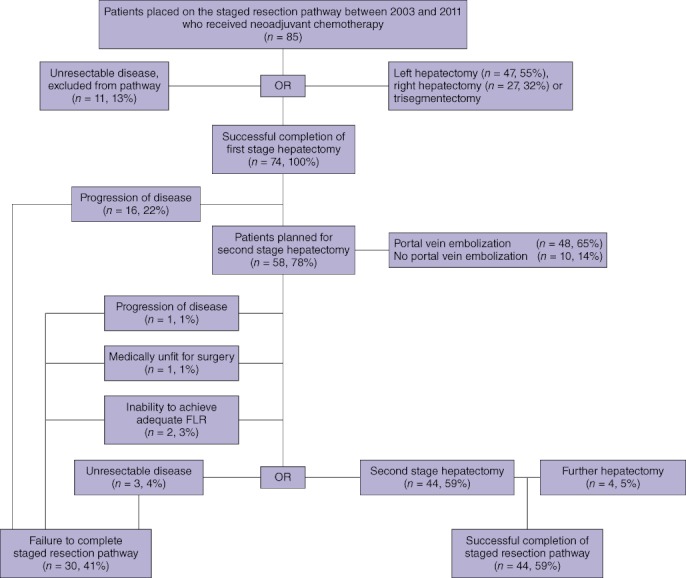
Flow chart showing the staged resection pathway in the failed and successful staged resection groups. FLR, future liver remnant
Overall and recurrence-free survival
Median follow-up in the 85 patients was 31 months (IQR: 22.1–51.2 months). At the time of last follow-up, 19 (22%) of the 85 patients placed on the staged resection pathway were alive. Rates of overall survival at 1, 3 and 5 years were 93%, 49% and 26%, respectively (Fig. 6). Median survival was 28.6 months (95% CI 21.0–48.6). Of the 30 patients who failed to complete the staged resection pathway [failed staged resection (FSR) group], only four remained alive at the last follow-up. In comparison, of the 44 patients who successfully completed the staged resection pathway [successful staged resection (SSR) group], 15 were alive (P < 0.001).
Figure 6.

Kaplan–Meier survival curves showing overall survival in the successful and failed staged resection groups
Median overall survival from the time of diagnosis of liver metastases was 46 months (95% CI 30.0–62.0) in the SSR group and 22 months (95% CI 19.0–29.0) in the FSR group (P < 0.001). Median survival in the unresectable (no hepatectomy) patient group was 21.6 months (95% CI 10.8–24.0).
Rates of 1-year survival were 95% in the SSR group, 93% in the FSR group and 82% in the unresectable group (P = 0.13). Rates of 3-year survival were 68% in the SSR group, 26% in the FSR group and 0% in the unresectable group (P < 0.001). Rates of 5-year survival were 42% in the SSR group and 22% in the FSR group (P = 0.011) (Fig. 6).
Factors associated with successful staged hepatectomy
Data for the 44 patients who successfully completed the staged resection (SSR group) were compared with data for the 30 patients in the FSR group. Univariate analysis showed that female gender, a lower number of lesions and a largest lesion size of <5 cm were significant factors predicting successful staged resection. Multivariable logistic regression analysis showed the presence of extrahepatic disease to be the only factor associated with the failure of staged resection (Table 3).
Table 3.
Univariate and multivariate logistic regression analysis for the success of staged resection (n = 74)
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age >65 years | 0.54 | 0.16–1.83 | 0.320 | |||
| Male gender | 0.26 | 0.08–0.78 | 0.022 | 0.32 | 0.05–1.51 | 0.165 |
| Node-positive primary disease | 0.99 | 0.21–4.30 | 0.987 | |||
| Disease-free interval <12 months | 1.24 | 0.40–4.10 | 0.717 | |||
| CEA >200 ng/ml | 0.67 | 0.19–2.36 | 0.525 | |||
| More than three lesions | 0.40 | 0.10–1.30 | 0.148 | 0.29 | 0.05–1.42 | 0.145 |
| Size of largest lesion >5 cm | 0.27 | 0.07–0.92 | 0.041 | 0.39 | 0.08–1.77 | 0.226 |
| Bilobar disease | 0.57 | 0.08–2.87 | 0.520 | |||
| Extrahepatic metastases | 0.43 | 0.15–1.14 | 0.098 | 0.15 | 0.02–0.78 | 0.038 |
| Interval between surgeries >150 days | 0.26 | 0.01–2.02 | 0.255 | |||
OR, odds ratio; 95% CI, 95% confidence interval; CEA, carcinoembryonic antigen.
