Nanostructure-Based Drug Delivery Systems for Brain Targeting.
Haque S, Md S, Alam MI, Sahni JK, Ali J, Sanjula Baboota S. Drug Dev Ind Pharm 2012;38:387–411
CONTEXT: It is a well-known fact that the blood brain barrier (BBB) hinders the penetrance and access of many pharmaco-therapeutic agents to the central nervous system (CNS). Many diseases of the CNS remain undertreated and the inability to treat most CNS disorders is not due to the lack of effective CNS drug discovery, rather, it is due to ineffective CNS delivery. Therefore, a number of nanostructured drug delivery carriers have been developed and explored over the past couple of years to transport the drugs to brain. OBJECTIVE: The present review will give comprehensive details of extensive research being done in the field of nanostructured carriers to transport the drugs through the BBB in a safe and effective manner. METHODS: The method includes both the polymeric- and lipid-based nanocarriers with emphasis on their utility, methodology, advantages, and the drugs which have been worked on using a particular approach to provide a noninvasive method to improve the drug transport through BBB. RESULTS: Polymeric- and lipid-based nanocarriers enter brain capillaries before reaching the surface of the brain microvascular endothelial cells without the disruption of BBB. These systems are further modified with specific ligands vectors and pegylation aiming to target and enhance their binding with surface receptors of the specific tissues inside brain and increase long circulatory time which favors interaction and penetration into brain endothelial cells. CONCLUSION: This review would give an insight to the researchers working on neurodegenerative and non-neurodegenerative diseases of the CNS including brain tumor.
Commentary
Medically refractory epilepsy is estimated to occur in about 500,000 individuals of the nearly 3 million in the United States with epilepsy. Medication resistance in epilepsy is due, in part, to tolerance related to molecular mechanisms that eliminate, or minimize the persistence of antiepileptic drug (AED) molecules in the brain. Currently, drug discovery for CNS disorders is almost exclusively limited to small molecular weight lipophilic molecules that can cross the blood brain barrier (BBB), and blood-cerebral spinal fluid barrier (BCSFB). This simple prerequisite eliminates a majority of potential AED molecule candidates early in the drug discovery process. The recent review by Haque et al. offers a comprehensive up-to-date understanding of the strategies that facilitate drug efficacy by focusing on creative drug delivery mechanisms rather than drug discovery. This article reviews the challenges of overcoming the array of active and passive transporter systems at the BBB and BCSFB that shuttle drug molecules toward and away from neural tissue. The authors compare drug molecule transport across the BBB and neural cell membranes in a number of disorder-based platforms including epilepsy. These transporters play the role of “gatekeepers” limiting access of molecules to the brain. Specifically, several transporter superfamilies mediate access of therapeutic drug molecules to the brain. The adenosine triphosphate binding cassette (ABC), and solute carrier (SLC) transporters comprise the two major known drug superfamily transporters in the brain. P-glycoprotein is the most extensively studied BBB transporter of the ABC family. P-glycoprotein in animal models can play a role in eliminating antiepileptic drug molecules, such as phenytoin, phenobarbital (1), and levetiracetam. However, the extent to which this transporter is involved in drug tolerance in humans remains unclear. The SLC transporter superfamily includes the organic anion transporters localized on neurons themselves. This system probably plays a significant role in eliminating valproic acid. Also noteworthy, is an associated monocarboxylate transporter, associated with CNS uptake of medium-chain fatty acids that play a dominant role in transporting valproic acid into the brain. The system L-transporters bidirectionally transport gabapentin and pregabalin. In addition, the multi-drug resistance-associated proteins (MRP) found at the BBB and BCSFB can restrict brain entry of phenytoin (2).
Manipulating these transporter gatekeepers can potentially increase the entry and persistence of drug molecules in the brain. Haque et al. focus on reviewing the growing field of nanotechnology utilized as innovative tools to deliver drugs across the BBB, and through the brain parenchyma. They do not extensively discuss drug molecule modifications currently used to minimize peripheral AED side effects. These latter approaches for the treatment of epilepsy include prodrugs (3) and efflux pump inhibition (6) (see Table). Utilization of nanocarriers can potentially bypass molecule transporters, increasing cell specificity, while improving diffusion of even hydrophilic molecules across the BBB and BCSFB. Drug molecules and other biologically active molecules can be potentially dissolved, entrapped, or encapsulated in these nanocarriers. Such nanoscale carriers can be used as biocompatible shuttles to ferry therapeutic molecules to their central targets. Candidate nanoparticles are similar in size to large biological molecules such as enzymes and cell membrane receptors. Because of their subcellular size, these nanosized drug carriers can interact with biomolecules on both cell surfaces and inside of them. Nanoparticles smaller than 50 nm can easily cross the BBB and enter neural cells themselves. The small size of the particles can dramatically increase the “surface area to volume” ratio. This means relatively large amounts of therapeutic agent can be associated with the nanocarriers facilitating shuttling drug to their target. Such an approach can minimize peripheral side effects by lowering systemic drug concentrations.
