Abstract
Damage to the endothelial glycocalyx, which helps maintain vascular homeostasis, heightens the sensitivity of the vasculature to atherogenic stimuli. Patients with renal failure have endothelial dysfunction and increased risk for cardiovascular morbidity and mortality, but the state of the endothelial glycocalyx in these patients is unknown. Here, we used Sidestream Darkfield imaging to detect changes in glycocalyx dimension in dialysis patients and healthy controls from in vivo recordings of the sublingual microcirculation. Dialysis patients had increased perfused boundary region and perfused diameters, consistent with deeper penetration of erythrocytes into glycocalyx, indicating a loss of glycocalyx barrier properties. These patients also had higher serum levels of the glycocalyx constituents hyaluronan and syndecan-1 and increased hyaluronidase activity, suggesting the shedding of these components. Loss of residual renal function had no influence on the imaging parameters but did associate with greater shedding of hyaluronan in blood. Furthermore, patients with higher levels of inflammation had more significant damage to the glycocalyx barrier. In conclusion, these data suggest that dialysis patients have an impaired glycocalyx barrier and shed its constituents into blood, likely contributing to the sustained endothelial cell activation observed in ESRD.
Patients with chronic renal failure have endothelial dysfunction and accelerated vascular disease leading to increased morbidity and mortality as a result of cardiovascular events.1–4 The mechanisms responsible are unclear, controversial, and presumed to be multifactorial. The vascular endothelium is coated on the luminal side by the glycocalyx, a negatively charged mesh of proteoglycans (PGs) and associated glycosaminoglycans.5 It is involved in mediating shear-induced release of nitric oxide and contributes to the endothelial permeability barrier, the regulation of redox state, and the inhibition of coagulation as well as leukocyte and platelet adhesion.6–9 Perturbation of glycocalyx occurs after provocation with inflammatory or atherogenic stimuli (such as ischemia reperfusion,10 infusion of oxidized LDL,9,11 administration of TNF-α12 or endotoxin,13 and during hyperglycemia14) and after stimulation with thrombin,15 atrial natriuretic peptide,16 or abnormal blood shear stress.17,18 Consequences of glycocalyx perturbation include a wide range of vascular abnormalities in experimental models, including increased vascular permeability followed by generation of tissue edema,19 increased rolling and adhesion of leukocytes,6 and increased platelet adhesion.9 Therefore, disruption of the glycocalyx leads to enhanced sensitivity of vasculature to atherogenic stimuli. Based on these observations, the importance of integrity of the endothelial glycocalyx in vascular homeostasis has become evident.
Attempts to assess the impairment of endothelial function in vivo are a challenge given the multifunctional nature of endothelial cells and lack of standardized tools to noninvasively assess endothelial function in a patient-friendly manner. We recently developed an imaging-based method to detect changes in glycocalyx dimension from in vivo recordings of the sublingual microcirculation, enabling us to assess the microvascular glycocalyx in vivo in patients. Previous studies have shown that, in healthy volunteers, the glycocalyx is disrupted by acute hyperglycemia.14 Subsequently, a significant reduction in glycocalyx volume was found in patients with type 1 diabetes.20 This disruption may contribute to the known predisposition of these patients to vascular disease.
No data are available on the state of the endothelial glycocalyx in patients with chronic renal failure. However, it is reasonable to hypothesize that the endothelial glycocalyx is affected in these patients given their predisposition to endothelial dysfunction and vascular disease. A damaged glycocalyx may lead to increased vulnerability and susceptibility of endothelial cells to vascular risk factors present in uremia. Therefore, the objective of this study was to answer the following questions. (1) Is the microvascular endothelial glycocalyx damaged in patients with ESRD on both hemodialysis (HD) and peritoneal dialysis (PD) compared with age- and sex-matched healthy controls? (2) Do dialysis patients have increased serum concentrations of glycocalyx constituents reflecting increased shedding? (3) Do the changes in endothelial glycocalyx correlate with other serum markers of endothelial activation, like sE-selectin?
Results
Clinical Characteristics
Clinical characteristics of the healthy controls and dialysis patients are listed in Table 1. Dialysis patients had significantly higher systolic and diastolic BPs, a different lipid profile, and increased levels of C-reactive protein (CRP) and malondialdehyde (MDA) compared with healthy controls. Residual renal function was present in 35% of our dialysis group. IL-6 levels were available in 24 dialysis patients and had a median of 8.6 (4.8–10.0) pg/ml. We found a positive correlation between IL-6 and CRP levels (r=0.58, P<0.01).
Table 1.
Baseline characteristics of study participants
| Parameter | Healthy Controls (n=21) | Dialysis Patients (n=40) | P Value |
|---|---|---|---|
| Sex male/female | 12/9 | 25/15 | 0.78 |
| Age (yr) | 44.1 (14.1) | 44.1 (15.1) | 0.99 |
| BMI (kg/m2) | 22.6 (2.5) | 23.0 (8.0) | 0.43 |
| Systolic BP (mmHg) | 121.7 (13.4) | 147.7 (26.2) | <0.01 |
| Diastolic BP (mmHg) | 75.0 (6.0) | 91.5 (17.4) | <0.01 |
| Time on dialysis (mo) | 50.6 (15.4–103.6) | ||
| Time on RRT (mo) | 55.6 (17.4–167.4) | ||
| Glucose (mmol/L) | 4.9 (0.5) | 5.2 (0.9) | 0.20 |
| Total cholesterol (mmol/L) | 5.1 (0.9) | 4.3 (0.9) | <0.01 |
| LDL cholesterol (mmol/L) | 3.1 (0.8) | 2.4 (0.7) | <0.01 |
| HDL cholesterol (mmol/L) | 1.6 (0.5) | 1.1 (0.3) | <0.01 |
| Triglycerides (mmol/L) | 0.9 (0.7–1.0) | 1.5 (1.1–2.1) | <0.01 |
| CRP (mg/L) | 1.0 (1.0–1.0) | 2.3 (1.0–9.1) | <0.01 |
| ALAT (U/L) | 22.0 (5.8) | 19.4 (10) | 0.27 |
| MDA (µM/L) | 1.8 (1.6–2.5) | 3.9 (3.1–17.4) | <0.01 |
| RRF present (%) | 35 |
Results are expressed as mean (SD) except for time on dialysis, time on RRT, triglycerides, CRP, and MDA, which are expressed as median (interquartile range). BMI, body mass index; ALAT, alanine aminotransferase.
