Abstract
Endothelial nitric oxide synthase (eNOS) deficiency may contribute to the pathogenesis of diabetic nephropathy in both experimental models and humans, but the underlying mechanism is not fully understood. Here, we studied two common sequelae of endothelial dysfunction in diabetes: glomerular capillary growth and effects on neighboring podocytes. Streptozotocin-induced diabetes increased glomerular capillary volume in both C57BL/6 and eNOS−/− mice. Inhibiting the vascular endothelial growth factor receptor attenuated albuminuria in diabetic C57BL/6 mice but not in diabetic eNOS−/− mice, even though it inhibited glomerular capillary enlargement in both. In eNOS−/− mice, an acute podocytopathy and heavy albuminuria occurred as early as 2 weeks after inducing diabetes, but treatment with either captopril or losartan prevented these effects. In vitro, serum derived from diabetic eNOS−/− mice augmented actin filament rearrangement in cultured podocytes. Furthermore, conditioned medium derived from eNOS−/− glomerular endothelial cells exposed to both high glucose and angiotensin II activated podocyte RhoA. Taken together, these results suggest that the combined effects of eNOS deficiency and hyperglycemia contribute to podocyte injury, highlighting the importance of communication between endothelial cells and podocytes in diabetes. Identifying mediators of this communication may lead to the future development of therapies targeting endothelial dysfunction in albuminuric individuals with diabetes.
One of the earliest features of kidney disease in diabetes is an alteration in the size- and/or charge-selective properties of the glomerular filtration barrier, a trilaminar structure that normally prevents the free passage of macromolecules into the urinary space. Manifested clinically as albuminuria, this disruption in the filtration barrier may result from structural or molecular changes in the fenestrated glomerular endothelial cells, podocytes, and/or the interpositioned glomerular basement membrane (GBM). High glucose-mediated molecular events1 and flow-mediated stress forces induce endothelial dysfunction in diabetes2 and diabetic nephropathy in particular.3 However, the mechanism by which such injury may translate to loss of glomerular permselectivity remains incompletely understood.
Endothelial dysfunction in diabetic nephropathy is perhaps most readily observed as “neoangiogenesis” of the glomerular capillaries.4,5 A number of experimental studies exploiting antiangiogenic therapies would appear, at first glance, to endorse a role for abnormal angiogenesis in the development of diabetic nephropathy.6 The proangiogenic growth factor, vascular endothelial growth factor (VEGF), for example, is abundantly expressed by podocytes within the diabetic glomerulus7 and strategies to block the activity of VEGF have been reported to attenuate albuminuria,8–10 despite evidence to the contrary in the nondiabetic setting.11,12 Podocytes act as the final barrier to macromolecular flow and, over recent years, injury to this specialized epithelial cell type has been acknowledged as playing a pivotal role in the pathogenesis of albuminuria in diabetes. Bidirectional crosstalk between glomerular endothelial cells and neighboring podocytes, accordingly, represents an alternative route via which endothelial injury may translate to urinary leakage of albumin.13,14
One of the major obstacles to the study of pathogenetic mechanisms in diabetic nephropathy has been the lack of an animal model that develops disease analogous to that seen in humans.15 However, augmented renal injury has recently been described in diabetic mice genetically deficient in endothelial nitric oxide synthase (eNOS), the major NOS isoform responsible for NO generation within the micro- and macrovasculature.16–20 In this study, we sought to establish the role that eNOS plays in glomerular capillary growth in diabetes and in the paradoxical response to anti-VEGF therapy, as well as the effects of eNOS deficiency on communication with neighboring podocytes and its response to “standard of care” with renin-angiotensin-aldosterone system (RAAS) blockade.
Results
Glomerular Capillary Volume Is Increased in Diabetic Wild-Type and eNOS−/− Mice and Is Reduced with VEGF Receptor Inhibition
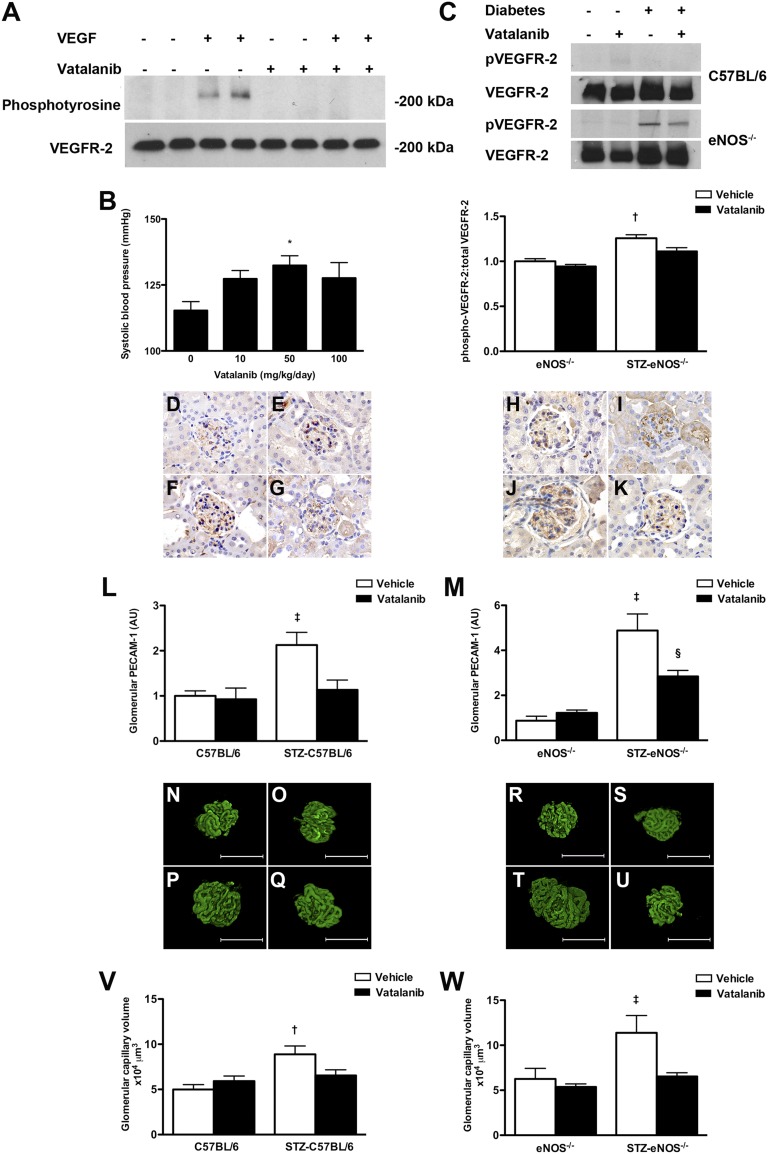
To evaluate the role of eNOS in glomerular capillary growth in diabetes, we administered a small molecule VEGF receptor (VEGFR) tyrosine kinase inhibitor, vatalanib (IC50 VEGFR-2 37 nM, VEGFR-1 77 nM, PDGFR-β 580 nM, c-kit 730 nM)21,22 to low-dose streptozotocin (STZ)-diabetic eNOS−/− mice. The efficacy of vatalanib in attenuating VEGF signaling was evaluated in three separate experiments. Treatment of cultured human glomerular endothelial cells12 with vatalanib prevented VEGF-induced VEGFR-2 phosphorylation (Figure 1A). To identify the optimal dose for in vivo studies, C57BL/6 mice were treated with varying doses of vatalanib for 1 week (n=5 per group) with the class effect rise in systolic BP (SBP)23 used as a bioassay of VEGF blockade (Figure 1B). These studies identified that the maximum rise in SBP occurred with vatalanib 50 mg/kg per day, which was therefore administered to control and STZ-diabetic C57BL/6 and eNOS−/− mice for 12 weeks. At the end of the study period, the effect of vatalanib in attenuating renal VEGF signaling was determined by immunoblotting (Figure 1C). These experiments revealed that basal renal VEGFR-2 phosphorylation was low in wild-type mice, although increased in eNOS−/− mice especially in the setting of diabetes. Treatment of STZ-eNOS−/− mice with vatalanib 50 mg/kg per day significantly reduced renal VEGFR-2 phosphorylation.
Figure 1.
