Abstract
Water is one of the prime necessities of life. We can hardly live for a few days without water. In a man's body, 70-80% is water. Cell, blood, and bones contain 90%, 75%, and 22% water, respectively. The general survey reveals that the total surface area of earth is 51 crore km2 out of which 36.1 crore km2 is covered sea. In addition to this, we get water from rivers, lakes, tanks, and now on hills. In spite of such abundance, there is a shortage of soft water in the world. Physicochemical parameter of any water body plays a very important role in maintaining the fragile ecosystem that maintains various life forms. Present research paper deals with various water quality parameter, chlorides, dissolved oxygen, total iron, nitrate, water temperature, pH, total phosphorous, fecal coli form bacteria, and adverse effect of these parameters on human being.
Keywords: Dissolved oxygen, drinking waters, fecal coliform bacteria, parameters, pesticides
INTRODUCTION
Drinking water is one of the basic needs of life and essential for survival. Still more than one billion people all over the world do not have ready access to an adequate and safe water supply and more than 800 million of those unsaved live in rural areas. In India, ground water is being used as raw water for 85% public water supply. (According to world health report 1998)water supply varies widely in terms of region and country. In 1970s, of the approximately 2.5 billion people in developing world, only 38% has safe drinking water. At the beginning of the 1980s, water supply coverage was 75% in urban areas and 46% in rural areas. In developing countries, 75% of the population had access to water supply. So they are always prone to loss of their lives or cost a big toll to save themselves from the occurrence of different water-borne disease. Water contamination due to pathogenic agents, chemicals, heavy metals, pesticides water disinfectants, and thereby product as a consequence of industrial and agricultural activities leaching from soil, rocks, and atmospheric deposition and other human activities has become a hazard to human health in several regions of world.
MATERIALS AND METHODS
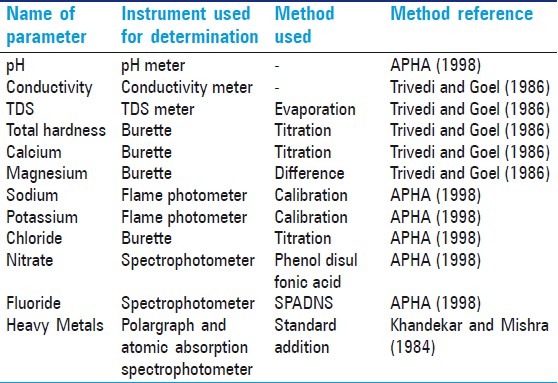
Permissible limits for drinking water quality according to American Public Health Association (APHA), World Health Organization (WHO), Indian Standard Institution (ISI), Central Pollution Control Board (CPCB) and Indian Council of Medical Research (ICMR) are compared in this review article. The following method are used for test water quality.[1]
Dissolved oxygen
For dissolved oxygen “Winkler's method with azide modification” is being followed (APHA, 1998; part 4500 – OC, p. 4-131).
Free carbon dioxide
Free carbon dioxide is being determined by NaOH titration method (APHA, 1998; part 4500-CO2, C, p. 4-31).
Chlorides
For the determination of Chlorides “Argentometric method” is being applied (APHA, 1998; part 4500-Cl B, p. 4-67).
Nitrates
For the estimation of nitrates “Brucine method” is being used (Trivedi and Goel, 1984, p. 59).
Phosphates
For the estimation of phosphates “Stannous chloride method” is being applied (APHA, 1998; part 4500 – P D, p. 4-145).
Silicates
Determination of silicates is being done by “Molybdosilicate method” (APHA, 1998; part 4500-SiO2 C, p. 4-156).
Sulfates
Determination of sulphates is being done by “Turbidimetric method” (APHA, 1998, part 4500 – SO42- E, p. 4-178).
Total alkalinity
Total alkalinity of the sample is being determined by standard titrimetric method.
Phenolphthalein alkalinity and methyl orange alkalinity (APHA, 1998, part 2320 B-CO2, D., p. 2-27).
