Abstract
Osteopontin is a proinflammatory cytokine and monocyte chemoattractant implicated in the pathogenesis of diabetic nephropathy. Synthetic agonists for liver X receptors (LXRs) suppress the expression of proinflammatory genes, including osteopontin, but whether LXR activation modulates diabetic nephropathy is unknown. We administered the LXR agonist T0901317 to mice with streptozotocin-induced diabetes and evaluated its effects on diabetic nephropathy. The LXR agonist decreased urinary albumin excretion without altering blood glucose levels and substantially attenuated macrophage infiltration, mesangial matrix accumulation, and interstitial fibrosis. LXR activation suppressed the gene expression of inflammatory mediators, including osteopontin, in the kidney cortex. In vitro, LXR activation suppressed osteopontin expression in proximal tubular epithelial cells by inhibiting AP-1–dependent transcriptional activation of the osteopontin promoter. Taken together, these results suggest that inhibition of renal osteopontin by LXR agonists may have therapeutic potential for diabetic nephropathy.
Osteopontin (OPN) is a secreted extracellular matrix protein and proinflammatory cytokine that has been identified as a key component of cell-mediated immunity.1 It is abundantly secreted by macrophages, and it mediates their recruitment and activation at sites of inflammation by regulating monocyte adhesion, migration, and inflammatory gene expression.2,3 OPN is expressed in atherosclerotic lesions, where it is abundantly synthesized by macrophages and to lesser extents, smooth muscle cells and endothelial cells.4 Using murine models, we and others have shown decreased atherosclerosis and macrophage accumulation in OPN-deficient mice,5,6 identifying OPN as a potential target for pharmacological intervention in atherosclerosis. In humans, the plasma OPN level is significantly correlated with the extent of coronary atherosclerosis.7,8 Moreover, the OPN levels are particularly elevated in patients with type 2 diabetes,9,10 and we showed that peroxisome proliferator-activated receptor α ligands suppress OPN expression in macrophages in vitro and that bezafibrate reduces plasma OPN levels in patients with type 2 diabetes.11 These clinical studies provide evidence that OPN may play a causal role in the development of cardiovascular disease in humans.
OPN is highly expressed in the kidney interstitium in models of diabetic nephropathy.12,13 Because OPN is a macrophage chemotactic protein, OPN expression and macrophage accumulation may play a role in the tubulointerstitial injury in diabetic nephropathy in rats13,14 and mice.15,16 Moreover, we and others have shown by DNA microarray studies that OPN gene expression is upregulated in the renal cortex and correlated with the degree of albuminuria in diabetic nephropathy,16–18 suggesting that regulation of OPN expression could be a potential target for therapeutic intervention in diabetic nephropathy. Furthermore, albuminuria and renal damage are ameliorated in OPN-deficient diabetic mice,19,20 also supporting the notion that OPN may be causally involved in the pathogenesis of diabetic nephropathy.
Liver X receptors (LXRs) are important regulators of cholesterol, free fatty acid, and glucose metabolism.21 In addition to their importance in lipid and glucose metabolism, LXR activation was recently found to regulate immune processes and inhibit inflammatory gene expression in macrophages.22,23 Synthetic LXR agonists were shown to prevent atherosclerosis in murine models and inhibit inflammation.22,24 We showed that LXR ligands T0901317 and GW3965 inhibit cytokine-induced OPN expression in macrophages.25 These effects are mediated by inhibition of the c-Jun/c-Fos DNA-binding activities to the proximal OPN promoter, which impairs activator protein-1 (AP-1)–dependent OPN transcription.25 These findings define a novel mechanism, where LXR ligands may impact macrophage inflammatory responses and atherosclerosis. However, the effects of LXR ligands on OPN expression in diabetic nephropathy remain unclear.
The purpose of the present study was to investigate the hypothesis that LXR activation prevents the development of diabetic nephropathy by inhibiting OPN expression and macrophage infiltration in the kidney interstitium.