VEGFR inhibition in control and diabetic wildtype and eNOS−/− mice. (A) Effect of vatalanib in cultured human renal glomerular endothelial cells. Cells were preincubated with either vatalanib (1 µM) or DMSO for 2 hours before stimulation with 20 ng/ml VEGF for 5 minutes. Cell lysates were immunoprecipitated for VEGFR-2 and immunoblotted for either phosphotyrosine or VEGFR-2. (B) SBP in C57BL/6 mice treated with vehicle or vatalanib (10 mg/kg, 50 mg/kg, 100 mg/kg daily) for 7 days. (C) pVEGFR-2 and total VEGFR-2 in kidney homogenates from control and STZ-diabetic wild-type (C57BL/6) and eNOS−/− mice treated with vehicle or vatalanib (50 mg/kg) for 12 weeks (n=3/group). (D–K) Glomerular PECAM-1 staining in mice treated with vehicle or vatalanib (50 mg/kg) for 12 weeks: (D) C57BL6 + vehicle, (E) C57BL/6 + vatalanib, (F) STZ-C57BL/6 + vehicle, (G) STZ-C57BL/6 + vatalanib, (H) eNOS−/− + vehicle, (I) eNOS−/− + vatalanib, (J) STZ-eNOS−/− + vehicle, and (K) STZ-eNOS−/− + vatalanib. Quantitation of glomerular PECAM-1 immunostaining in (L) C57BL/6 mice and (M) eNOS−/− mice. (N–U) Glomerular capillary volume determined by FMA in mice treated with vehicle or vatalanib (50 mg/kg) for 12 weeks: (N) C57BL6 + vehicle, (O) C57BL/6 + vatalanib, (P) STZ-C57BL/6 + vehicle, (Q) STZ-C57BL/6 + vatalanib, (R) eNOS−/− + vehicle, (S) eNOS−/− + vatalanib, (T) STZ-eNOS−/− + vehicle, and (U) STZ-eNOS−/− + vatalanib. Quantitation of glomerular capillary volume in (V) C57BL/6 and (W) eNOS−/− mice. *P<0.05 versus 0 mg/kg per day, †P<0.05 versus all other groups, ‡P<0.01 versus all other groups, §P<0.05 versus eNOS−/− + vehicle. AU, arbitrary units. Scale bar, 30 µm. Original magnification, ×400.
After treatment with either vatalanib or vehicle for 12 weeks, glomerular capillary growth was assessed using both conventional immunostaining for the endothelial marker platelet endothelial cell adhesion molecule-1 (PECAM-1) (Figure 1, D–M) and the novel technique of fluorescent microangiography (FMA) (Figure 1, N–W).24 By either method, glomerular capillary volume was found to be increased with diabetes in both wild-type and eNOS−/− mice and was reduced with vatalanib (Figure 1, D–W), demonstrating that eNOS is not essential for either glomerular capillary enlargement or its response to antiangiogenic therapy.
Albuminuria Is Reduced with VEGFR Inhibition in Diabetic Wild-Type but Not eNOS−/− Mice
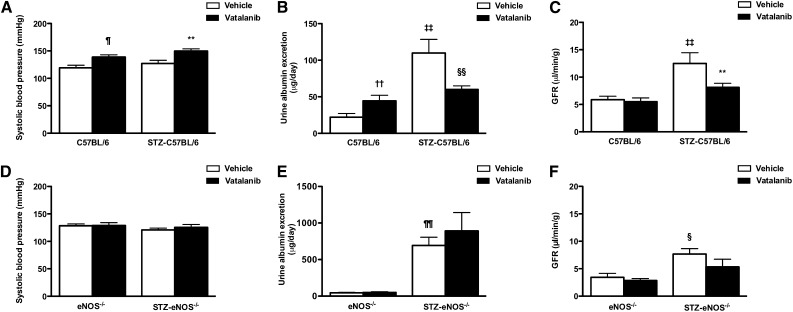
The effects of VEGFR inhibition on renal function, structure, ultrastructure, and gene expression are shown in Table 1, Figure 2, and Supplemental Figure 1. Although vatalanib treatment attenuated diabetes-induced glomerular capillary growth in both wild-type and eNOS−/− mice, the effect on renal function was strikingly different. Treatment with vatalanib caused a rise in SBP in both control and diabetic wild-type mice (Figure 2A). However, whereas this was associated with an increase in albuminuria in nondiabetic mice, the opposite effect was observed in STZ-C57BL/6 mice (Figure 2B), with a reduction in albumin excretion rate (AER) associated with attenuation in diabetes-associated hyperfiltration (Figure 2C). In contrast, VEGFR inhibition had no effect on SBP in the setting of eNOS deficiency (Figure 2D), whereas the heavy albuminuria that was observed in STZ-eNOS−/− mice was similarly unaffected (Figure 2E). Although GFR was modestly increased with diabetes in eNOS−/− mice, it did not reach the level observed in STZ-C57BL/6 mice and was nonsignificantly reduced with vatalanib (Figure 2F). Thus, just as the hypertensive effect of anti-VEGF therapy is mediated through NOS-dependent pathways,25 so too are its attenuating effects on diabetic albuminuria and hyperfiltration.
Table 1.
Metabolic parameters, ultrastructure, and gene expression in control and STZ-diabetic wild-type (C57BL/6) and eNOS−/− mice treated with vehicle or vatalanib for 12 weeks
| Body Weight (g) | Kidney Weight (g) | Kidney Weight: Body Weight (%) | HbA1c (%) | Vv (mes/glom) | BMT (nm) | VEGF-A mRNA (AU) | VEGFR-2 mRNA (AU) | |
|---|---|---|---|---|---|---|---|---|
| C57BL/6 + vehicle | 30.8±0.3 | 0.161±0.003 | 0.52±0.01 | 4.6±0.2 | 0.22±0.01 | 204±11 | 1.04±0.13 | 1.03±0.11 |
| C57BL/6 + vatalanib | 27.0±1.1a | 0.164±0.01 | 0.61±0.05 | 4.4±0.1 | 0.21±0.01 | 190±8 | 1.22±0.15 | 1.13±0.06 |
| STZ-C57BL/6 + vehicle | 24.9±0.7b,c | 0.227±0.006b,c | 0.91±0.03b,c | 10.1±0.2b,c | 0.28±0.01 | 198±4 | 0.81±0.08 | 1.43±0.17 |
| STZ-C57BL/6 + vatalanib | 24.4±0.5c,d | 0.193±0.011d,e | 0.77±0.04c,f,g | 9.5±0.3b,c | 0.23±0.03 | 211±7 | 1.00±0.09 | 1.26±0.04 |
| eNOS−/− + vehicle | 29.3±0.7 | 0.144±0.005 | 0.50±0.01 | 4.9±0.1 | 0.23±0.02 | 224±7 | 0.69±0.03 | 1.14±0.12 |
| eNOS−/− + vatalanib | 29.1±0.5 | 0.134±0.005 | 0.46±0.07 | 4.8±0.2 | 0.25±0.01 | 234±11 | 0.86±0.11 | 1.04±0.08 |
| STZ-eNOS−/− + vehicle | 22.3±0.9h,i | 0.169±0.007j | 0.75±0.04h,i | 10.9±0.6h,i | 0.25±0.02 | 224±13 | 1.18±0.16 | 1.30±0.20 |
| STZ-eNOS−/− + vatalanib | 22.1±0.8h,i | 0.145±0.011 | 0.66±0.06j,k | 9.8±0.8h,i | 0.23±0.02 | 226±11 | 1.15±0.18 | 1.16±0.09 |
HbA1c, hemoglobin A1c; Vv (mes/glom), mesangial fractional volume; BMT, basement membrane thickness; AU, arbitrary units.
P<0.01 versus C57BL/6 + vehicle.
P<0.001 versus C57BL/6 + vatalanib.
P<0.001 versus C57BL/6 + vehicle.
P<0.05 versus C57BL/6 + vatalanib.
P<0.05 versus C57BL/6 + vehicle.
P<0.01 versus C57BL/6 + vatalanib.
P<0.05 versus STZ-C57BL/6 + vehicle.
P<0.001 versus eNOS−/− + vehicle.
P<0.001 versus eNOS−/− + vatalanib.