Total hardness and calcium hardness
These are being determined by “EDTA titrimetric method” (APHA, 1998, part 2340, C, p. 2-36).
Pesticides estimation method
Pesticides estimation has been done with gas liquid chromatography. Gas liquid chromatography is a partition chromatography in which stationary phase is liquid and mobile phase is gas where partition of compound takes place between two phases. The requirement of GLC is that compound or its decomposed stable product should be volatile at the working (column) temperature. The column, oven, and the detector are three important components of gas chromatography. The column is the heart of gas chromatography. It is used to hold the separating media in a fixed position relative to a constant carrier gas flow through media. It is composed of long narrow, metal, or glass tube filled with a packing material consisting of liquid stationary phase coated on a solid support. The liquid phase should have a boiling point more than over the working temperature (200-300°C). The stationary phase is mostly organic silicon.
DISCUSSION
pH
pH is a measure of the acidic or basic (alkaline) nature of a solution. The concentration of the hydrogen ion [H+] activity in a solution determines the pH. Mathematically this is expressed as:
pH = –log [H+]
The pH value is the negative power to which 10 must be raised to equal the hydrogen ion concentration. A pH range of 6.0 to 9.0 appears to provide protection for the life of fresh water fish and bottom dwelling invertebrates. The Table 1 gives some special effects of pH on fish and aquatic life.
Table 1.
The methods of analysis of different parameters of water quality
Temperature
Water temperature regulates the metabolism of the aquatic ecosystem. High water temperature stress aquatic ecosystem by reducing the ability of water to hold essential dissolved gases like oxygen often summer head can cause fish kills in water bodies because high temperature reduce available oxygen in the water.
Hardness
It is defined as the sum of calcium and magnesium concentrations and is a measure of the capacity of water to precipitate soap
Alkalinity
Alkalinity is primarily due to carbonate, bicarbonate and hydroxide contents. It is used in the interpretations and control of water and waste water processes.
Dissolved Oxygen
Dissolved oxygen analysis measures the amount of gaseous oxygen (O2) dissolved in an aqueous solution. Oxygen gets into water by diffusion from the surrounding air, by aeration (rapid movement) and as a waste product of photosynthesis. Environmental impact of total dissolved solids gas concentration in water should not exceed 110% (above 13-14 mg/l). Concentration above this level can be harmful to aquatic life. Fish in waters containing excessive dissolved gases may suffer from “gas bubble disease”; however, this is a very rare occurrence. The bubbles or emboli block the flow of blood through blood vessels causing death. External bubbles emphysema can also occur and be seen on fins, on skin and on other tissue. Aquatic invertebrate are also affected by “gas bubble disease,” but at levels higher than those lethal to fish. Adequate dissolved oxygen is necessary for good water quality
Nitrate and Nitrite
Nitrate and Nitrite are naturally occurring ions that are part of nitrogen cycle. In general, vegetables are the main source of nitrate intake when level in drinking water is below 10 mg/l. When nitrate level in drinking water exceeds 50 mg/l, drinking water becomes the main source of total nitrate intake. The presence of nitrate indicates an old contamination provided nitrites are absent.
High level of nitrate in drinking water due to excessive use of agriculture fertilizers, decayed vegetable water, domestic effluent, sewage disposal industrial discharges, leachable from refuse dumps, atmospheric and atmospheric precipitation has become a serious problem (Makhijani and Manoharan 1999)[2]
Excess concentration of nitrate causes disease. Methemoglobinemia oxygen transport depends on the maintenance of intra cellular hemoglobin in the reduced (Fe2+) state. When hemoglobin is oxidized to methemoglobin, the heme iron becomes (Fe3+) and is incapable of binding oxygen. Methemoglobinemia is suspected in any cyanotic patient with no evidence of heart and lung disease of cyanosis is due to decreased oxygen saturation.