TABLE.
Epilepsy-Related Drug-Molecule Carrier Systems and Delivery Strategies
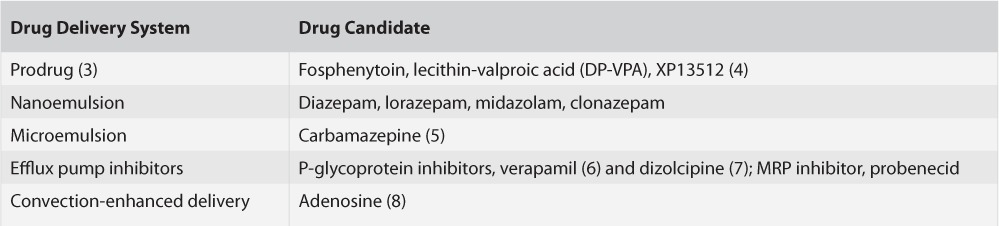
Moreover, these nanocarrier systems have been shown to protect drug molecules from in vivo degradation, while reducing neural toxicity resulting from lower effective concentrations in the brain. Drug-delivery systems include nanoemulsions for intranasal drug delivery, including a number of benzodiazepines, and intravenous administration of AED-microemulsions, including carbamazepine, may emerge in the near future as a rapid delivery option for efficiently targeting epileptic networks (see Table).
Haque et al. have comprehensively outlined the extent to which nanostructure-based drug delivery systems are already available for disorders outside of epilepsy, most notably cancer. The novel delivery mechanisms already in practice for the treatment of cancers highlight the underutilization of these treatment approaches for epilepsy. It is promising that first-generation drug delivery approaches are at various levels of development and deployment for the treatment of epilepsy. However, it is important to aggressively pursue next-generation drug delivery systems. Such novel mechanisms will facilitate access to potentially more effective molecules aimed at stabilizing so-called medication-resistant epilepsy.
Footnotes
Editor's Note: Authors have a Conflict of Interest disclosure which is posted under the Supplemental Materials (204.8KB, docx) link.
References
- 1.Volk HA, Loescher W. Multidrug resistance in epilepsy: Rats with drug-resistant seizures exhibit enhanced brain expression of P-glycoprotein compared with rats with drug responsive seizures. Brain. 2005;128:1358–1368. doi: 10.1093/brain/awh437. [DOI] [PubMed] [Google Scholar]
- 2.Potschka H, Fedrowitz M, Loscher W. Multidrug resistance protein MRP2 contributes to blood-brain barrier function and restricts anti-epileptic drug activity. J Pharmacol Exp Ther. 2003;306:124–131. doi: 10.1124/jpet.103.049858. [DOI] [PubMed] [Google Scholar]
- 3.Bennewitz MF, Saltzman WM. Nanotechnology for the delivery of drugs to the brain for epilepsy. Neurotherapeutics. 2009;6:323–336. doi: 10.1016/j.nurt.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rautio J, Kumpulainen H, Heimback T. Prodrugs: Design and clinical applications. Nat Rev Drug Discov. 2008;7:225–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 5.Madhusudhan B, Rambhau D, Apte SS, Gopinath D. 1-O-alkylglycerol stabilized carbamazepine intravenous o/w nanoemulsions for drug targeting in mice. J Drug Target. 2007;15:154–161. doi: 10.1080/10611860601141150. [DOI] [PubMed] [Google Scholar]
- 6.Iannetti P, Spalice A, Parisi P. Calcium-channel blocker verapamil administration in prolonged and refractory status epilepticus. Epilepsia. 2005;46:967–969. doi: 10.1111/j.1528-1167.2005.59204.x. [DOI] [PubMed] [Google Scholar]
- 7.Bankstahl JP, Hoffman K, Bethman K, Loscher W. Glutamate is critically involved in seizure-induced overexpression of P-glycoprotein in the brain. Neuropharmacology. 2008;54:1006–1016. doi: 10.1016/j.neuropharm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Yildirim M, Marangoz C. Anticonvulsant effects of focal and intracere-broventricular adenosine on penicillin-induced epileptiform activity in rats. Brain Res. 2007;1127:193–200. doi: 10.1016/j.brainres.2006.10.024. [DOI] [PubMed] [Google Scholar]