P<0.01 versus eNOS−/− + vatalanib.
P<0.05 versus eNOS−/− + vehicle.
Figure 2.
Effect of VEGFR inhibition on renal function in control and diabetic wildtype and eNOS−/− mice. Systolic BP (A and D), urine albumin excretion (B and E), and GFR (C and F) in control and STZ-diabetic (A–C) wild-type (C57BL/6) and (D–F) eNOS−/− mice treated with vehicle or vatalanib (50 mg/kg) for 12 weeks. ¶P<0.05 versus C57BL/6 + vehicle, **P<0.01 versus STZ-C57BL/6 + vehicle, ††P<0.01 versus C57BL/6 + vehicle, ‡‡P<0.001 versus C57BL/6 + vehicle, §§P<0.05 versus STZ-C57BL/6 + vehicle, ¶¶P<0.001 versus eNOS−/− + vehicle, §P<0.05 versus eNOS−/− + vehicle.
Albuminuria in STZ-eNOS−/− Mice Is Associated with an Acute Podocytopathy
Whereas albuminuria was attenuated by VEGFR blockade in STZ-C57BL/6 mice, it was unaffected in STZ-eNOS−/− mice. Consequently, we next sought to investigate alternative mechanisms that could account for the large increase in urine albumin excretion that occurred in these animals. In the first instance, we considered the interval over which albuminuria developed. Mice were treated with a daily intraperitoneal injection of STZ for 5 days and AER was determined 14 days after the initial injection (Table 2). At this early time point, albuminuria was profoundly increased in STZ-eNOS−/− mice, whereas glomerular volume and GFR were unaffected (Figure 3, A and B and Table 2).
Table 2.
Metabolic parameters in control and STZ-diabetic C57BL/6 and eNOS−/− mice at 2 weeks
| Body Weight (g) | Blood Glucose (mmol/L) | Kidney Weight (g) | Kidney Weight: Body Weight (%) | Glomerular Volume (×105) (μm3) | GFR (µl/min per g) | Renal AngII (pg/mg) | |
|---|---|---|---|---|---|---|---|
| C57BL/6 | 21.7±0.3 | 10.5±0.7 | 0.140±0.005 | 0.65±0.03 | 2.41±0.07 | 5.7±0.6 | 31.1±3.3 |
| STZ-C57BL/6 | 21.7±0.4 | 23.6±1.4a | 0.137±0.005 | 0.62±0.02 | 2.41±0.09 | 6.3±1.3 | 48.2±3.6b |
| eNOS−/− | 21.2±0.7 | 10.6±1.1 | 0.111±0.004c | 0.52±0.02c | 2.57±0.22 | 4.6±1.0 | 29.9±4.3 |
| STZ-eNOS−/− | 20.6±0.3 | 24.0±1.2a | 0.118±0.004d | 0.57±0.02 | 2.55±0.31 | 4.4±2.1 | 28.7±1.7 |
P<0.01 versus respective nondiabetic control groups.
P<0.01 versus all other groups.
P<0.05 versus either C57BL/6 or STZ-C57BL/6.
P<0.05 versus C57BL/6.
Figure 3.
Acute albuminuria and podocytopathy in STZ-eNOS−/− mice. (A) Urine albumin excretion and (B) albumin/creatinine ratio in control and STZ-diabetic wild-type (C57BL/6) and eNOS−/− mice, 2 weeks after the initial intraperitoneal injection of STZ. (C–F) Podocyte ultrastructure in control and diabetic C57BL/6 and eNOS−/− mice 2 weeks after the initial intraperitoneal injection of STZ: (C) C57BL/6, (D) STZ-C57BL/6, (E) eNOS−/−, and (F) STZ-eNOS−/−. The black triangle marks cytoplasmic vacuoles in a podocyte from an STZ-eNOS−/− mouse. (G) Percentage abnormal podocytes displaying pseudocysts, adsorption droplets, or cytoplasmic vacuoles. (H–K) Podocyte foot processes in control and diabetic C57BL/6 and eNOS−/− mice 2 weeks after the initial intraperitoneal injection of STZ: (H) C57BL/6, (I) STZ-C57BL/6, (J) eNOS−/−, and (K) STZ-eNOS−/−. (L) Podocyte foot process width. (M–P) Endothelial morphology in control and diabetic C57BL/6 and eNOS−/− mice 2 weeks after the initial intraperitoneal injection of STZ: (M) C57BL/6, (N) STZ-C57BL/6, (O) eNOS−/−, and (P) STZ-eNOS−/−. Despite areas of foot process widening (thick arrows) endothelial fenestrations (thin arrows) were preserved in STZ-eNOS−/− mice. ***P<0.001 versus all other groups, †††P<0.05 versus C57BL/6, ‡‡‡P<0.01 versus all other groups, §§§P<0.01 versus C57BL/6.
Structurally, the kidneys of both nondiabetic and newly diabetic eNOS−/− mice demonstrated variable mesangial proliferative GN with occasional globally sclerosed glomeruli or localized cortical scarring. Despite a 5- to 10-fold increase in albuminuria in eNOS−/− mice with new-onset diabetes (Figure 3, A and B), no additional structural changes were observed by light microscopy, although there was an incremental increase in albumin immunostaining within the tubular cells of the pars convoluta (Supplemental Figure 2). Ultrastructurally, a focal podocytopathy was evident in STZ-eNOS−/− mice with new-onset diabetes, including the presence of cytoplasmic vacuoles and pseudocysts (Figure 3, C–F) affecting approximately 15% of podocytes (Figure 3G) and associated with absence of cytoplasmic actin filaments (Supplemental Figure 3). Foot process width, by contrast, was incrementally although minimally increased with diabetes and eNOS deficiency (Figure 3, H–L). Despite acute podocyte injury, glomerular endothelial ultrastructure was preserved in albuminuric STZ-eNOS−/− mice (Figure 3, M–P).
Ultrastructural Evidence of Podocyte Injury Is an Early Event in db/db eNOS−/− Mice
Albuminuria was not increased in STZ-eNOS−/− mice that failed to develop diabetes (data not shown) and podocytopathy was not observed in STZ-C57BL/6 mice (Figure 3). Nevertheless, to further exclude a role for STZ toxicity in the podocyte injury observed in diabetic eNOS−/− mice, we also examined glomerular ultrastructure in double knockout diabetic db/db eNOS−/− mice (Supplemental Table 1).18 By light microscopy, renal injury was more pronounced in db/db eNOS−/− mice aged 12 weeks, than STZ-eNOS−/− mice after 12 weeks of diabetes, with expansion of the mesangial matrix, dilated tubules with casts and focal areas of inflammatory cell infiltration (Supplemental Figure 4). Consistent with the podocytopathy observed in STZ-eNOS−/− mice, ultrastructural evidence of podocyte injury was also present in db/db eNOS−/− mice between 8 and 12 weeks of age, including accumulation of vacuoles, pseudocysts, and electron-dense droplets within the cytoplasm together with foot process effacement (Figure 4 and Supplemental Figure 5).
Figure 4.
Podocyte ultrastructure in db/db eNOS−/− mice at 8–12 weeks of age. Representative photomicrographs showing a (A, black square) podocyte pseudocyst, (B) cytoplasmic vacuole, and (C, arrow) focal areas of foot process effacement.