Imaging of the Microcirculation
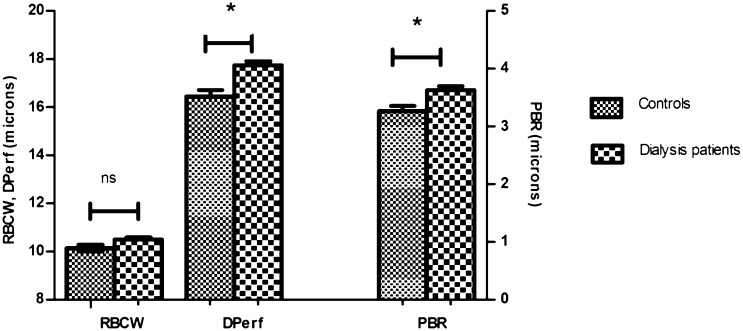
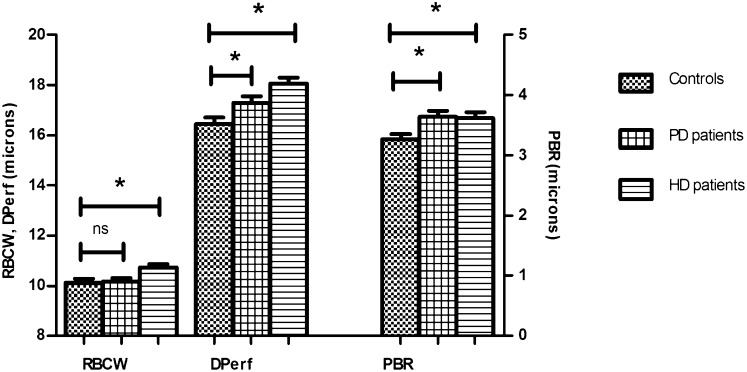
The perfused diameter (DPerf) and perfused boundary region (PBR) were increased in patients compared with healthy controls: DPerf, 17.7 (1.1) versus 16.4 (1.3) µm, P<0.01; PBR, 3.6 (0.5) versus 3.3 (0.4) µm, P<0.01 (Figure 1). No significant difference was found in red blood cell column width (RBCW): 10.5 (0.7) versus 10.1 (0.6) µm, P=0.06. To test whether both PD and HD patients behaved the same way, subgroup analysis was performed (Figure 2). Both PD and HD patients showed a significant increase in DPerf and PBR compared with healthy controls: DPerf, 16.4 (1.3) µm in controls, 17.3 (1.0) µm in PD (P=0.03), and 18.1 (1.1) µm in HD (P<0.01); PBR, 3.3 (0.4) µm in controls, 3.6 (0.4) µm in PD (P<0.01), and 3.6 (0.5) µm in HD (P=0.02). We found no correlation between the imaging parameters and BP, parameters of lipid profile, CRP, time on renal replacement therapy (RRT), or time on dialysis (data not shown).
Figure 1.
Results of imaging parameters in dialysis patients and healthy controls. Dialysis patients had increased RBC DPerf and PBR compared with healthy controls. *P<0.05. ns, nonsignificant.
Figure 2.
Results of imaging parameters in PD patients, HD patients, and healthy controls. Both PD (n=17) and HD (n=23) patients had significant alterations of RBC DPerf and PBR compared with healthy controls (n=21). *P<0.05.
Glycocalyx Constituents and Their Regulating Enzymes
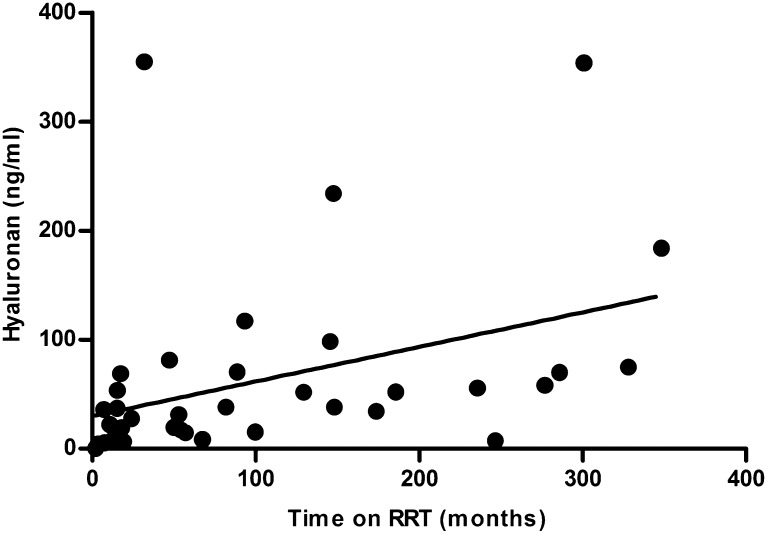
Serum levels of hyaluronan (HA), hyaluronidase activity, syndecan-1, and E-selectin were higher in patients compared with healthy controls (Table 2). We did not find any correlation between these parameters and the imaging parameters, CRP, or parameters of lipid profile (data not shown). In dialysis patients, HA levels positively correlated with total time on RRT (Figure 3) and time on dialysis (r2=0.24, P<0.01), and hyaluronidase activity was positively correlated with MDA levels (r=0.57, P<0.01). There was no correlation between HA levels and hyaluronidase activity (data not shown).
