Abstract
Erythroid bursts from cord or adult blood were grown in methylcellulose cultures (3 international units of erythropoietin per plate). On day 13, single bursts were picked up and reincubated for 16-24 hr with [3H]leucine. Radioactive globin chains [alpha,beta,G gamma, and A gamma (Ala-136)] were analyzed by either isoelectric focusing on polyacrylamide gels and fluorography or carboxymethylcellulose chromatography. In all cases, alpha to non-alpha globin radioactivity ratios were close to 1. In single cord blood bursts, the values of both gamma-to-beta and G gamma-to-A gamma ratios were spread over a large spectrum and further characterized by a continuous rather than a bimodal distribution. Morever, the G gamma-to-A gamma ratios demonstrated in single bursts appeared to be directly correlated with the respective gamma-to-beta ratios. These data suggest that both the gamma leads to beta and the G gamma leads to A gamma switches are mediated via mechanisms modulating the relative activities of the different genes in the non-alpha globin gene cluster rather than via selection of clones committed to the preferential synthesis of beta and A gamma globins. In contrast with the results obtained with cord blood, individual adult blood bursts synthesize a lower and hence relatively more uniform amount of gamma globin chains.
Full text
PDF

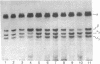
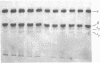
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Belding T. K., Margolet L., Noyes A. N. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science. 1975 Apr 25;188(4186):361–363. doi: 10.1126/science.804182. [DOI] [PubMed] [Google Scholar]
- Clarke B. J., Housman D. Characterization of an erythroid precursor cell of high proliferative capacity in normal human peripheral blood. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1105–1109. doi: 10.1073/pnas.74.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Dan M., Hagiwara A. Detection of two types of hemoglobin (HbA and HbF) in single erythrocytes by fluorescent antibody technique. Exp Cell Res. 1967 Jun;46(3):596–598. doi: 10.1016/0014-4827(67)90385-0. [DOI] [PubMed] [Google Scholar]
- FRASER I. D., RAPER A. B. Observations on the change from foetal to adult erythropoiesis. Arch Dis Child. 1962 Jun;37:289–296. doi: 10.1136/adc.37.193.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Ogawa M. Erythropoietic precursors in murine blood. Exp Hematol. 1977 May;5(3):161–165. [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Harris H., Gravely M. The chemical heterogeneity of the fetal hemoglobin in normal newborn infants and in adults. Mol Cell Biochem. 1977 Aug 19;17(1):45–55. doi: 10.1007/BF01732554. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Efremov G. D., Duma H., Mladenovski B., Hyman C. B., Rachmilewitz E. A., Bouver N., Miller A., Brodie A. The present status of the heterogeneity of fetal hemoglobin in beta-thalassemia: an attempt to unify some observations in thalassemia and related conditions. Ann N Y Acad Sci. 1974;232(0):107–124. doi: 10.1111/j.1749-6632.1974.tb20576.x. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Felice A., Powars D., Ringelhann B. Anomaly in the gamma chain heterogeneity of the newborn. Nature. 1977 Jan 6;265(5589):63–65. doi: 10.1038/265063a0. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Wrightstone R. N., Wilson J. B., Schroeder W. A., Kendall A. G. Hemoglobin Kenya, the product of fusion of amd polypeptide chains. Arch Biochem Biophys. 1972 Dec;153(2):850–853. doi: 10.1016/0003-9861(72)90408-0. [DOI] [PubMed] [Google Scholar]