Acute Podocytopathy and Albuminuria Development Is Prevented by RAAS Blockade in STZ-eNOS−/− Mice
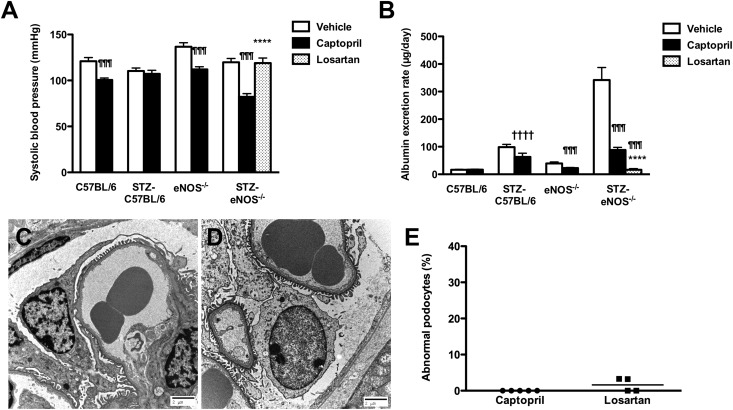
In patients with diabetic nephropathy, albuminuria is widely considered to be indicative of underlying endothelial dysfunction and is often responsive to RAAS blockade. To determine whether the podocyte injury and heavy albuminuria that occurred in STZ-eNOS−/− mice were similarly sensitive to renin-angiotensin system (RAS) blockade, control and STZ-diabetic wild-type and eNOS−/− mice were treated with the angiotensin converting enzyme (ACE) inhibitor captopril, commenced on the same day as the first intraperitoneal injection of STZ and continued for 2 weeks (Supplemental Table 2). At this early time point, captopril treatment markedly reduced AER in STZ-eNOS−/− mice, such that urinary albumin excretion was equivalent to that seen in STZ-C57BL/6 mice (Figure 5, A and B). Because SBP was lower in captopril-treated animals than in their vehicle-treated counterparts (Figure 5A) and because ACE inhibition may exert angiotensin II (AngII) independent effects, STZ-eNOS−/− mice were also treated with the AngII receptor blocker losartan at a dose that did not lower BP (Figure 5A). Consistent with the observations in ACE inhibitor–treated STZ-eNOS−/− mice, the AER in STZ-eNOS−/− mice that received losartan was similarly reduced compared with vehicle-treated animals (Figure 5B). Ultrastructurally, both captopril and losartan treatment abrogated podocyte injury in STZ-eNOS−/− mice (Figure 5, C–E). Administration of the mineralocorticoid receptor antagonist, spironolactone, resulted in an intermediate response, with AER in STZ-eNOS−/− mice being reduced compared with vehicle-treated animals, but not to levels achieved with either captopril or losartan (STZ-eNOS−/−+ spironolactone, blood glucose 24.7±3.5 mmol/L, SBP 96±4 mmHg, AER 161.8×/÷1.2 [P<0.05 versus vehicle or captopril, P<0.001 versus losartan]).
Figure 5.
Effect of RAS blockade on acute albuminuria and podocytopathy in STZ-eNOS−/− mice. (A) Systolic BP and (B) urine albumin excretion in control and STZ-diabetic wild-type (C57BL/6) and eNOS−/− mice treated with vehicle (drinking water), captopril (20 mg/kg per day), or losartan (10 mg/kg per day) for 2 weeks commencing with the first intraperitoneal injection of STZ. (C and D) Podocyte ultrastructure in STZ-eNOS−/− mice treated with (C) captopril or (D) losartan. (E) Percentage abnormal podocytes displaying pseudocysts, adsorption droplets, or cytoplasmic vacuoles. ¶¶¶P<0.001 versus vehicle, ****P<0.001 versus STZ-eNOS−/− + captopril, ††††P<0.05 versus vehicle.
Secreted Factors from STZ-eNOS−/− Mice Augment Podocyte Injury In Vitro and Are Attenuated by ACE Inhibition
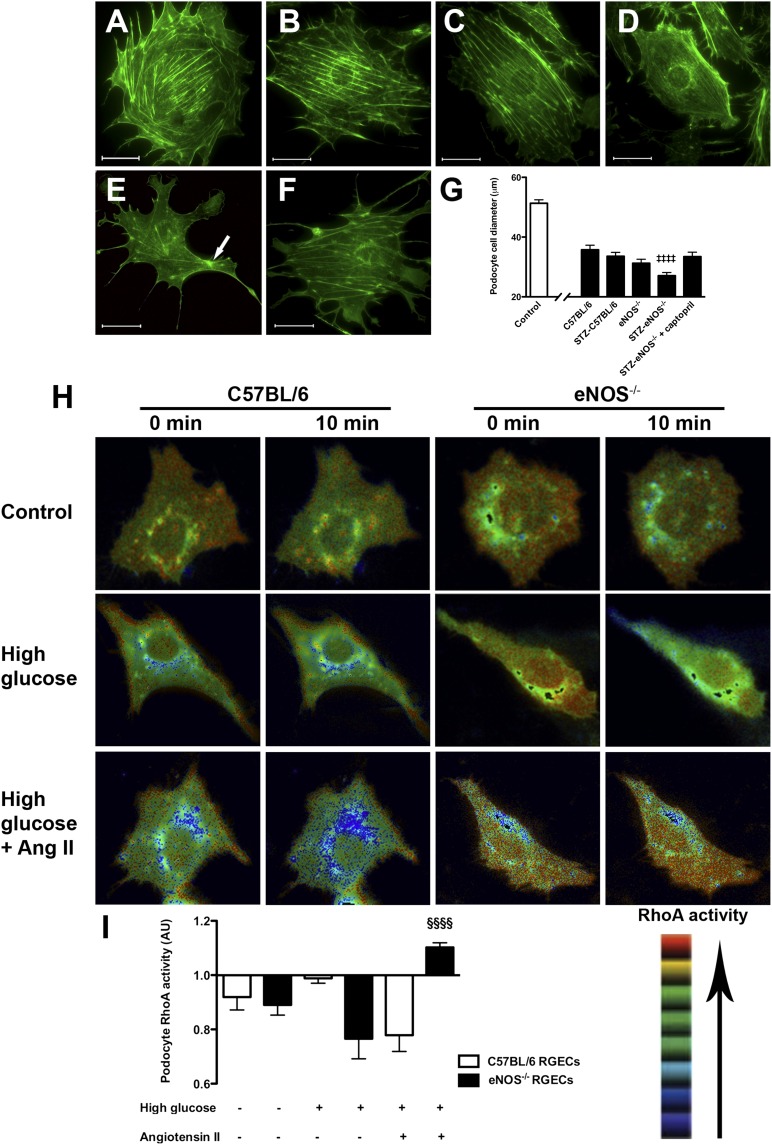
Although acute podocyte injury in STZ-eNOS−/− mice was RAAS sensitive, renal AngII levels were similar between normoglycemic and hyperglycemic eNOS−/− mice, in contrast to the rise in renal AngII seen in STZ-C57BL/6 mice (Table 2). Because the ultrastructural lesions that occurred with albuminuria development in STZ-eNOS−/− mice occurred in podocytes, yet these cells are not known to express eNOS,26 we hypothesized that the podocytopathy observed was a consequence of altered RAAS-dependent secreted factors. To determine whether podocyte morphology could be directly affected by circulating factors released in the setting of diabetes and eNOS deficiency, cultured podocytes were exposed to serum from the various study groups. Labeling podocytes under basal conditions for filamentous actin (F-actin) revealed the presence of cortical bundles of subplasmalemmal F-actin together with transversely arranged cytosolic actin stress fibers (Figure 6A). Compared with cells under basal conditions, exposure of cultured podocytes to 1% mouse serum caused disruption in cortical F-actin and a reduction in cell size (Figure 6, B–G). Moreover, among serum-treated podocytes, there was an incremental decrease in cell body diameter in the setting of diabetes and eNOS deficiency (Figure 6, B–E), such that radially arranged F-actin arising from an actin rich center could be observed in occasional podocytes treated with serum from STZ-eNOS−/− mice (Figure 6E). These cytoskeletal rearrangements, analogous to those observed in response to mechanical stretch,27 were associated with the development of long, protruberant lamellipodia, with a significantly greater effect observed with the serum of vehicle-treated STZ-eNOS−/− mice than captopril-treated STZ-eNOS−/− mice (Figure 6, E–G). Pretreatment of podocytes with losartan did not alter the cytoskeletal rearrangements induced by STZ-eNOS−/− serum (fold change in cell body diameter versus vehicle, 0.98±0.04).
Figure 6.
Podocyte cytoskeleton and RhoA activity. (A–F) Fluorescent microscopy images of Alexa 488 phalloidin stained immortalized mouse podocytes cultured (A) under basal conditions or (B) in the presence of serum from control and STZ-diabetic wild-type (C57BL/6) and eNOS−/− mice: (B) C57BL/6, (C) STZ-C57BL/6, (D) eNOS−/−, (E) STZ-eNOS−/−, and (F) STZ-eNOS−/− + captopril. Arrow marks actin-rich center. (G) Cell body diameter. (H) Podocyte RhoA activity determined by FRET in live cells after exposure for 10 minutes to media conditioned by C57BL/6 and eNOS−/− RGECs under basal conditions or after incubation with high (25 mM) glucose for 48 hours with or without AngII for 4 hours. (I) Podocyte RhoA activity shown as the mean relative FRET intensity normalized to time zero. AU, arbitrary units. ‡‡‡‡P<0.01 versus all other groups, §§§§P<0.05 versus C57BL/6 RGECs. Scale bar, 30 µm.