Table 2.
Serum levels of glycocalyx constituents, hyaluronidase activity, and sE-selectin in the study groups
| Parameter | Healthy Controls | Dialysis Patients | P Value |
|---|---|---|---|
| HA (ng/ml) | 16.8 (6.4–29.5) | 35.9 (14.5–70) | 0.02 |
| Hyaluronidase activity (U/ml) | 24.1 (20.9–26.9) | 28.2 (23.9–36.0) | 0.01 |
| Syndecan-1 (ng/ml) | 27.5 (18.9–33.7) | 111.0 (79.8–154.0) | <0.01 |
| sE-selectin (ng/ml) | 20.9 (14.3–30.6) | 47.6 (27.1–67.6) | <0.01 |
Results are presented as median (interquartile range).
Figure 3.
Relationship between serum levels of HA and total time on RRT in dialysis patients. A positive correlation was present between serum levels of HA and time on RRT (r2=0.32, P=0.0002).
Effect of Residual Renal Function
Within our dialysis group, the presence of residual renal function (RRF) seemed to have no influence on the degree of alterations in microcirculation as determined by Sidestream Darkfield (SDF) imaging. Patients without RRF had increased levels of HA and decreased hyaluronidase activity and sE-selectin compared with those patients with preserved RRF. Furthermore, this group of patients had a longer time on RRT (Table 3).
Table 3.
Effect of RRF on outcome parameters
| Parameter | Dialysis (RRF+) | Dialysis (RRF−) | P Value |
|---|---|---|---|
| Imaging parameters | |||
| RBCW (µm) | 10.40 (0.74) | 10.55 (0.69) | 0.49 |
| DPerf (µm) | 17.71 (1.15) | 17.75 (1.13) | 0.90 |
| PBR (µm) | 3.69 (0.45) | 3.58 (0.45) | 0.44 |
| Biochemical parameters | |||
| HA (ng/ml) | 19.2 (6.6–61.1) | 44.7 (28.5–71.4) | 0.04 |
| Hyaluronidase activity (U/ml) | 31.6 (26.4–38.9) | 24.9 (23.4–31.7) | 0.04 |
| Syndecan-1 (ng/ml) | 95.3 (68.1–159.5) | 116.6 (90.7–152.8) | 0.69 |
| sE-selectin (ng/ml) | 56.6 (12.9–154.0) | 43.8 (23.5–53.2) | 0.03 |
| Time on RRT (mo) | 17.9 (11.5–39.5) | 137.5 (64.8–238.5) | <0.01 |
Results of imaging parameters are presented as mean (SD). Results of biochemical parameters and time on RRT are presented as median (interquartile range). RRF+, RRF is present; RRF−, RRF is absent.
Effect of Inflammation
Patients were divided in two groups based on CRP levels higher or lower than 10 mg/L. Patients with higher levels of CRP seemed to have a significant increase in PBR (3.91 [0.56] versus 3.55 [0.39] µm, P=0.03) and sE-selectin (55.4 [44.1–81.2] versus 45.6 [26.1–61.1] ng/ml, P=0.04). No significant differences were observed with regard to the other biochemical or imaging parameters (Table 4).
Table 4.
Effect of inflammation on outcome parameters
| Parameter | Dialysis (CRP<10 mg/L) | Dialysis (CRP>10 mg/L) | P Value |
|---|---|---|---|
| Imaging parameters | |||
| RBCW (µm) | 10.57 (0.75) | 10.22 (0.45) | 0.19 |
| DPerf (µm) | 17.68 (1.17) | 17.92 (0.97) | 0.48 |
| PBR (µm) | 3.55 (0.39) | 3.91 (0.56) | 0.03 |
| Biochemical parameters | |||
| HA (ng/ml) | 34.9 (15.0–69.3) | 51.5 (7.2–84.1) | 0.92 |
| Hyaluronidase activity (U/ml) | 28.3 (24.1–34.6) | 26.4 (22.9–37.5) | 0.71 |
| Syndecan-1 (ng/ml) | 111.0 (76.8–165.5) | 107.0 (80.5–144.5) | 0.74 |
| sE-selectin (ng/ml) | 45.6 (26.1–61.1) | 55.4 (44.1–81.2) | 0.04 |
| Time on RRT (mo) | 52.6 (16.7–147.8) | 129.3 (15.2–281.4) | 0.52 |
Results of imaging parameters are presented as mean (SD). Results of biochemical parameters and time on RRT are presented as median (interquartile range).
Cardiovascular Disease
Six patients (15%) had a history of documented cardiovascular disease (CVD): coronary artery disease, cerebrovascular disease, or peripheral vascular disease. These patients seemed to have a significant increase in PBR and CRP compared with those patients without CVD (PBR, 4.04 [3.6–4.4] versus 3.5 [3.3–3.9] µm, P=0.04; CRP, 16.8 [4.5–34.5] versus 1.9 [1.0–4.9] mg/L, P=0.02). No significant differences were observed with regard to other biochemical or imaging parameters (Table 5).