Nitrites can produce a serious condition in fish called “brown blood disease” Nitrites also react directly with hemoglobin in human blood and other warm-blooded animals to produce methemoglobin. Methemoglobin destroys the ability of red blood cell to transport oxygen. This condition is especially serious in babies under three months of age. It causes a condition known as methemoglobinemia or “blue baby disease”. Water with nitrite levels exceeding 1.0 mg/l should not be used for feeding babies Nitrite/nitrogen levels below 90 mg/l and nitrate levels below 0.5 mg/l seem to have no effect on warm water fish.
Chlorides
Chlorides are the inorganic compound resulting from the combination of the chlorine gas with metal. Some common chlorides include sodium chloride (NaCl) and magnesium chloride (MgCl2). Chlorine alone as (Cl2) highly toxic, and it is often used a disinfectant. In combination with a metal such as sodium, it becomes essential for life. Small amounts of chlorides are required for normal cell functions in plant and animal life.
Environmental impact of chloridesare not usually harmful to human health; however, the sodium part of the table salt has been linked to heart and kidney diseases. Sodium chloride may impact a salty taste at 250 mg/l; however, calcium or magnesium chloride is usually detected by taste until levels of 1000 mg/l are reached. Public drinking water standards require chloride level not to exceed 250 mg/l. Chlorides may get into surface water from several sources including: rocks contain chlorides, agricultural run-off, waste water from industries, oil well wastes, and effluent waste water from waste water treatment plants. Chlorides can corrode metals and affect the taste of food products. Chlorides can contaminate fresh water streams and lakes. Fish and aquatic communities cannot survive in high level of chlorides. Therefore, water that is used in industry or proceeds for any use has a recommended maximum chloride level.
Fluoride[3]
According to WHO 1984 and Indian standard drinking water specification 1991 the maximum permissible limit of fluoride in drinking water is 1.5 ppm and highest desirable limit is 1.0 ppm. Fluoride concentrations above 1.5 ppm in drinking water cause dental fluorosis and much higher concentration skeletal fluorosis. Low concentration (approximately 0.5 ppm) provides protection against dental caries. India is among the 23 nations around the globe where health problems occur due to the consumption of fluoride contamination water and the extent of fluoride contamination in water varies from 1.0 to 400 mg/l. In India, 20 million people are severely affected by fluorosis and 40 million people are exposed to risk of endemic fluorosis (Chinoy J. N. 1991). In India fluoride endemic states one Andhra Pradesh, Karnataka, Tamil Nadu, Punjab, Haryana, Maharashtra, Gujarat, Rajasthan, Uttar Pradesh, Kerala, Jammu and Kashmir, and Delhi.
Arsenic
Arsenic contamination in drinking water has been reported in different region of the world mainly in china (WHO1996). In India, it had been found to be wide spread in different region of the West Bengal due to dissolution of arsenic containing bed rocks. WHO has prescribed a provisional guideline value of As 10 μg/l in drinking water and according to India standard drinking water specification 1991, the highest desirable limit is 50 μg/l and no relaxation for maximum permissible level. Early clinical symptoms of acute intoxication include abdominal pain, vomiting, diarrhea, muscular pain, and with flushing of the skin. These symptoms are often followed by numbers and tingling of the extremities, muscular cramping and the appearance of a popular erythematous rash.
Chronic exposure due to arsenic contaminated drinking water includes dermal lesions, peripheral neuropathy, skin cancer, and peripheral vascular disease. Major dermatological signs are nelano-keratosis, melanosis, spotted and diffuse keratosis, leucomelanosis, and dorsal keratosis (Saha et al 1999).[4]
Lead[5]
From a drinking water perspective, the almost universal use of lead compounds in plumbing fittings and as solder in water distribution systems is important.[5] Lead pipes may be used in older distribution systems and plumbing. Lead is present in tap water to some extent as a result of its dissolution from natural sources but primarily from household plumbing systems in which the pipes, solder, fittings, or service connections to homes contain lead. PVC pipes also contain lead compounds that can be leached from them and result in high lead concentration in drinking water. According to India standard drinking water specification 1991, highest desirable limit of lead in drinking water is 0.05 ppm and no relaxation for maximum permissible limit. Provisional tolerable weekly intake of 25 μg/l lead per kg body wt or 93.5 μg/kg body wt/day for all age group was established (WHO 1993). Lead is a cumulative general poison and associated with several health hazards like anemia (Moore. 1988),[5] reproductive effects (Wildt et al. 1983)[6] (Cullen et al. 1984).[7]
Phosphorus
Phosphorus is one of the key elements necessary for growth of plants and animals. Phosphorus in elemental form is very toxic and is subject to bioaccumulation. Phosphate PO43- formed from this element. Phosphate exists in three forms: Orthophosphate, met-pho sulfate, and organically bound phosphate. Each compound contains phosphorus in a different chemical formula orthoform are produced by natural processes and are found in sewage. Poly forms are used for treating boiler water and in detergents in water they change into the ortho form organic phosphates are important in nature. Their occurrence may result from the breakdown of organic pesticides which contain phosphates. They may exit in solution, as particles, loose fragments, or in the bodies of aquatic organisms.