- Iscove N. N. The role of erythropoietin in regulation of population size and cell cycling of early and late erythroid precursors in mouse bone marrow. Cell Tissue Kinet. 1977 Jul;10(4):323–334. doi: 10.1111/j.1365-2184.1977.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Kidoguchi K., Ogawa M., Karam J. D., McNeil J. S., Fitch M. S. Hemoglobin biosynthesis in individual bursts in culture: studies of human umbilical cord blood. Blood. 1979 Mar;53(3):519–522. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Miller D. M., Turner P., Nienhuis A. W., Axelrod D. E., Gopalakrishnan T. V. Active conformation of the globin genes in uninduced and induced mouse erythroleukemia cells. Cell. 1978 Jul;14(3):511–521. doi: 10.1016/0092-8674(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Nute P. E., Pataryas H. A., Stamatoyannopoulos G. The G and A hemoglobin chains during human fetal development. Am J Hum Genet. 1973 May;25(3):271–276. [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Grush O. C., O'Dell R. F., Hara H., MacEachern M. D. Circulating erythropoietic precursors assessed in culture: characterization in normal men and patients with hemoglobinopathies. Blood. 1977 Dec;50(6):1081–1092. [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Bunn H. F., Stamatoyannopoulos G. Cellular distribution of hemoglobin F in a clonal hemopoietic stem-cell disorder. N Engl J Med. 1978 Jan 12;298(2):72–75. doi: 10.1056/NEJM197801122980203. [DOI] [PubMed] [Google Scholar]
- Peschle C., Cillo C., Rappaport I. A., Magli M. C., Migliaccio G., Pizzella F., Mastroberardino G. Early fluctuations of BFU-E pool size after transfusion or erythropoietin treatment. Exp Hematol. 1979 Feb;7(2):87–93. [PubMed] [Google Scholar]
- SHEPARD M. K., WEATHERALL D. J., CONLEY C. L. Semi-quantitative estimation of the distribution of fetal hemoglobin in red cell populations. Bull Johns Hopkins Hosp. 1962 Jun;110:293–310. [PubMed] [Google Scholar]
- Saglio G., Ricco G., Mazza U., Camaschella C., Pich P. G., Gianni A. M., Gianazza E., Righetti P. G., Giglioni B., Comi P. Human T gamma globin chain is a variant of A gamma chain (A gamma Sardinia). Proc Natl Acad Sci U S A. 1979 Jul;76(7):3420–3424. doi: 10.1073/pnas.76.7.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. A., Huisman T. H., Shelton J. R., Shelton J. B., Kleihauer E. F., Dozy A. M., Robberson B. Evidence for multiple structural genes for the gamma chain of human fetal hemoglobin. Proc Natl Acad Sci U S A. 1968 Jun;60(2):537–544. doi: 10.1073/pnas.60.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. R., Shelton J. B., Apell G., Huisman T. H., Bouver N. G. World-wide occurrence of nonallelic genes for the -chain of human foetal haemoglobin in newborns. Nat New Biol. 1972 Dec 27;240(104):273–274. doi: 10.1038/newbio240273a0. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B., Wood W. G. A model for the persistence or reactivation of fetal haemoglobin production. Lancet. 1976 Sep 25;2(7987):660–663. doi: 10.1016/s0140-6736(76)92469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Stamatoyannopoulos G., Lim G., Nute P. E. F-cells in the adult: normal values and levels in individuals with hereditary and acquired elevations of Hb F. Blood. 1975 Nov;46(5):671–682. [PubMed] [Google Scholar]
- Wood W. G., Weatherall D. J., Clegg J. B. Interaction of heterocellular hereditary persistence of foetal haemoglobin with beta thalassaemia and sickle cell anaemia. Nature. 1976 Nov 18;264(5583):247–249. doi: 10.1038/264247a0. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Whittaker J. H., Clegg J. B., Weatherall D. J. Haemoglobin synthesis in human bone marrow culture. Biochim Biophys Acta. 1972 Aug 25;277(2):413–420. doi: 10.1016/0005-2787(72)90421-2. [DOI] [PubMed] [Google Scholar]
- Young N. S., Benz E. J., Jr, Kantor J. A., Kretschmer P., Nienhuis A. W. Hemoglobin switching in sheep: only the gamma gene is in the active conformation in fetal liver but all the beta and gamma genes are in the active conformation in bone marrow. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5884–5888. doi: 10.1073/pnas.75.12.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]