Table 5.
Effect of presence of CVD on outcome parameters
| Parameter | Dialysis (CVD+) | Dialysis (CVD−) | P Value |
|---|---|---|---|
| Imaging parameters | |||
| RBCW (µm) | 10.19 (9.66–10.44) | 10.50 (10.00–11.03) | 0.14 |
| DPerf (µm) | 17.75 (17.35–18.24) | 17.59 (17.15–18.24) | 0.87 |
| PBR (µm) | 4.04 (3.57–4.40) | 3.50 (3.32–3.87) | 0.05 |
| Biochemical parameters | |||
| HA (ng/ml) | 58.1 (3.8–107.6) | 34.9 (15.02–69.18) | 0.83 |
| Hyaluronidase activity (U/ml) | 33.1 (23. –39.9) | 27.8 (24.0–32.7) | 0.45 |
| Syndecan-1 (ng/ml) | 101.2 (64.1–149.0) | 116.5 (84.7–158.3) | 0.52 |
| sE-selectin (ng/ml) | 51.8 (38.6–76.8) | 45.9 (26.9–63.7) | 0.38 |
| CRP (mg/L) | 16.8 (4.5–34.5) | 1.9 (1.0–4.9) | 0.02 |
| Time on RRT (mo) | 119.4 (9.8–292.1) | 53.6 (17.4–154.6) | 0.68 |
Results are presented as median (interquartile range).
Discussion
Analysis of the spatial and temporal variations of erythrocyte column width in the sublingual microvasculature reveals a significant increase in the dimension of the erythrocyte-permeable region bordering the red blood cell (RBC) column in dialysis patients compared with healthy controls. The increased PBR and the corresponding increased dimension of the erytrocyte DPerf are consistent with deeper penetration of RBCs into glycocalyx on the luminal surface of the endothelium (i.e., loss of glycocalyx barrier properties). Additionally, we found increased serum levels of HA and syndecan-1 in patients, consistent with shedding of these components from the vascular wall. To our knowledge, this study is the first to examine the endothelial glycocalyx using noninvasive microvascular imaging and report increased serum levels of glycocalyx constituents and regulating enzyme in patients with ESRD.
The presence of glycocalyx prevents RBCs from approximating the endothelium very closely.5,21,22 Previous studies, in which microvascular glycocalyx was assessed in humans by SDF imaging of the sublingual microvasculature,20 together with previous experimental studies11,23 have led to the concept that perturbation of the glycocalyx is associated with an impairment of its RBCs-excluding properties, affecting the temporal and spatial variations of the microvascular RBC column width. Similarly, recent experimental studies showed that enzymatic degradation of glycocalyx is followed by increases in PBR.24,25 The PBR reflects how far RBCs can penetrate into the erythrocyte-accessible part of the cell-free layer. The increase in PBR found in dialysis patients is consistent with increased penetration of RBCs into glycocalyx. Overall, our findings imply that analysis of dynamic microvascular RBCs column width variations can be used to detect pathophysiological changes near the microvascular wall in dialysis patients.
In the setting of CKD, several factors may contribute to the alteration of the endothelial glycocalyx; however, the exact mechanisms responsible still remain to be elucidated. The vascular endothelial dysfunction in CKD is associated with a chronic inflammatory state.26 In our study, patients with higher levels of inflammation as measured by CRP had an increased PBR compared with those patients with lower levels of CRP. This finding is indicative for a more pronounced alteration in glycocalyx barrier properties in this group. CKD is also associated with a chronic deficiency in antioxidant systems.27 Increased oxidative stress acts as a major contributor to severe atherosclerosis and cardiovascular morbidity and mortality found in these patients,26 and reactive oxygen species can mediate alterations of the endothelial glycocalyx.10 Overhydration is another common problem in dialysis patients, especially after loss of RRF; also, hypervolemia can alter the endothelial surface layer potentially through atrial natriuretic peptide, which causes shedding of glycocalyx constituents in blood.16 Loss of RRF is associated with progressive impairment of endothelial function and is a risk factor of cardiovascular mortality in dialysis patients.28 In the present study, we found significantly higher levels of HA in the anuric group compared with patients with RRF, but the SDF imaging of the microcirculation did not detect significant differences between the two groups.
One of the earliest changes in the endothelium upon activation is an alteration in the glycocalyx composition, with shedding of its constituents into the circulation. Here, we investigated the plasma levels of the glycocalyx constituents HA, syndecan-1, and the regulating enzyme hyaluronidase. HA, a high mol wt polysaccharide, is an important component of the endothelial glycocalyx. It serves as a mechano-shear sensor, regulates NO release, and maintains vascular permeability.29–31 In vitro studies have shown that inflammatory cytokines, particularly IL-1, IL-6, and TNF-α, as well as reactive oxygen species are involved in the degradation of HA.32–34 High serum levels of HA have been reported in CKD35–37 and seem to be a risk predictor of poor survival in dialysis.38 However, the mechanisms underlying the increased levels have not been fully explained. They may reflect altered connective tissue metabolism in uremia. Shedding of HA from the endothelial glycocalyx on the vascular wall may be another cause. In the present study, the high HA levels found in patients were positively correlated with the duration of dialysis treatment, confirming previously reported data.39,40 Although the correlation was highly significant, the explained variance was small: only 24% of the variability of HA was explained by its relationship with the duration of the dialysis treatment. This finding makes it likely that complex mechanisms are responsible for the perturbation of HA metabolism. In general, increased HA production and turnover are associated with increased hyaluronidase levels, and our results support these findings. The fact that other glycosaminoglycans than HA, like heparan sulfate (HS) or chondroitin sulfate, are bound by hyaluronidase may explain the lack of correlation between HA levels and hyaluronidase activity. Syndecans are a family of four transmembrane HSPGs that is the major source of cellular HS.41 Syndecan-1 can be shed through a proteolitic42 or oxidative mechanism43,44 and functions as soluble HSPGs in the extracellular milieu. Syndecan shedding is stimulated in vitro by inflammatory factors and activated in vivo under inflammatory conditions.45 The increased heparanase activity in HD patients46,47 may contribute to shedding of syndecan-1 from the endothelial glycocalyx48 and may lead to acceleration of the atherosclerotic process. To our knowledge, this study is the first to specifically investigate the syndecan-1 levels in dialysis patients.