Secreted Factors from eNOS−/− Renal Glomerular Endothelial Cells Exposed to High Glucose Concentrations and AngII Induce RhoA Activation in Podocytes
Because the RAAS-dependent podocyte cytoskeletal rearrangements induced by STZ-eNOS−/− mouse serum were reminiscent of those previously described after RhoA activation,28 we finally sought to determine whether podocyte RhoA activation may also be induced by secretory products derived from glomerular endothelial cells. Renal glomerular endothelial cells (RGECs) were therefore derived from C57BL/6 and eNOS−/− mice (Supplemental Figure 7) and preincubated with high glucose concentrations (25 mM) ± 100 nM AngII, before applying the conditioned media generated to cultured mouse podocytes and measuring RhoA activity by fluorescence resonance energy transfer (FRET) in live cells. Although podocyte RhoA activity was reduced by control conditioned media, it was increased by exposure of cells to media conditioned by eNOS−/− RGECs treated with high glucose and AngII (Figure 6, H and I).
Discussion
Although the relationship between endothelial dysfunction and albuminuria has been appreciated for >20 years,29 the mechanisms by which a primary endothelial injury may predispose to urinary leakage of albumin remain incompletely resolved. At the same time, recent observations in clinical studies30 and experimental models20 have suggested that deficiency of an ostensibly endothelial-specific gene, eNOS, may predispose to the development of renal injury in diabetes. In this study, we identified that the combination of hyperglycemia and eNOS knockout results in an acute podocytopathy and ensuing heavy albuminuria. In the context of emerging evidence for a bidirectional communication across the filtration barrier, these observations highlight the importance of endothelial-podocyte communication and its sensitivity to RAAS-blockade in regulating albuminuria in diabetes.
In attempting to elucidate the role of eNOS in both glomerular capillary growth and podocyte structure/function in diabetes, the following four major observations were made: (1) the antialbuminuric effects of anti-VEGF agents in diabetes are eNOS dependent; (2) in contrast, both glomerular capillary growth and its response to VEGFR blockade may occur through eNOS-independent events; (3) the development of acute hyperglycemia in the setting of eNOS deficiency leads to podocyte injury; and (4) this podocytopathy can be prevented by RAAS blockade.
Clinical experience has revealed that both hypertension and proteinuria are class effects of anti-VEGF therapy,23 the former being recently shown to be mediated through decreased NOS activity.25 This study extends these initial observations and now demonstrates that the attenuating effects of VEGFR blockade on diabetic albuminuria and hyperfiltration are similarly also mediated through eNOS-dependent pathways. VEGF-induced increases in vascular permeability are regulated by eNOS in an Akt-dependent manner.31 Variable expression or activity of eNOS therefore likely explains the variable effects of VEGF blockade previously reported in experimental models of diabetic nephropathy.8,32 Although the absence of eNOS affected the response to VEGFR blockade with respect to albuminuria development in diabetes, it did not affect either diabetes-associated glomerular capillary growth or its response to anti-VEGF therapy. eNOS is a positive regulator of angiogenesis under most circumstances,33–35 whereas conversely deficiency of the enzyme has also been implicated in enhanced endothelial proliferation in diabetes.17 The term angiogenesis refers to the growth of new blood vessels from pre-existing ones. Increased glomerular capillary volume (one, but not the sole, cause of glomerular hypertrophy in diabetes) occurs as a consequence of an increase in the number, length, and surface area of glomerular capillaries and is mediated by an increase in both cell size and cell number.5,36 The lack of effect of eNOS deficiency on this trophic response highlights that glomerular capillary growth in diabetes, although responsive to anti-VEGF therapy, is not strictly analogous to angiogenesis that occurs in other vascular beds, during development or in other disease states. That at least some VEGF-mediated processes may occur independently of downstream eNOS is readily illustrated by the observations that although eNOS−/− mice are viable and fertile, VEGF deletion results in embryonic lethality.37,38 The increase in renal VEGFR-2 phosphorylation observed in STZ-eNOS−/− mice relative to wild-type animals may be indicative of a compensatory response circumventing some of the restrictions on cellular signaling and function imposed by eNOS deficiency. Thus, although VEGF activity accounts, at least in part, for the modest albuminuria that occurs in diabetic wild-type mice, eNOS-dependent, but VEGF-independent, pathways likely account for the robust filtration barrier defect in STZ-eNOS−/− mice.
The presence of heavy albuminuria in STZ-eNOS−/− mice despite a reduction in glomerular capillary volume with VEGFR blockade prompted us to search for alternative mechanisms that may mediate altered glomerular permselectivity in these animals. Of particular note is the immediacy at which the increase in albuminuria occurred. This observation is consistent with a previous study of STZ-eNOS−/− mice that reported the largest incremental rise in albumin excretion at the earliest measured time point, 4 weeks after the onset of diabetes.19 Although, with a longer duration of diabetes, the kidneys of eNOS−/− mice may develop a range of histopathological lesions, including mesangiolysis and endothelial injury,19,39 in this study, onset of heavy albuminuria preceded such changes. Indeed, the major ultrastructural abnormality was podocyte pseudocyst and adsorption droplet formation, indicative of cellular injury and degeneration,40,41 and similar to that previously described to accompany AngII-mediated podocyte actin remodeling.42 The early development of podocytopathy in db/db eNOS−/− mice and the increase in albumin content of the pars convoluta tubular cells in STZ-eNOS−/− mice both support the supposition that podocyte injury is the cause and not the consequence of increased albumin excretion in these animals. Nevertheless, this does not preclude an additional significant role for downstream tubular reabsorption defects. Moreover, increased albumin immunostaining within the pars convoluta may reflect impaired albumin return to the systemic circulation. Thus, the precise role that eNOS deficiency may play in regulating tubular function in diabetes remains to be resolved.