Factors associated with disease-free survival
Univariate analysis showed that in the 44 patients who successfully completed the staged resection, node-positive primary disease, a largest lesion size of <5 cm and the presence of extrahepatic metastasis were factors associated with lower rates of recurrence-free survival. Multivariate logistic regression analysis (Table 4) showed only the presence of extrahepatic disease to be associated with lower disease-free survival.
Table 4.
Univariate logistic regression analysis for recurrence-free survival (n = 31)
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age >65 years | 1.85 | 0.33–10.35 | 0.488 | 1.04 | 0.16–6.71 | 0.968 |
| Male gender | 1.04 | 0.28–3.86 | 0.947 | 3.82 | 0.96–15.20 | 0.086 |
| Node-positive primary disease | 0.15 | 0.04–0.59 | 0.014 | 0.61 | 0.09–4.02 | 0.615 |
| Disease-free interval <12 months | 0.37 | 0.09–1.50 | 0.175 | 0.58 | 0.12–2.88 | 0.522 |
| CEA >200 ng/ml | 0.45 | 0.05–3.88 | 0.476 | 0.12 | 0.01–1.39 | 0.120 |
| More than three lesions | 1.17 | 0.28–4.90 | 0.831 | 0.31 | 0.07–1.40 | 0.158 |
| Extrahepatic metastases | 0.24 | 0.08–0.76 | 0.021 | 0.12 | 0.02–0.62 | 0.030 |
| Interval between surgeries >150 days | 1.21 | 0.30–4.86 | 0.787 | |||
OR, odds ratio; 95% CI, 95% confidence interval; CEA, carcinoembryonic antigen.
Discussion
Staged hepatectomy for the management of bilobar CRLM is possible and appears to offer good patient survival when both resections are successfully completed. In this series, the SSR group achieved significantly better overall and disease-free survival than the FSR group. The staged hepatectomy was completed in 44 of 85 patients (52%), which is lower than rates reported in other series.5,8–11 Patients entered into this pathway had been deemed initially unresectable at presentation.
Higher success rates might be achieved if the criteria for entry into the staged resection pathway were more restrictive. However, in the current series, the 30 patients who failed to complete the second resection achieved better survival than those who underwent only exploratory laparotomy. Although these patients never achieved a disease-free status and continued to be treated with chemotherapy and locoregional therapy, this does not appear to have adversely affected their overall survival rates compared with those of patients who received palliative chemotherapy only. In fact, perhaps because of the continued stop-and-go chemotherapy and the locoregional therapies administered after hepatectomy in the FSR group, survival in this group appears to have been slightly better than that in patients receiving palliative chemotherapy alone.4
Previous criteria to determine resectability focused on the number and size of metastases and the chronology of the occurrence of liver disease in relation to that of the primary cancer, along with the spatial arrangement of the metastases. The Memorial Sloan–Kettering Cancer Center group refined these criteria with a clinical risk score, which enabled the prediction of survival after liver resection and helped to expand the criteria for resectability by identifying a subset of patients in whom better outcomes might be achieved.12 Adam et al. demonstrated the feasibility of converting unresectable CRLM patients to a potentially resectable group by using neoadjuvant chemotherapy.8
Various researchers have focused their attention on the FLR.13 In general, an FLR equivalent to 20% of liver volume can be viable in a normal liver resection, but, in patients who have received chemotherapy, an FLR of ≥ 30% of hepatic volume is recommended. Therefore, liver resection is feasible regardless of the number and sizes of metastases as long as 30% of the liver volume can be retained and can include a draining hepatic vein and an adequate portal and arterial supply with intact biliary drainage.