Serum E-selectin, a surrogate marker of endothelial activation, is also increased in dialysis patients.49,50 Increased synthesis/release after cytokine stimulation51 and inadequate clearance because of renal failure can both be responsible for the high levels. We found similar increased levels of sE-selectin in our dialysis group but no relationship with the changes in imaging parameters or serum levels of glycocalyx constituents.
Several recent studies support the hypothesis that disturbance of the glycocalyx accelerates atherosclerosis. The inhibition of HA synthesis in a murine model of atherosclerosis led to enhanced inflammatory and thrombotic responses and increased atherosclerosis.52 In another murine model, the glycocalyx was decreased both after an atherogenic diet and at lesion-prone locations in the vascular tree, and this thin glycocalyx was accompanied by greater intima-to-media ratios.53 These findings support the hypothesis that perturbation of the glycocalyx interferes with its protective function and contributes to increased vascular vulnerability. This may be also true for CKD, where a damaged endothelial glycocalyx may increase the susceptibility of the endothelial cells to vascular risk factors present in uremia.
Interestingly, we found that patients with CVD had increased PBR compared with patients without CVD. These findings were not supported by a significant increase in plasma levels of HA or syndecan-1. This suggests that the alterations of the endothelial glycocalyx present in dialysis patients are already so severe that the presence of CVD is not accompanied by additional significant shedding of its constituents. Whether our imaging method is able to identify a more severe alteration of the glycocalyx in this group of patients needs to be confirmed by other studies. Here, the number of patients is too small to allow any definite conclusions.
One of the limitations of our study is the cross-sectional design. Future studies should focus on specific longitudinal effects of dialysis treatment on the glycocalyx. Furthermore, ESRD is associated with alterations in RBC mechanical properties and changes in rheology (hematocrit and plasma viscosity),54–56 and additional studies are needed to investigate their effect on PBR as measured by SDF imaging. Here, we did not find any correlation between the imaging parameters and the hemoglobin levels of the patients (results not shown). Another issue that needs additional investigation is the effect of local hematocrit in microvasculature on the imaging parameters. To answer this question, a stepwise controlled reduction in hematocrit independent of endothelial glycocalyx dimension should be obtained in a controlled experimental setting. A strong point of our study is the well documented phenotype of all dialysis patients, including measurement of RRF.
In conclusion, in this study, we show that dialysis patients have loss of glycocalyx barrier properties as estimated by analysis of the dynamic variations of erythrocyte column width in the sublingual microcirculation. Additionally, we found high levels of HA and syndecan-1 in blood, consistent with increased shedding of glycocalyx from the vascular wall. Impaired glycocalyx barrier properties, together with shedding of its constituents into blood, are consistent with sustained pathogenic endothelial cell activation in dialysis patients and probably contribute to the aggressive vascular pathology present in this group of patients.
The SDF imaging of the microcirculation provides a direct, noninvasive, and fast method for the assessment of the endothelial glycocalyx, whereas the plasma levels of glycocalyx constituents are only an indirect measure of its integrity. The state of endothelial glycocalyx and its circulating components show great promise as markers of endothelial dysfunction, and their measurement may be of value in the clinical setting. They could provide valuable tools to monitor vascular vulnerability, detect early stages of disease, evaluate risk, and judge the response of patients with kidney disease to treatment.
Concise Methods
Study Design and Subjects
We performed a single-center cross-sectional observational study to determine the status of endothelial glycocalyx and plasma levels of glycocalyx constituents in 17 PD patients, 23 HD patients, and 21 age- and sex-matched healthy controls. All patients were recruited from the Dialysis Center of the Academic Medical Center, University of Amsterdam, between August of 2008 and May of 2009. Patients were excluded if one of the following applied: diabetes mellitus, any acute inflammatory episode, use of antioxidants, use of statins 6 weeks before the measurements, or use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers on the day of the measurements.
The study was carried out in accordance with the principles of the Declaration of Helsinki. Approval was obtained from the Committee of Medical Ethics of the Academic Medical Center, University of Amsterdam, and all participants gave written informed consent.
Blood Sampling and General Laboratory Measurements
Blood samples were drawn after an overnight fast from healthy volunteers and most of the patients. Patients who were scheduled for HD in the afternoon were asked to fast for at least 3 hours before the measurement, and blood was drawn before the dialysis session. BP was measured in triplicate, and the last two measurements were averaged to determine systolic and diastolic BPs.
All standard laboratory measurements were performed on a Hitachi P-800 (Roche Diagnostics, Mannheim, Germany). All reagents were provided by the same company. Glucose, creatinine, alanine aminotransferase, urea, and triglycerides were measured by standard enzymatic methods. Total cholesterol and HDL cholesterol were measured with colorimetry. LDL cholesterol was calculated using the Friedewald formula. CRP was measured using an immunoturbidimetric assay. IL-6 levels were measured with a commercially available ELISA (R&D Systems Europe Ltd., Abingdon, United Kingdom).