Nephropathy is considered a “microvascular” complication of diabetes, highlighting that frequently the primary cell subjected to hyperglycemic insult is the endothelial cell.43 However, recent attention has focused on a primary role for podocyte injury in mediating albuminuria development in diabetes.44 Previous detailed histologic studies localized eNOS in the kidney to the endothelium of arterioles and glomerular capillaries,45 whereas podocytes are considered not to express this enzyme.26 Podocyte injury, in this setting, therefore likely involves endocrine and/or paracrine effects. Conventional RAAS-dependent endocrine effects are supported by the in vitro effects on podocyte cytoskeletal structure induced by serum derived from STZ-eNOS−/− mice and by responsiveness of albuminuria (albeit partial) to aldosterone antagonism. However, increased podocyte RhoA activity induced by conditioned media mimicking the diabetic eNOS−/− state suggests that paracrine mediators may also play a role. Bidirectional communication between endothelial cells and podocytes is not only important in diabetes, but is also essential for normal renal development.46,47 As such, a primarily podocyte-restricted pathology in the presence of global eNOS deficiency may reflect impaired development of these cells, with an unmasking of insidious effects with new-onset diabetes. Endothelial-podocyte communication is perhaps best exemplified by the VEGF–VEGFR-2 system, in which VEGF is expressed primarily by podocytes7 and VEGFR-2 primarily (or exclusively14) by endothelial cells. Beyond the VEGF–VEGFR-2 pathway, other mediators of this crosstalk include the SDF-1/CXCR4 system47,48 and the angiopoietins; in the latter case, podocyte-specific overexpression of angiopoietin-2 has been shown to promote endothelial apoptosis and proteinuria.49 NO itself, meanwhile, has been reported to paradoxically increase glomerular permeability to albumin.50
Sensitivity of acute podocyte injury and albuminuria to RAAS blockade may indicate either activation of the RAAS in STZ-eNOS−/− mice or heightened RAAS sensitivity of target cells. In the case of the former, a number of RAAS components have been found to be upregulated with diabetes in the kidneys of eNOS−/− mice.51 RAAS activation has also been described in the hearts of triply deficient n/i/eNOS−/− mice, although the mechanisms underlying this response have similarly not been defined.52 In this study, we observed an approximate 50% increase in renal AngII with new-onset diabetes in wild-type mice and no change in eNOS−/− animals. Although increased intraglomerular AngII concentrations (not measured in this study) may occur in STZ-eNOS−/− mice, our results suggest that eNOS is likely required for the increase in renal AngII with diabetes. In support of the latter postulate of heightened RAAS-sensitivity, podocyte RhoA activation occurred in response to conditioned medium from eNOS−/− RGECs pretreated with high glucose concentrations and AngII. In contrast, the opposite effect was observed with medium from wild-type cells under the same conditions. Thus, in the context of our in vivo observations, eNOS deficiency is sufficient to alter the secretory response of endothelial cells exposed to high glucose, even in the presence of normal AngII concentrations, whereas this altered secretome may directly affect podocyte function. To note, although we focused on RhoA activity, lamellipodia development and actin fiber rearrangement are reminiscent of a migratory phenotype. Accordingly, other Rho GTPases (i.e., Rac/Cdc42) that mediate these effects are also likely to be implicated in the podocyte response to endothelial secretory products. Among the growing catalog of secretomic mediators of endothelial-podocyte communication, currently identified candidates are predominantly podocyte-derived factors that act on endothelial-bound receptors, likely reflecting their requirement to be present in greater abundance to induce their biologic effects when acting contrary to urinary flow. Endothelial-derived regulators of podocyte function may not only be present in relatively low abundance but may also not be restricted to protein-based molecules, with exciting evidence emerging for pivotal effects of both paracrine lipid-mediators53 and nucleic acids.54
Over recent years, considerable efforts have been invested in identifying an animal model of diabetic nephropathy that recapitulates human disease. In this regard, the diabetic eNOS−/− mouse has been viewed as an advance given the range of histopathological lesions reported.55 Although both STZ- and db/db- eNOS−/− mice are characterized by albuminuria, we observed strikingly worse renal injury in db/db eNOS−/− mice. Moreover, a recent report also described that this renal injury is similarly sensitive to RAS blockade.56 Conversely, despite the presence of heavy albuminuria, changes in classic structural parameters of diabetic nephropathy in STZ-eNOS−/− mice, such as basement membrane thickening and mesangial expansion, are relatively minor.
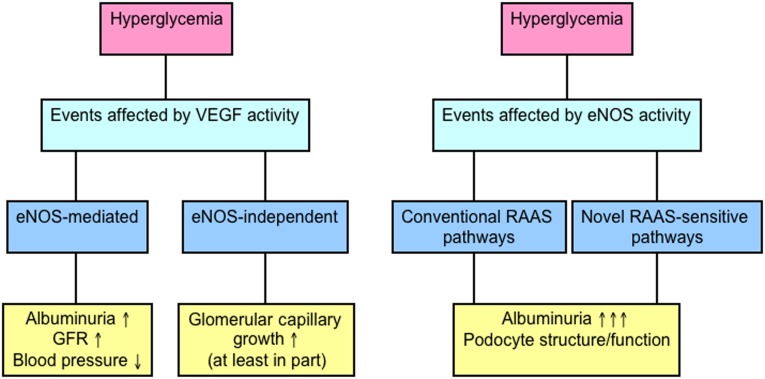
In summary, the detrimental effects of VEGF in experimental diabetes may occur through mechanisms that are eNOS dependent (albuminuria and hyperfiltration) and eNOS independent (glomerular capillary growth, in part). Correspondingly, eNOS deficiency itself in diabetes leads to acute podocyte injury and heavy albuminuria in diabetes through either RAAS-mediated or RAAS-sensitive pathways (Figure 7). Altered endothelial-podocyte crosstalk is likely to be a major mechanism by which endothelial injury results in increased albumin excretion in diabetes.
Figure 7.
Illustrating the role of VEGF and eNOS in regulating renal function and renal injury in diabetes. VEGF may mediate changes in renal structure and function in diabetes through either eNOS-dependent (albuminuria, hyperfiltration) or independent mechanisms (glomerular capillary growth). Although some of the detrimental effects of VEGF are eNOS-mediated, eNOS deficiency itself exerts profound effects on podocyte integrity and albuminuria. These effects are RAAS sensitive and may occur through conventional RAAS-dependent mechanisms or novel RAAS-sensitive pathways that regulate endothelial/podocyte function and the communication between these two cell types.
Concise Methods
In Vivo Studies
Long-Term Study
Male C57BL/6 mice and eNOS−/− mice (aged 7 weeks, n=12–16/group; Jackson Laboratories) were randomized to receive a daily intraperitoneal injection of either 0.1 M citrate buffer (pH 4.5) or 50 mg/kg STZ in citrate buffer for 5 consecutive days according to the protocol recommended by the Animal Models of Diabetes Complications Consortium (AMDCC).55 Two weeks after the initial intraperitoneal injection, diabetes was confirmed (Accu-check Advantage; Roche, Mississauga, ON) and only animals with a blood glucose >16 mmol/L were considered diabetic. Mice were subsequently randomized to receive either the VEGFR tyrosine kinase inhibitor vatalanib (50 mg/kg) (LC Laboratories, Woburn, MA) or distilled water alone by daily oral gavage for 12 weeks.
SBP was measured in anesthetized mice (2% isoflurane) using a CODA noninvasive BP system (Kent Scientific, Torrington, CT) and with external body temperature maintained at 36.0–36.5°C (Infrared thermometer, Waltham, MA). Invasive SBP was measured via the right carotid artery, with a 1.4 F Millar Mikro-tip pressure catheter (Model SPR-839; Millar Instruments, Houston, TX). SBP determined via the tail-cuff technique correlated closely with invasive measurements (r2=0.83, P<0.02, slope 1.04±0.23; Supplemental Figure 6). Hemoglobin A1c was measured using A1cNow+ (Bayer, Sunnyvale, CA). Urinary albumin was determined after 24-hour metabolic caging with AssayMax mouse albumin ELISA (Assaypro, St. Charles, MO). GFR was determined by FITC-inulin clearance (n≥6/group) using an adaptation of the protocol recommended by the AMDCC, with FITC-inulin delivery achieved via the tail-vein.
Short-Term Study
C57BL/6 mice and eNOS−/− mice received a daily intraperitoneal injection of either 0.1 M citrate buffer (pH 4.5) or 50 mg/kg STZ as described (n=16–20/group). Two weeks after the initial intraperitoneal injection, animals were housed in metabolic cages for 24 hours and albuminuria determined as described. Urine creatinine was determined with Creatinine Companion (Exocell, Philadelphia, PA).
db/db eNOS−/− Mice
db/db eNOS−/− were generated as previously described.18 Genotyping was performed by PCR. Animals were maintained for 8–12 weeks.
RAAS Blockade
C57BL/6 mice and eNOS−/− mice received a daily intraperitoneal injection of either 0.1 M citrate buffer (pH 4.5) or 50 mg/kg STZ as described. Commencing on the day of the first intraperitoneal injection of STZ, mice were randomized to receive either vehicle (drinking water) or captopril (20 mg/kg per day; Sigma) (n=12/group). In additional experiments, a further 12 STZ-eNOS−/− mice received losartan (10 mg/kg per day; Sigma) and a further 5 STZ-eNOS−/− mice received spironolactone (50 mg/kg per day; Sigma), both in drinking water. Mice were studied 2 weeks after the first intraperitoneal injection of STZ.
All experimental procedures adhered to the guidelines of the Canadian Council on Animal Care and were approved by St. Michael’s Hospital Animal Care Committee.
Immunoblotting
Human RGECs (ScienCell, Carlsbad, CA)12 were preincubated with 1 µM vatalanib or DMSO for 2 hours, before the addition of recombinant human VEGF (20 ng/ml) (Millipore, Billerica, MA) for 5 minutes. Cell lysates were subjected to immunoprecipitation for VEGFR-2 (Santa Cruz Biotechnology, Santa Cruz, CA) before immunoblotting for either phosphotyrosine (4G10; Upstate Biotechnology, Lake Placid, NY) or VEGFR-2 (Santa Cruz Biotechnology). Immunoblotting kidney tissue was performed using the following antibodies: VEGFR-2 Tyr951 (New England Biolabs, Ipswich, MA) and β-actin (Abcam, Cambridge, MA). Densitometry was performed using Image J software (version 1.39; National Institutes of Health, available at http://rsb.info.nih.gov/ij/).