Rainfall can cause varying amounts of phosphates to wash from farm soils into nearby waterways. Phosphate will stimulate the growth of plankton and aquatic plants which provide food for fish this increased growth may cause an increase in the fish population and improve the overall water quality. However, if an excess of phosphate enters the water way, algal and aquatic plants will grow wildly, choke up the water way, and use up large amounts of oxygen. This condition is known as Eutrophication or over-fertilization of receiving waters. The rapid growth of aquatic vegetation can cause the death and decay of vegetation- and quality life because of the decrease in dissolved oxygen levels. Phosphates are not toxic to people or animals unless they are present in very high levels. Digestive problem could occur from extremely high level of phosphate.
Iron[8]
Iron is the most abundant element, by weight, in the earth's crust. Iron is the second most abundant metal in earth's crust. It is an essential element in human nutrition. The minimum daily requirement of iron is ranged from about 10 to 50 mg/day (FAO/WHO 1988)
Natural water contains variable amounts of iron despite its universal distribution and abundance. Iron in ground water is normally present in the ferrous or bivalent from (Fe++) or insoluble Iron urban exposure to air. Iron is a race elements required by both plants and animal. It is a vital oxygen transport mechanism in the blood all vertebrate and some invertebrate animals.
Iron in water may be present in varying qualities depending upon the geological area and other chemical component of the water way. Ferrous Fe++ and ferric F+++ irons are primary forms of concern in the aquatic environment other forms may be in either organic or inorganic waste water streams. The ferrous form Fe2+ can persist in water void of dissolved oxygen and usually originates from ground water or ---- that are pumped or drained. Iron in domestic water supply system, stains laundry and porcelain. It appears to be more of a nuisance than a potential health hazard. Taste thresholds of iron in water 0.1 mg/l for ferrous iron and 0.2 mg/l ferric Iron, giving a bitter or an astringent taste. Water used in industrial processes usually contain less than 0.2 mg/l iron. Black or Brown swaps water may contain iron concentration of several mg/l in the presence or the absence of dissolved oxygen, but this iron form has little effect on aquatic life. The current aquatic life standard is 1.0 mg/l based on toxic effects.
Microbiological Parameter
Fecal coliform bacteria
Fecal coliform bacteria are a collection of relatively harmless microorganisms that live in large number in the intestines of the warm and cold blooded animals. They aid in the digestion of food. A specific subgroups of this collection is the fecal coliform bacteria, the most common member being Escherichia coli. These organisms may be repeated from the total coliform group by their ability to grow at elevated temperatures and are associated only with the fecal material of warm blooded animals.
The presence of fecalcoliform bacteria in aquatic environmental indicate that the water has been contaminated with the fecal material of man or other animals. At the time this occurred, the source water may have been contaminated by pathogens or disease-producing bacteria or viruses which can also exist in fecal material. Some water-borne pathogenic diseases include typhoid fever, viral, and bacterial gastroenteritis and hepatitis A. The presence of fecal contamination is an indicator that a potential health risk exists for individuals exposed to this water.