For additional analysis, plasma aliquots were snap-frozen and stored at −80°C. RRF was expressed as the residual GFR, which was calculated as the mean of creatinine and urea clearance obtained from a 24-hour urine collection in dialysis patients. Presence of RRF was defined as residual GFR≥1 ml/min.
Measurements of Glycocalyx Constituents and Regulating Enzyme
HA (Corgenix Inc., Broomfield, CO), hyaluronidase activity (ELISA previously described in the work by Nieuwdorp et al.20), and syndecan-1 (Diaclone; Gen-Probe Inc., CA) were determined in all participants by ELISAs.
Measurements of Endothelial Activation
Serum E-selectin was measured in all participants by a commercially available ELISA (Quantikine; R&D Systems, Ltd., United Kingdom).
Oxidative Stress
Plasma MDA concentrations were determined by HPLC using a previously described method57 with some adaptations (Supplemental Data 1 has a detailed description).
Imaging of the Microcirculation
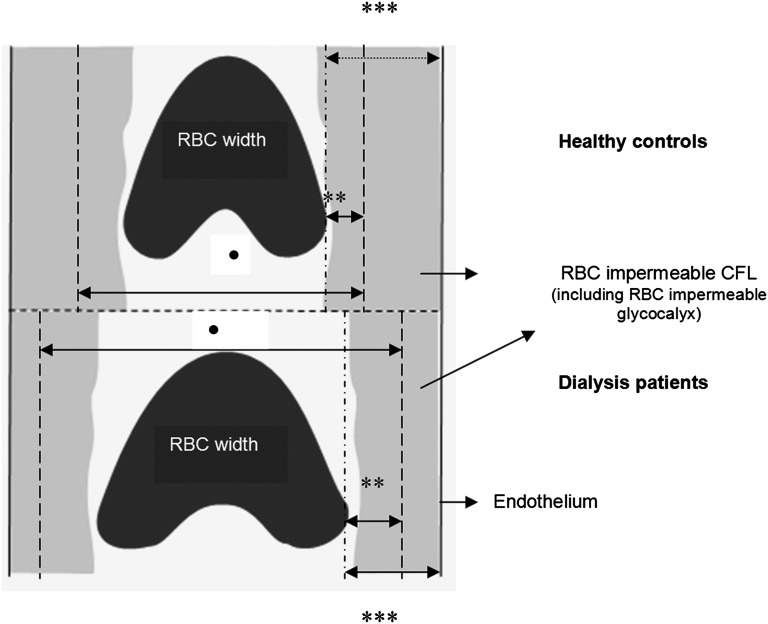
We performed intravital microscopic imaging of the sublingual microvasculature using an SDF MicroScan videomicroscope (MicroVision Medical Inc., Wallingford, PA) (Supplemental Data 2). In HD patients, the measurements were performed before the dialysis session. Images were collected with a 5× objective with a 0.2 NA, providing a 325-fold magnification on screen, and they were sized 720×576 pixels. The frame rate was 23/s. Video sequences of 2 seconds each were recorded using Streampix software (Norpix Inc., Montreal, Canada) in at least 10 areas close to the frenulum. Movies consisted of 40 consecutive frames of 950×700 µm sublingual tissue surface area. The first frame of each movie was used to automatically identify all available microvessels, and measurement lines perpendicular to the vessel direction were placed automatically every 10 µm along each visible microvessel. All vessels with a diameter of 50 µm and larger were excluded. Each line represented a measurement site; at each measurement site, a total of 21 parallel (every ±0.5 μm) intensity profiles was plotted using ImageJ (National Institutes of Health, Bethesda, MD), and RBCW (full width half maximum) was determined at each line for all 40 consecutive frames in a movie, revealing a total of 840 RBCW measurements at a measurement site (21 profiles×40 frames). The RBCW showed considerable variation in these 40 frames. The associated (cumulative) distributions of the RBCWs for these 840 measurements were used to determine median RBCW (P50) as well as lower and upper percentiles of the RBCW distribution. To assess the position of the outer edge of the RBC perfused lumen at each measurement site, the RBC DPerf was derived from the RBCW distribution by linear extrapolation of all RBCW percentiles between P25 and P75. The PBR was defined as the distance of median (P50) RBCW to the outer edge of the extrapolated Dperf as shown in Figure 4. Approximately 100–300 measurement sites were indentified per video recording, giving approximately 1000–3000 measurements of median RBCW, PBR, and DPerf per patient.
Figure 4.
Schematic illustration of endothelial glycocalyx imaging method. In dialysis patients, perturbation of glycocalyx allows the erythrocytes to approach the vessel wall, leading to increased DPerf and PBR compared with healthy controls. RBC width, median RBCW. •DPerf is the perfused diameter (RBC perfused lumen). **PBR is the perfused boundary region (RBC-permeable part of the cell-free layer including cell-permeable glycocalyx). ***Cell-free layer.
Reproducibility data were acquired by performing SDF imaging on two separate days in 16 healthy volunteers (unpublished data) (Supplemental Data 3). The observed changes in dialysis patients were significantly greater than the differences between visits of healthy volunteers.