Results
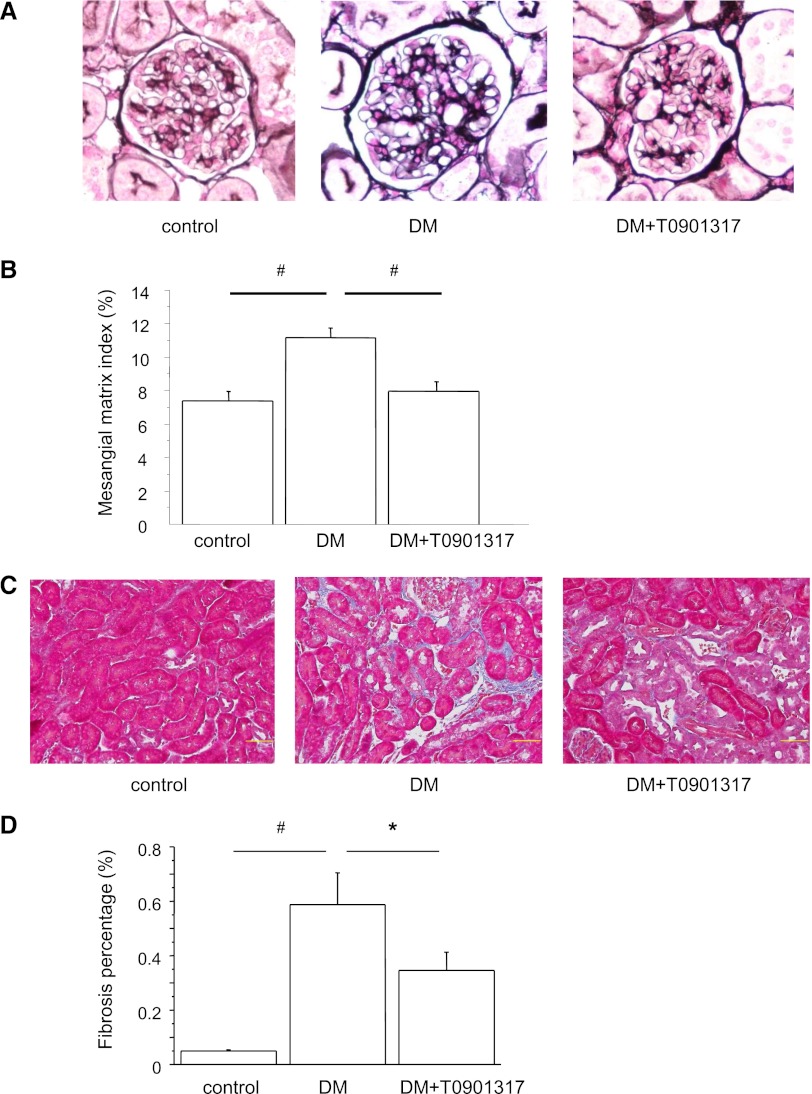
LXR Ligand Ameliorates Diabetic Nephropathy
The metabolic data are summarized in Table 1. At 8 weeks after diabetes induction by streptozotocin (STZ), there were no significant differences in the systolic BPs, kidney weights, and triglyceride levels between the three groups of nondiabetic control mice (control), STZ-induced diabetic mice (DM), and diabetic mice treated with LXR agonist T0901317 (DM+T0901317). Hemoglobin A1c (HbA1c) was significantly higher in the DM group than the control group, but there was no significant difference between the DM and DM+T0901317 groups. Urinary albumin excretion (UAE) increased in the diabetic mice during the study. However, T0901317 treatment significantly reduced the mean UAE compared with the DM group at 8 weeks after diabetes induction. Representative glomeruli in periodic acid–methenamine silver (PAM) -stained sections are shown in Figure 1A. Glomerular hypertrophy and mesangial matrix expansion were observed in the DM group at the end of the 8-week observation period. However, these changes were ameliorated in the DM+T0901317 group compared with the DM group (Figure 1B). Interstitial fibrosis was examined in Masson’s trichrome-stained renal sections (Figure 1C). The fibrotic area in the interstitium was larger in the DM group than the control group. The fibrotic area was markedly reduced in the DM+T0901317 group compared with the DM group (Figure 1D). Because LXR activation is known to increase lipogenesis, we measured the triglyceride levels and expressions of lipogenic enzymes, including sterol regulatory element-binding protein 1c (SREBP-1c), acetyl-coenzyme A carboxylase α, and fatty acid synthase. As shown in Supplemental Figure 1A, the gene expressions of SREBP-1c and acetyl-coenzyme A carboxylase α were increased in the DM+T0901317 group compared with the DM group. However, there were no significant differences in the triglyceride levels in serum (Table 1) and renal tissues (Supplemental Figure 1B) between the control, DM, and DM+T0901317 groups.
Table 1.
