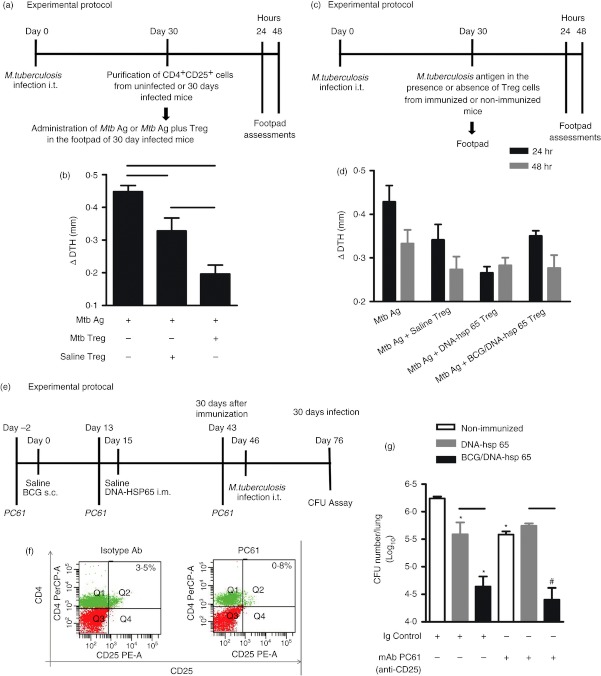
Figure 4.
Evaluation of the regulatory T cells in vivo after immunization with the DNA-heat-shock protein (hsp) 65 or BCG/DNA-hsp 65 vaccine. BALB/c mice were infected intratracheally with 1 × 105 bacilli. Thirty days after infection, 50 μg Mycobacterium tuberculosis antigen (Mtb Ag) in the presence or absence of CD4+ CD25+ cells, which were obtained from infected or uninfected mice, were administered in the right footpad. The left footpad received saline (a). Oedema measurement was performed 48 hr after the administration of antigen (b). BALB/c mice were infected, and 30 days after infection, they received 50 μg M. tuberculosis antigens in the right footpad in the presence or absence of CD4+ CD25+ cells that were obtained from immunized or non-immunized mice (c). Oedema measurements were performed at 24 and 48 hr (d) after antigen administration. BALB/c mice were immunized with BCG/DNA-hsp 65 and challenged 30 days after prime boost vaccination. Three days before immunization and challenge, the mice were treated with the monoclonal antibody (mAb) PC61 (e). Representative flow cytometry data demonstrating the percentages of CD4+ CD25+ lymphocytes in the blood of mice treated with 500 μg mAb PC61 during immunization (f). Colony-forming unit (CFU) counts were evaluated 30 days after challenge (g). The results are expressed as the mean ± SEM. The data are representative of two experiments. For the delayed type hypersensitivity (DTH) assay, with CD4+ CD25+ cells purified from uninfected or infected mice, seven mice were used. For the DTH assay, with CD4+ CD25+ cells purified from immunized or non-immunized mice, 8–14 mice were used. For the treatment with anti-CD25, eight or nine mice were used. Horizontal bars represent the significant difference between the groups. *P < 0·05 compared with non-immunized, infected and treated with immunoglobulin control mice; #P < 0·05 compared with mice non-immunized, infected and treated with mAb PC61.