Pesticides
Most commonly used Pesticides which have been detected in drinking water, ground water well water and surface water in many countries are alchlor (Ritter 1990), aldicarb (Heibseh 1988)[9,10] Carbofuran (Ritter 1990).[11]
CONCLUSION
It was found that all parameters of permissible limit of drinking water were not set for all by different agencies i.e. (APHA), (WHO), (ISI), (CPCB), and (ICMR). Public health officers, doctors, and researchers follow these norms. However, there is a bias in permissible limit of drinking water quality set by different agencies (APHA, 1998; part 4500 – OC, p. 4-131). Permissible limits for drinking water parameters such as pH, temperature, hardness, alkalinity, dissolved oxygen, nitrate and nitrite, chlorides, fluoride, arsenic, lead, cadmium, mercury, chromium, phosphorus, iron and microbiological parameter like of fecal coliform bacteria are compared for different agencies in the Tables 2 and 3.[12] The permissible limits of different parameter set by different agencies do not show uniformity. pH (mg/l): 6.85-9.2 show uniformity set by different agencies, Turbidity (NTU): given by ISI 10NTU, ICMR 25NTU, CPCB 10 NTU, conductivity 200 mg/l and alkalinity 600 mg/l is given only central pollution controlboard not set up by USEPA, WHO, ISI, and ICMR,[13] Total hardness and Iron (mg/l): WHO 500, 0.1, ISI 300, 0.1, ICMR 600,1.0, CPCB 600, 1.0 but not set up by USEPA, there is a great difference in chlorides contents set by different agencies Chlorides (mg/l): USEPA250WHO200ISI250 ICMR 1000 CPCB 1000 nitrates and sulfates are much important parameters which show the status of water bodies nitrates show the trophic status and pollution in water quality. Nitrates and sulfates are not set by USEPA and WHO. Residual free chlorine (mg/l) 0.02 set up only by ISI, Calcium and magnesium (mg/l) WHO 75, 50 ISI 75, 30 ICMR 200 CPCB 200,100. Calcium and magnesium are significant to detect hardness of water, Copper (mg/l): USEPA 1.3, WHO 1.0, ISI0.05, ICMR 1.5, CPCB 1.5, Fluorides (mg /l): USEPA 4.0, WHO 1.5, ISI 0.6-1.2, ICMR 1.5, CPCB 1.5, Mercury mg/l:USEPA 0.001, WHO 0.002,ISI 0.001, ICMR 0.001, CPCB no relaxation,Mercury shows similarity with different agencies but no relaxation given by CPCB, Cadmium (mg/l):USEPA 0.005, WHO 0.005, ISI 0.01, ICMR 0.01, CPCB no relaxation, Selenium (mg/l):USEPA 0.05, WHO 0.01, CPCB no relaxation Arsenic (mg/l) USEPA 0.05, WHO 0.05,ISI 0.05, ICMR0.05, CPCB no relaxation Lead (mg/l): USEPA WHO 0.05 ISI 0.10 ICMR 0.05 CPCB no relaxation Zinc (mg/l): WHO 5.0,ISI 5.0, ICMR 0.10, CPCB 15.0, Chromium (mg/l): USEPA 0.1, ISI 0.5 CPCB no relaxation, E. coli (MPN/100 ml) CPCB no relaxation. E. coli shows water quality and pollution status but this permissible limit not given by different agencies. There is great confusion to the researcher, public health officers, and doctors to following the permissible limits when they do research. The WHO and MCI should fix the uniformity permissible which are helpful to the society.
Table 2.
Permissible limits of drinking water quality
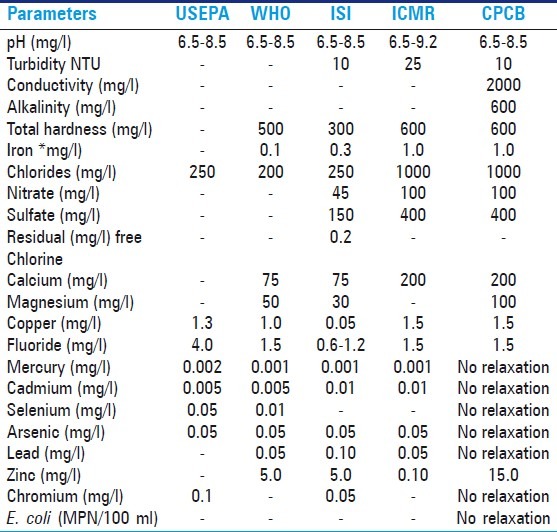
Table 3.