This programme has evolved to enable all patients with predominantly liver disease to be considered as potentially resectable. Decisions on whether patients are scheduled for extended hepatectomy with or without PVE, multiple wedge resections, resection combined with local ablation, or staged hepatectomy are based on findings in re-scanning after chemotherapy. Data from this series and others5,8–11 indicate that the most common reason why patients do not proceed to a second hepatic resection is that their disease progresses during the interval between the two resections. In some patients who have been referred from other centres, this may occur because they have already received multiple cycles of chemotherapy prior to commencing the staged hepatectomy protocol, whereas others may demonstrate liver regeneration stimulated by PVE and/or the first resection.14
The only factor found to be significantly associated with the failure of staged resection was the presence of extrahepatic disease. Number of lesions was associated with the success of staged hepatectomy, but showed a trend towards statistical significance only in multivariate analysis. This finding is similar to data reported in previous studies showing that age and the presence of three or more metastases in the FLR were associated with staged hepatectomy failure.10
The current study has demonstrated the feasibility and safety of the staged hepatectomy strategy, as well as its ability to achieve a cure and improve overall and disease-free survival. The present findings are strengthened by the size of the series and the complete survival data obtained from the provincial death registry. Weaknesses of the present study include those inherent in a largely retrospective study design and the fact that multivariate analysis was performed using data for a relatively low number of patients. In conclusion, staged hepatectomy is a feasible and safe technique for the management of bilobar CRLM. When staged hepatectomy is successfully completed, 5-year survival exceeds 40%. This strategy can be used in patients for whom maintaining an adequate FLR would not be possible in a single resection. For this patient population, staged resection may represent the only chance for cure. Future studies should focus on comparative investigations of outcomes in patients in whom staged resection is not attempted, and on the quality of life of patients undergoing aggressive resectional interventions. In addition, future studies should be directed at defining the role of chemotherapy between resections.
Conflicts of interest
None declared.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch. Surveillance, Epidemiology and End Results (SEER) Program. 2007. http://www.seer.cancer.gov. [Accessed 1 March 2012]
- 3.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. American Joint Committee on Cancer. Cancer Staging Manual. 7th edn. New York, NY: Springer; 2010. p. 143. [Google Scholar]
- 4.Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, et al. Outcome of primary tumour in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–3384. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adam R, Miller R, Pitombo M, Wicherts DA, de Haas RJ, Bitsakou G. Two-stage hepatectomy approach for initially unresectable colorectal hepatic metastases. Surg Oncol Clin N Am. 2007;16:525–536. doi: 10.1016/j.soc.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Chan G, Hassanain M, Chaudhury P, Vrochides D, Neville A, Cesari M, et al. Pathological response grade of colorectal liver metastases treated with neoadjuvant chemotherapy. HPB. 2010;12:277–284. doi: 10.1111/j.1477-2574.2010.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict longterm survival. Ann Surg. 2004;240:644–658. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowers KA, O’Reilly D, Bond-Smith GE, Hutchins RR. Feasibility study of two-stage hepatectomy for bilobar liver metastases. Am J Surg. 2011;203:691–697. doi: 10.1016/j.amjsurg.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Narita M, Oussoultzoglou E, Jaeck D, Fuchschuber P, Rosso E, Pessaux P, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg. 2011;98:1463–1475. doi: 10.1002/bjs.7580. [DOI] [PubMed] [Google Scholar]
- 11.Jaeck D, Bachellier P, Nakano H, Oussoultzoglou E, Weber JC, Wolf P, et al. One- or two-stage hepatectomy combined with portal vein embolization for initially non-resectable colorectal liver metastases. Am J Surg. 2011;185:221–229. doi: 10.1016/s0002-9610(02)01373-9. [DOI] [PubMed] [Google Scholar]
- 12.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 14.Simoneau E, Aljiffry M, Salman A, Abualhassan N, Cabrera T, Valenti D, et al. Portal vein embolization stimulates tumour growth in patients with colorectal cancer liver metastasis: a retrospective case–control study. HPB. 2012;14:461–468. doi: 10.1111/j.1477-2574.2012.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