Real-Time PCR
Real-time PCR was performed using SYBR green on an ABI Prism 7900HT Fast PCR System (Applied Biosystems, Foster City, CA).39 Primer sequences (ACGT Corp., Toronto, ON) were as follows: VEGFR-2, forward TCAGAGACACTGAGCATGGAA, reverse GTTTTCAGCTCTTCTGAGGCAA; VEGF-A, forward GGAGAGATGAGCTTCCTACAGCA, reverse CCTCGGCTTGTCACATTTTTCT; and RPL13a, forward GCTCTCAAGGTTGTTCGGCTGA, reverse AGATCTGCTTCTTCTTCCGATA. Experiments were performed in triplicate and data analysis was performed using the Applied Biosystems Comparative CT method.
Immunohistochemistry
Immunohistochemistry was performed with antibodies directed against PECAM-1 (Santa Cruz Biotechnology) and albumin (Bethyl Laboratories Inc., Montgomery, TX). Glomerular PECAM-1 and pars convoluta albumin were quantified using Aperio ImageScope software (Aperio Technologies Inc., Vista, CA), with PECAM-1 immunostaining determined in 30 glomeruli from each kidney section and albumin quantified in proximal tubules that could be seen directly arising from glomeruli (approximately 20/kidney section). Data are expressed as the fold change relative to nondiabetic wild-type mice.
Glomerular Volume
Glomerular volume (GV) was calculated on 4-μm periodic acid–Schiff-stained kidney sections using the formula GV=(β/k)(GA)3/2, where β=1.38 pertains to the sphere and k=1.10 is the distribution coefficient.57
FMA
FMA was performed using a 1% agarose solution admixed with fluorescent microbeads (10%, 20 nm diameter) (Invitrogen, Carlsbad, CA) as previously described.24 Kidney cross-sections (200 μm) (VT100SR; Leica, Nussloch, Germany) were mounted with Mowiol (Calbiochem, San Diego, CA) containing 2.5% (wt/vol) DABCO antifading agent (Sigma) and serial images (0.8141 µm steps) were collected by confocal microscopy (Leica TCS SL; Leica, Richmond Hill, CA). Glomerular capillary volumes were measured with Image J software (version 1.39) and reconstructions were generated using Neurolucida (MBF Bioscience, Williston, VT). Glomerular capillary volume was calculated in 12 glomerular Z-stacks taken from at least three mice per group.
Electron Microscopy
Transmission electron microscopy was performed on kidney cortical tissue from four to six mice per group (Philips CM100 transmission electron microscope; Newcastle University). At least three glomeruli per case were examined for the assessment of podocyte morphology, endothelial morphology, foot process width, basement membrane thickness, and mesangial fractional volume as previously described.12,58
Renal AngII
For determination of renal AngII, kidneys were rapidly collected, snap frozen in liquid nitrogen, and stored at −80°C. To provide adequate sample volume, kidney tissues were pooled from one to two mice. AngII was determined in six pooled samples from each group by RIA at the Hypertension Core Laboratory at Wake Forest School of Medicine (Winston-Salem, NC) as previously described.59
Conditionally Immortalized Mouse Podocytes
Mouse podocytes were maintained and differentiated in RPMI as previously described.27 C57BL/6 mice and eNOS−/− mice received either citrate buffer or STZ (n=3/group). Fourteen days later, pooled serum was collected by cardiac puncture and, after filtering, applied to cultured podocytes at a concentration of 1% for 24 hours. In separate experiments, podocytes were pretreated with losartan (100 μM) for 4 hours. Cells were permeabilized with 0.1% Triton X-100 before staining with 25 µg/ml Alexa Flour 488 phalloidin (Invitrogen). Podocyte morphology was observed (Zeiss Axioskop fluorescence microscope) in at least 60 podocytes from each condition (n=3).
Measurement of Podocyte RhoA Activity by FRET
C57BL/6 and eNOS−/− mice RGECs were obtained from Cell Biologics (Chicago, IL). RGEC isolation (Cell Biologics) entailed perfusion of mice with Dynabeads, extraction of glomeruli with a magnetic particle concentrator, and harvesting of endothelial cells with anti-ICAM-2 antibody conjugated Dynabeads. RGECs were characterized by their cobblestone morphology; tube formation after plating on LDEV-Free Basement Membrane Matrix (BD Biosciences, Franklin Lakes, NJ) in the presence of recombinant mouse VEGF (50 ng/ml, Millipore) for 6 hours; and the ability to take up Dil-Ac-LDL (Biomedical Technologies, Stoughton, MA) after incubation with 10 µg/ml for 5 hours at 37°C (Supplemental Figure 7, A–F). Flow cytometry using polyclonal anti-mouse VE-cadherin antibody (R&D Systems, Minneapolis, MN) revealed that >94% C57BL/6 and eNOS−/− RGECs stained positive for VE-cadherin (Cell Biologics; Supplemental Figure 7, G and H). Cells were cultured in mouse endothelial cell medium (Cell Biologics) under basal conditions or in the presence of 25 mM glucose for 48 hours with or without 100 nM human AngII (Sigma) for an additional 4 hours. Cells were washed twice in PBS and then incubated overnight in mouse endothelial cell medium containing 10% FBS to generate conditioned media. FRET measurement in live cells was carried out as previously described.60 Differentiated mouse podocytes were plated onto glass-bottom plates (MatTek Corp., Ashland, MA) coated with type I collagen at a density of 2×105 cells/plate. The following day, cells were transfected with lipofectamine 2000 with 0.5 µg per well Raichu 1298× encoding a FRET probe for RhoA61 (kind gift from Dr. M. Matsuda, Kyoto University, Japan). FRET measurement was carried out 48 hours after transfection following overnight serum starvation, using an inverted fluorescent microscope (IX81; Olympus). After the acquisition of the basal image, conditioned media prewarmed to 37°C was added to the cells at a final concentration of 50% (v/v). Corrected FRET (cFRET) intensity was calculated at each pixel using Metamorph Software (Molecular Devices, Sunnyvale, CA) using the following formula: (rawFRET-background)/(CFP-background). cFRET was converted to pseudocolor pixel to pixel and the average cFRET intensity from each cell was calculated and normalized to time zero.
Statistical Analyses
Data are presented as mean ± SEM. Statistical significance was determined by one-way ANOVA with a Newman–Keuls post hoc comparison. Statistical analyses were performed using GraphPad Prism software (version 5.00; GraphPad Software, San Diego, CA).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Ms. Bridgit Bowskill, Mr. Li-Hao Chen, Mr. Kodie Lee, and Ms. Christine Kuliszewski for their excellent technical assistance and Dr. Richard Gilbert for helpful discussions.
D.A.Y. was sponsored by a KRESCENT postdoctoral fellowship and is currently the recipient of a Canadian Society of Transplantation postdoctoral fellowship. A.A. is a Canadian Diabetes Association Clinician Scientist and a recipient of a Novo Nordisk Diabetes Innovation Award and these studies were supported, in part, by the Canadian Diabetes Association (OG-3-10-2949-AA) and the Novo Nordisk Diabetes Innovation Award program. These studies were supported by a grant from the Physicians’ Services Incorporated Foundation to A.A. and, in part, by CIHR grants. K.A.C. is supported by a Clinician Scientist Award from the Heart and Stroke Foundation of Ontario.
Portions of this work were presented in abstract form at the annual meeting of the American Society of Nephrology, November 16–21, 2010, in Denver, Colorado.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011121170/-/DCSupplemental.