Outcome Measures
The primary outcome was the difference in sublingual endothelial glycocalyx dimension as determined by SDF imaging between the dialysis patients and healthy volunteers. Our study parameters were median RBCW, DPerf, and PBR. We also tested whether the treatment modality (HD or PD) had any influence on the degree of alterations of the imaging parameters. Secondary outcomes were the differences in plasma levels of glycocalyx constituents and a marker of endothelial activation between the dialysis patients and controls. We measured HA, syndecan-1, hyaluronidase activity, and sE-selectin. The correlations between the outcome parameters and total time on RRT and time on dialysis were tested in the dialysis patients. Additionally, we investigated the effect of RRF, inflammation, oxidative stress, and presence of CVD on the outcome parameters.
Statistical Methods
Results are expressed as means (SD) or medians (interquartile range) depending on the distribution of the data. For baseline characteristics, continuous variables were compared with the use of either an unpaired t test or Mann-Whitney test depending on the distribution of the data. Categorical variables were compared with the use of the chi-squared or Fisher exact test where appropriate. Analysis was performed using SPSS version 16.0 (Chicago, IL). A P value<0.05 was defined as statistically significant. The correlations between different parameters were tested using either Pearson or Spearman correlation test depending on the distribution of the data. Only correlations with a P value<0.01 were considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the Nephron Foundation for the financial support. Dr. J.A. Bijlsma, Dr. I. Keur, Dr. W. Smit, and Dr. J.M.R. Willemsen are gratefully acknowledged for their contribution to the inclusion of patients. We also thank D. Lopes-Barreto, J. Sierts, S.L. Yong, and D.R. de Waart for their excellent laboratory support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011121181/-/DCSupplemental.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Schiffrin EL, Lipman ML, Mann JFE: Chronic kidney disease: Effects on the cardiovascular system. Circulation 116: 85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Wheeler DC, Townend JN: Abnormalities of endothelial function in patients with predialysis renal failure. Heart 83: 205–209, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Pries AR, Secomb TW, Gaehtgens P: The endothelial surface layer. Pflugers Arch 440: 653–666, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Constantinescu AA, Vink H, Spaan JAE: Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol 23: 1541–1547, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Nieuwdorp M, Meuwese MC, Vink H, Hoekstra JB, Kastelein JJ, Stroes ES: The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr Opin Lipidol 16: 507–511, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG: The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch 454: 345–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vink H, Constantinescu AA, Spaan JAE: Oxidized lipoproteins degrade the endothelial surface layer: Implications for platelet-endothelial cell adhesion. Circulation 101: 1500–1502, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Rubio-Gayosso I, Platts SH, Duling BR: Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 290: H2247–H2256, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Constantinescu AA, Vink H, Spaan JAE: Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am J Physiol Heart Circ Physiol 280: H1051–H1057, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Henry CBS, Duling BR: TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 279: H2815–H2823, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Nieuwdorp M, Meuwese MC, Mooij HL, van Lieshout MHP, Hayden A, Levi M, Meijers JCM, Ince C, Kastelein JJP, Vink H, Stroes ESG: Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis 202: 296–303, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Nieuwdorp M, van Haeften TW, Gouverneur MCLG, Mooij HL, van Lieshout MHP, Levi M, Meijers JCM, Holleman F, Hoekstra JBL, Vink H, Kastelein JJP, Stroes ESG: Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55: 480–486, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Subramanian SV, Fitzgerald ML, Bernfield M: Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem 272: 14713–14720, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, Becker BF: Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol 289: H1993–H1999, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H: Vasculoprotective properties of the endothelial glycocalyx: Effects of fluid shear stress. J Intern Med 259: 393–400, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Gouverneur M, Spaan JAE, Pannekoek H, Fontijn RD, Vink H: Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 290: H458–H462, 2006 [DOI] [PubMed] [Google Scholar]
- 19.van den Berg BM, Vink H, Spaan JAE: The endothelial glycocalyx protects against myocardial edema. Circ Res 92: 592–594, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JAE, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JBL, Kastelein JJP, Stroes ESG, Vink H: Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55: 1127–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Pries AR, Secomb TW, Jacobs H, Sperandio M, Osterloh K, Gaehtgens P: Microvascular blood flow resistance: Role of endothelial surface layer. Am J Physiol 273: H2272–H2279, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Desjardins C, Duling BR: Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol 258: H647–H654, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Van Teeffelen JW, Brands J, Stroes ES, Vink H: Endothelial glycocalyx: Sweet shield of blood vessels. Trends Cardiovasc Med 17: 101–105, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Potter D, van den Berg B, Vink H: Inhibiting the production of hyaluronan disrupts the endothelial glycocalyx and impairs arteriogenesis [Abstract]. FASEB J 25: 633.3, 2011 [Google Scholar]
- 25.Eskens B, Cobelens H, Vink H, van Teeffelen J: Enzymatic glycocalyx treatment impairs insulin-mediated recruitment of microvascular blood volume and decreases insulin sensitivity in rats [Abstract]. FASEB J 25: 1023.13, 2011 [Google Scholar]