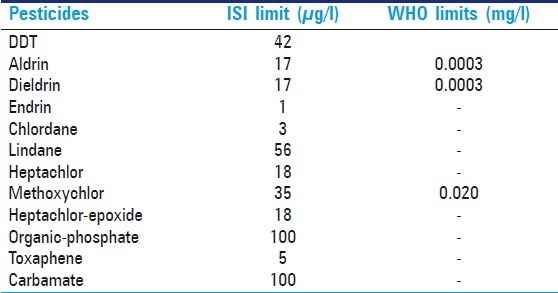
Permissible limits of pesticides in drinking water
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.20th ed. New York: American Public Health Association; 1998. APHA. Standard methods for the examination of water and waste\water. [Google Scholar]
- 2.Makhijani SD, Manoharan A. Nitrate pollution problem in drinking water sources: Monitoring and surveillance. Paper presented in the workshop water quality field test kits for Arsenic, Fluoride and Nitrate held from 8-9 Sept. 1999 at ITRC, Lucknow [Google Scholar]
- 3.Chinoy JN. Effects of fluoride on physiology of animals and human beings. Indian J Environ Toxico. 1991;1:17–32. [Google Scholar]
- 4.Saha KC, Dikshit AK, Bandyopadhyay MA. A review of arsenic poisoning and its effect onhuman health. CritRev Environ Sci Technol. 1999;29:281–313. [Google Scholar]
- 5.Moore MR. Haematological effects of lead. Sci Total Environ. 1988;71:419–31. doi: 10.1016/0048-9697(88)90214-8. [DOI] [PubMed] [Google Scholar]
- 6.Wildt K, Eliasson R, Borlin M. Effects of occupational exposure to lead on sperm and semen. In: Clarbson TW, Nordberg GF, Sager PR, editors. Reproductive and developmental toxicity of metals. Proceeding of joint meeting in Rochester. New York: Plenum press; 1983. pp. 279–300. [Google Scholar]
- 7.Cullen MR, Kayne RD, Robins JM. Endocrine andreproductive dysfunction in men a social with occupation inorganic lead intoxication. Arch Environ Health. 1984;39:431–40. doi: 10.1080/00039896.1984.10545877. [DOI] [PubMed] [Google Scholar]
- 8.FAO/WHO. Requirement of Vitamin A, Iron, Folate and Vitamin B12. Report of a joint FAO/WHO expend consultation Rome, Food and Agricultural organization of the united nations (FAO Food and Nutrition series No. 23) 1988 [Google Scholar]
- 9.Hiebsch S. The occurrence of thirty five pesticides in Canadian drinking water and surface water. Ottawa, Canada: Department of National Healthand Welfare, Evironmental Health Directorate; 1988. [Google Scholar]
- 10.Khandekar RN, Mishra UC. Environmental lead exposure of an urban Indian population. Sci Total Environ. 1984 Dec;40:269–78. doi: 10.1016/0048-9697(84)90356-5. [DOI] [PubMed] [Google Scholar]
- 11.Ritter WF. Pesticide contamination of ground water in the United States – A review. Environ Sci Health. 1990;25:1–29. doi: 10.1080/03601239009372674. [DOI] [PubMed] [Google Scholar]
- 12.Trivedi RK, Goel PK. Chemical and Biological Methods for Water Pollution Studies. Karad: Environmental Publications; 1986. [Google Scholar]
- 13.Evaluation of certain food additives and contaminants: Forty first report of joint FAO/WHO. Expert committee on food additives. Geneva: world health organization; 1993. WHO. WHO technical report series No.837. [PubMed] [Google Scholar]


