Abstract
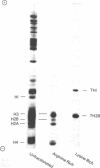
The distribution and synthesis of the testis-specific variants of histones H1 and H2B, TH1 and TH2B, respectively, and of the somatic histones were studied in rat testis cells. Rat testis cells were labeled in vivo with intratesticular injections of [3H]lysine. The cells and nuclei were then separated into different developmental classes by velocity sedimentation and the histones were analyzed. TH1 and TH2B, as well as the somatic histones, were present in spermatocytes and round spermatids, but none of them were detectable in elongated spermatids. The synthesis and nuclear accumulation of TH1 and TH2B took place throughout pachytene, as well as in earlier stages, but not in the round spermatids. In addition, there was synthesis during pachytene of a histone that migrates electrophoretically with H2A. However, somatic histone synthesis, with the possible exception of H2A and H2B, was not detectable at the pachytene stage. In vivo treatment of rats with hydroxyurea reduced DNA synthesis in the testis to 1% of control values and significantly reduced the synthesis of H3, H2B, H2A, and H4, with the greatest effect being on H3 and H4. However, the hydroxyurea treatment did not significantly decrease the synthesis of TH1, H1, or TH2B. These results prove that the synthesis of several histones during the meiotic prophase is not dependent upon concurrent S-phase DNA synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., Gross P. R. Noncoincidence of histone and DNA synthesis in cleavage cycles of early development. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5016–5020. doi: 10.1073/pnas.74.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson R. E., Grimes S. R., Jr, Yonuschot G., Irvin J. L. The histones of rat testis. Arch Biochem Biophys. 1975 Jun;168(2):403–412. doi: 10.1016/0003-9861(75)90269-6. [DOI] [PubMed] [Google Scholar]
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- Grabske R. J., Lake S., Gledhill B. L., Meistrich M. L. Centrifugal elutriation: separation of spermatogenic cells on the basis of sedimentation velocity. J Cell Physiol. 1975 Aug;86(1):177–189. doi: 10.1002/jcp.1040860119. [DOI] [PubMed] [Google Scholar]
- Grimes S. R., Jr, Meistrich M. L., Platz R. D., Hnilica L. S. Nuclear protein transitions in rat testis spermatids. Exp Cell Res. 1977 Nov;110(1):31–39. doi: 10.1016/0014-4827(77)90266-x. [DOI] [PubMed] [Google Scholar]
- Grimes S. R., Jr, Platz R. D., Meistrich M. L., Hnilica L. S. Partial characterization of a new basic nuclear protein from rat testis elongated spermatids. Biochem Biophys Res Commun. 1975 Nov 3;67(1):182–189. doi: 10.1016/0006-291x(75)90300-9. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Tres L. L. Structural and transcriptional features of the mouse spermatid genome. J Cell Biol. 1975 May;65(2):258–270. doi: 10.1083/jcb.65.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Geroch M. E. An unusual pattern of lysine rich histone components is associated with spermatogenesis in rat testis. Biochem Biophys Res Commun. 1975 Mar 17;63(2):378–384. doi: 10.1016/0006-291x(75)90699-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meistrich M. L., Bruce W. R., Clermont Y. Cellular composition of fractions of mouse testis cells following velocity sedimentation separation. Exp Eye Res. 1973 Apr;79(1):213–227. [PubMed] [Google Scholar]
- Meistrich M. L., Eng V. W. Separation of nuclei of mouse testis cells by sedimentation velocity. Exp Cell Res. 1972 Jan;70(1):237–242. doi: 10.1016/0014-4827(72)90205-4. [DOI] [PubMed] [Google Scholar]
- Meistrich M. L., Reid B. O., Barcellona W. J. Meiotic DNA synthesis during mouse spermatogenesis. J Cell Biol. 1975 Jan;64(1):211–222. doi: 10.1083/jcb.64.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meistrich M. L. Separation of spermatogenic cells and nuclei from rodent testes. Methods Cell Biol. 1977;15:15–54. doi: 10.1016/s0091-679x(08)60207-1. [DOI] [PubMed] [Google Scholar]
- Meistrich M. L., Trostle P. K. Separation of mouse testis cells by equilibrium density centrifugation in renografin gradients. Exp Cell Res. 1975 Apr;92(1):231–244. doi: 10.1016/0014-4827(75)90656-4. [DOI] [PubMed] [Google Scholar]
- Oliver D., Sommer K. R., Panyim S., Spiker S., Chalkley R. A modified procedure for fractionating histones. Biochem J. 1972 Sep;129(2):349–353. doi: 10.1042/bj1290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Platz R. D., Grimes S. R., Meistrich M. L., Hnilica L. S. Changes in nuclear proteins of rat testis cells separated by velocity sedimentation. J Biol Chem. 1975 Aug 10;250(15):5791–5800. [PubMed] [Google Scholar]
- Platz R. D., Meistrich M. L., Grimes S. R., Jr Low-molecular-weight basic proteins in spermatids. Methods Cell Biol. 1977;16:297–316. doi: 10.1016/s0091-679x(08)60107-7. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shires A., Carpenter M. P., Chalkley R. A cysteine-containing H2B-like histone found in mature mammalian testis. J Biol Chem. 1976 Jul 10;251(13):4155–4158. [PubMed] [Google Scholar]
- Shires A., Carpenter M. P., Chalkley R. New histones found in mature mammalian testes. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2714–2718. doi: 10.1073/pnas.72.7.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari A. J., Moses M. J. The structure of the central region in the synaptonemal complexes of hamster and cricket spermatocytes. J Cell Biol. 1973 Jan;56(1):145–152. doi: 10.1083/jcb.56.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström K. O., Parvinen M. DNA synthesis during male meiotic prophase in the rat. Hereditas. 1976;82(1):25–28. doi: 10.1111/j.1601-5223.1976.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Takai S., Borun T. W., Muchmore J., Lieberman I. Concurrent synthesis of histone and deoxyribonucleic acid in liver after partial hepatectomy. Nature. 1968 Aug 24;219(5156):860–861. doi: 10.1038/219860a0. [DOI] [PubMed] [Google Scholar]