- 26.Bolton CH, Downs LG, Victory JGG, Dwight JF, Tomson CRV, Mackness MI, Pinkney JH: Endothelial dysfunction in chronic renal failure: Roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant 16: 1189–1197, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, Nguyen AT, Thévenin M, Jaudon MC, Zingraff J, Verger C, Jungers P, Descamps-Latscha B: Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med 21: 845–853, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, Movilli E, Pola A, d’Avolio G, Gelatti U: Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant 10: 2295–2305, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Henry CBS, Duling BR: Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol Heart Circ Physiol 277: H508–H514, 1999 [DOI] [PubMed]
- 30.Thi MM, Tarbell JM, Weinbaum S, Spray DC: The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: A “bumper-car” model. Proc Natl Acad Sci U S A 101: 16483–16488, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC: Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A 100: 7988–7995, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moseley R, Waddington RJ, Embery G: Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. Biochim Biophys Acta 1362: 221–231, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Vigetti D, Genasetti A, Karousou E, Viola M, Moretto P, Clerici M, Deleonibus S, De Luca G, Hascall VC, Passi A: Proinflammatory cytokines induce hyaluronan synthesis and monocyte adhesion in human endothelial cells through hyaluronan synthase 2 (HAS2) and the nuclear factor-kappaB (NF-kappaB) pathway. J Biol Chem 285: 24639–24645, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yung S, Coles GA, Davies M: IL-1 beta, a major stimulator of hyaluronan synthesis in vitro of human peritoneal mesothelial cells: Relevance to peritonitis in CAPD. Kidney Int 50: 1337–1343, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Woodrow G, Turney JH, Davison AM, Cooper EH: Serum hyaluronan concentrations predict survival in patients with chronic renal failure on maintenance haemodialysis. Nephrol Dial Transplant 11: 98–100, 1996 [PubMed] [Google Scholar]
- 36.Pannekeet MM, Zemel D, Koomen GC, Struijk DG, Krediet RT: Dialysate markers of peritoneal tissue during peritonitis and in stable CAPD. Perit Dial Int 15: 217–225, 1995 [PubMed] [Google Scholar]
- 37.van Esch S, Zweers MM, Jansen MA, de Waart DR, van Manen JG, Krediet RT: Determinants of peritoneal solute transport rates in newly started nondiabetic peritoneal dialysis patients. Perit Dial Int 24: 554–561, 2004 [PubMed] [Google Scholar]
- 38.Stenvinkel P, Heimbürger O, Wang T, Lindholm B, Bergström J, Elinder CG: High serum hyaluronan indicates poor survival in renal replacement therapy. Am J Kidney Dis 34: 1083–1088, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Lipkin GW, Forbes MA, Cooper EH, Turney JH: Hyaluronic acid metabolism and its clinical significance in patients treated by continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 8: 357–360, 1993 [PubMed] [Google Scholar]
- 40.Turney JH, Davison AM, Forbes MA, Cooper EH: Hyaluronic acid in end-stage renal failure treated by haemodialysis: Clinical correlates and implications. Nephrol Dial Transplant 6: 566–570, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M: Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68: 729–777, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Park PW, Wilson CL, Parks WC: Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111: 635–646, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, Oury TD: Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem 284: 3537–3545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kliment CR, Tobolewski JM, Manni ML, Tan RJ, Enghild J, Oury TD: Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid Redox Signal 10: 261–268, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartlett AH, Hayashida K, Park PW: Molecular and cellular mechanisms of syndecans in tissue injury and inflammation. Mol Cells 24: 153–166, 2007 [PubMed] [Google Scholar]
- 46.Cohen-Mazor M, Sela S, Mazor R, Ilan N, Vlodavsky I, Rops AL, van der Vlag J, Cohen HI, Kristal B: Are primed polymorphonuclear leukocytes contributors to the high heparanase levels in hemodialysis patients? Am J Physiol Heart Circ Physiol 294: H651–H658, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Sela S, Shurtz-Swirski R, Cohen-Mazor M, Mazor R, Chezar J, Shapiro G, Hassan K, Shkolnik G, Geron R, Kristal B: Primed peripheral polymorphonuclear leukocyte: A culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol 16: 2431–2438, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD: Heparanase enhances syndecan-1 shedding: A novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem 282: 13326–13333, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Bonomini M, Reale M, Santarelli P, Stuard S, Settefrati N, Albertazzi A: Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron 79: 399–407, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Malatino LS, Stancanelli B, Cataliotti A, Bellanuova I, Fatuzzo P, Rapisarda F, Leonardis D, Tripepi G, Mallamaci F, Zoccali C: Circulating E-selectin as a risk marker in patients with end-stage renal disease. J Intern Med 262: 479–487, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Pigott R, Dillon LP, Hemingway IH, Gearing AJH: Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun 187: 584–589, 1992 [DOI] [PubMed] [Google Scholar]
- 52.Nagy N, Freudenberger T, Melchior-Becker A, Röck K, ter Braak M, Jastrow H, Kinzig M, Lucke S, Suvorava T, Kojda G, Weber AA, Sörgel F, Levkau B, Ergün S, Fischer JW: Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: Novel insights into the role of hyaluronan synthesis. Circulation 122: 2313–2322, 2010 [DOI] [PubMed] [Google Scholar]
- 53.van den Berg BM, Spaan JAE, Rolf TM, Vink H: Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol 290: H915–H920, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Brimble KS, McFarlane A, Winegard N, Crowther M, Churchill DN: Effect of chronic kidney disease on red blood cell rheology. Clin Hemorheol Microcirc 34: 411–420, 2006 [PubMed] [Google Scholar]
- 55.Srour MA, Bilto YY, Juma M: Susceptibility of erythrocytes from non-insulin-dependent diabetes mellitus and hemodialysis patients, cigarette smokers and normal subjects to in vitro oxidative stress and loss of deformability. Clin Hemorheol Microcirc 22: 173–180, 2000 [PubMed] [Google Scholar]
- 56.Nowak-Piszczek E, Wyrwicz G, Dabrowski Z, Smoleński O, Spodaryk K: Deformability and enzymes activities of red blood cells in hemodialysed and peritoneal dialysed patients with chronic renal disease. Clin Hemorheol Microcirc 28: 251–257, 2003 [PubMed] [Google Scholar]
- 57.Agarwal R, Chase SD: Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 775: 121–126, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.