References
- 1.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 2.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM: Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balakumar P, Chakkarwar VA, Krishan P, Singh M: Vascular endothelial dysfunction: A tug of war in diabetic nephropathy? Biomed Pharmacother 63: 171–179, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Nyengaard JR: Number and dimensions of rat glomerular capillaries in normal development and after nephrectomy. Kidney Int 43: 1049–1057, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Nyengaard JR, Rasch R: The impact of experimental diabetes mellitus in rats on glomerular capillary number and sizes. Diabetologia 36: 189–194, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA: Abnormal angiogenesis in diabetic nephropathy. Diabetes 58: 1471–1478, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, Casley DJ, Bach LA, Kelly DJ, Gilbert RE: Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes 48: 2229–2239, 1999 [DOI] [PubMed] [Google Scholar]
- 8.de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH: Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol 12: 993–1000, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S: Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol 17: 3093–3104, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R: Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes 51: 3090–3094, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Advani A, Kelly DJ, Advani SL, Cox AJ, Thai K, Zhang Y, White KE, Gow RM, Marshall SM, Steer BM, Marsden PA, Rakoczy PE, Gilbert RE: Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci U S A 104: 14448–14453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP: Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13: 1349–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Sison K, Eremina V, Baelde H, Min W, Hirashima M, Fantus IG, Quaggin SE: Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol 21: 1691–1701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breyer MD, Böttinger E, Brosius FC, 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K, AMDCC : Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Mohan S, Reddick RL, Musi N, Horn DA, Yan B, Prihoda TJ, Natarajan M, Abboud-Werner SL: Diabetic eNOS knockout mice develop distinct macro- and microvascular complications. Lab Invest 88: 515–528, 2008 [DOI] [PubMed]
- 17.Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ, Croker B: Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol 18: 539–550, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC: Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, Harris RC, Takahashi T: Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol 170: 1473–1484, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CH, Li F, Hiller S, Kim HS, Maeda N, Smithies O, Takahashi N: A modest decrease in endothelial NOS in mice comparable to that associated with human NOS3 variants exacerbates diabetic nephropathy. Proc Natl Acad Sci U S A 108: 2070–2075, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rösel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F: PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 60: 2178–2189, 2000 [PubMed] [Google Scholar]
- 22.Drevs J, Müller-Driver R, Wittig C, Fuxius S, Esser N, Hugenschmidt H, Konerding MA, Allegrini PR, Wood J, Hennig J, Unger C, Marmé D: PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res 62: 4015–4022, 2002 [PubMed] [Google Scholar]
- 23.Izzedine H, Rixe O, Billemont B, Baumelou A, Deray G: Angiogenesis inhibitor therapies: Focus on kidney toxicity and hypertension. Am J Kidney Dis 50: 203–218, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Advani A, Connelly KA, Yuen DA, Zhang Y, Advani SL, Trogadis J, Kabir MG, Shachar E, Kuliszewski MA, Leong-Poi H, Stewart DJ, Gilbert RE: Fluorescent microangiography is a novel and widely applicable technique for delineating the renal microvasculature. PLoS ONE 6: e24695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM: Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 54: 652–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sörensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ: Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol 13: 2639–2647, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K: Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T: Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1621–1630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen T, Bjerre-Knudsen J, Feldt-Rasmussen B, Deckert T: Features of endothelial dysfunction in early diabetic nephropathy. Lancet 1: 461–463, 1989 [DOI] [PubMed] [Google Scholar]
- 30.He Y, Fan Z, Zhang J, Zhang Q, Zheng M, Li Y, Zhang D, Gu S, Yang H: Polymorphisms of eNOS gene are associated with diabetic nephropathy: A meta-analysis. Mutagenesis 26: 339–349, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Six I, Kureishi Y, Luo Z, Walsh K: Akt signaling mediates VEGF/VPF vascular permeability in vivo. FEBS Lett 532: 67–69, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Kim HW, Lim JH, Kim MY, Chung S, Shin SJ, Chung HW, Choi BS, Kim YS, Chang YS, Park CW: Long-term blockade of vascular endothelial growth factor receptor-2 aggravates the diabetic renal dysfunction associated with inactivation of the Akt/eNOS-NO axis. Nephrol Dial Transplant 26: 1173–1188, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Babaei S, Teichert-Kuliszewska K, Zhang Q, Jones N, Dumont DJ, Stewart DJ: Angiogenic actions of angiopoietin-1 require endothelium-derived nitric oxide. Am J Pathol 162: 1927–1936, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D: NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest 115: 1816–1827, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK: Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A 98: 2604–2609, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo M, Ricardo SD, Deane JA, Shi M, Cullen-McEwen L, Bertram JF: A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J Anat 207: 813–821, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW: Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439–442, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC: Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Advani A, Huang Q, Thai K, Advani SL, White KE, Kelly DJ, Yuen DA, Connelly KA, Marsden PA, Gilbert RE: Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am J Pathol 178: 2205–2214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mundel P: Urinary podocytes: Lost and found alive. Kidney Int 64: 1529–1530, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Gassler N, Elger M, Kränzlin B, Kriz W, Gretz N, Hähnel B, Hosser H, Hartmann I: Podocyte injury underlies the progression of focal segmental glomerulosclerosis in the fa/fa Zucker rat. Kidney Int 60: 106–116, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Hsu HH, Hoffmann S, Endlich N, Velic A, Schwab A, Weide T, Schlatter E, Pavenstädt H: Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J Mol Med (Berl) 86: 1379–1394, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Brownlee M: The pathobiology of diabetic complications: A unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Wolf G, Chen S, Ziyadeh FN: From the periphery of the glomerular capillary wall toward the center of disease: Podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Bachmann S, Bosse HM, Mundel P: Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol 268: F885–F898, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, Imai E, Nagasawa T, Rakugi H, Isaka Y: The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol 20: 1714–1723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayyed SG, Hägele H, Kulkarni OP, Endlich K, Segerer S, Eulberg D, Klussmann S, Anders HJ: Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia 52: 2445–2454, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Davis B, Dei Cas A, Long DA, White KE, Hayward A, Ku CH, Woolf AS, Bilous R, Viberti G, Gnudi L: Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol 18: 2320–2329, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Li B, Yao J, Morioka T, Oite T: Nitric oxide increases albumin permeability of isolated rat glomeruli via a phosphorylation-dependent mechanism. J Am Soc Nephrol 12: 2616–2624, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Kosugi T, Heinig M, Nakayama T, Matsuo S, Nakagawa T: eNOS knockout mice with advanced diabetic nephropathy have less benefit from renin-angiotensin blockade than from aldosterone receptor antagonists. Am J Pathol 176: 619–629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakata S, Tsutsui M, Shimokawa H, Suda O, Morishita T, Shibata K, Yatera Y, Sabanai K, Tanimoto A, Nagasaki M, Tasaki H, Sasaguri Y, Nakashima Y, Otsuji Y, Yanagihara N: Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation 117: 2211–2223, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Stitt-Cavanagh EM, Faour WH, Takami K, Carter A, Vanderhyden B, Guan Y, Schneider A, Breyer MD, Kennedy CR: A maladaptive role for EP4 receptors in podocytes. J Am Soc Nephrol 21: 1678–1690, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fichtlscherer S, Zeiher AM, Dimmeler S: Circulating microRNAs: Biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol 31: 2383–2390, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Brosius FC, 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T, Animal Models of Diabetic Complications Consortium : Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang MZ, Wang S, Yang S, Yang H, Fan X, Takahashi T, Harris RC: Role of blood pressure and the renin-angiotensin system in development of diabetic nephropathy (DN) in eNOS-/- db/db mice. Am J Physiol Renal Physiol 302: F433–F438, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilbert RE, Huang Q, Thai K, Advani SL, Lee K, Yuen DA, Connelly KA, Advani A: Histone deacetylase inhibition attenuates diabetes-associated kidney growth: Potential role for epigenetic modification of the epidermal growth factor receptor. Kidney Int 79: 1312–1321, 2011 [DOI] [PubMed] [Google Scholar]
- 58.White KE, Bilous RW: Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol 11: 1667–1673, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Allred AJ, Chappell MC, Ferrario CM, Diz DI: Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol 279: F636–F645, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Aoki K, Matsuda M: Visualization of small GTPase activity with fluorescence resonance energy transfer-based biosensors. Nat Protoc 4: 1623–1631, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Nakamura T, Kurokawa K, Kiyokawa E, Matsuda M: Analysis of the spatiotemporal activation of rho GTPases using Raichu probes. Methods Enzymol 406: 315–332, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.