Metabolic data at 8 weeks after the induction of diabetes
| Control (n=8) | DM (n=8) | DM+T0901317 (n=8) | |
|---|---|---|---|
| Systolic BP (mmHg) | 102.7±6.7 | 115.9±3.8 | 113.2±2.7 |
| HbA1c (%) | 4.25±0.05 | 9.03±0.46a | 9.14±0.46a |
| Body weight (g) | 26.57±0.35 | 18.66±1.23a | 20.23±0.79a |
| Kidney weight (mg) | 273.7±7.3 | 311.3±20.0 | 278.6±10.1 |
| Relative kidney weight (mg/g body weight) | 11.23±0.46 | 17.22±1.54a | 13.86±0.52b |
| Triglycerides (mg/dl) | 25.3±2.1 | 43.0±14.7 | 42.9±8.1 |
| UAE (μg/24 h) | 9.3±1.2 | 82.8±11.7a | 52.9±7.5a,b |
Data are means ± SEM. Control, nondiabetic control mice; DM, untreated diabetic mice; DM+T0901317, T0901317-treated diabetic mice; HbA1c, hemoglobin A1c; UAE, urinary albumin excretion.
P<0.01 versus the control group.
P<0.01 versus the DM group.
Figure 1.
PAM staining and Masson’s trichrome staining of kidney sections. (A) Representative glomeruli of PAM-stained kidneys from control, DM, and DM+T0901317 mice. Glomerular hypertrophy and mesangial matrix expansion are evident in the DM group. Original magnification, ×400. (B) MMI in glomeruli. T0901317 suppresses the increase in the MMI compared with the DM group. Data are means ± SEM. *P<0.001. (C) Representative photomicrographs of kidney sections with Masson’s trichrome staining. Interstitial fibrosis is significantly increased in the DM group compared with the control group and significantly decreased in the DM+T0901317 group compared with the DM group. Original magnification, ×400. (D) Percentages of fibrosis in glomeruli. Data are means ± SEM. #P<0.001, *P<0.05.
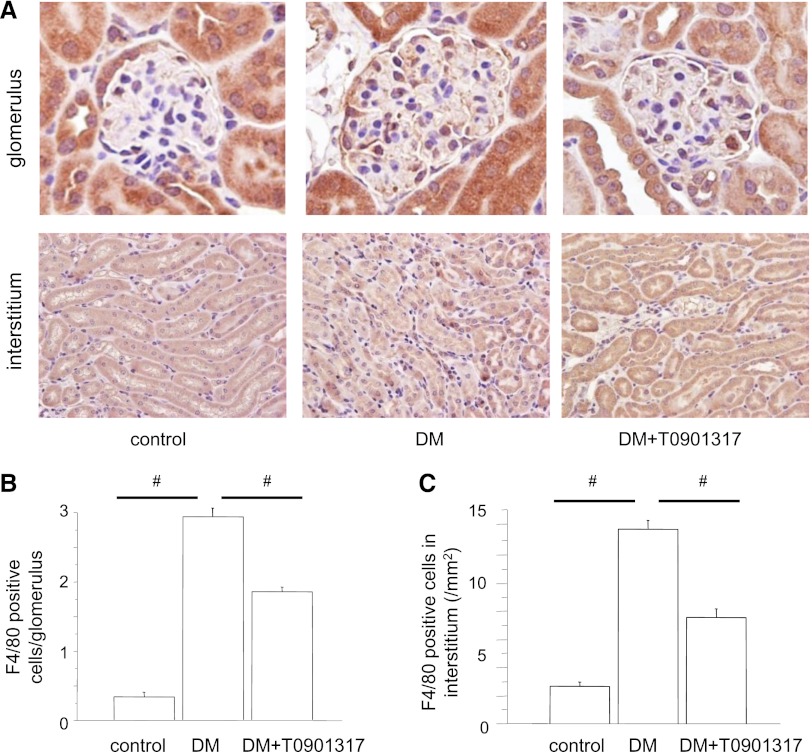
LXR Ligand Inhibits Macrophage Infiltration in the Kidney
The number of macrophages in the glomeruli was remarkably higher in the DM group than the control group. Interestingly, the macrophage infiltration into the glomeruli was significantly reduced in the DM+T0901317 group compared with the DM group (Figure 2, A and B). Similarly, the macrophage infiltration into the interstitium was increased in the DM group but suppressed in the DM+T0901317 group (Figure 2, A and 2C).
Figure 2.
Macrophage infiltration into the kidney. (A) The macrophage infiltration into the glomeruli and interstitium is remarkable in the DM group and suppressed in the DM+T0901317 group. Original magnification, ×400. (B) Numbers of intraglomerular macrophages. Data are means ± SEM. #P<0.001. (C) Numbers of macrophages in the interstitium. Data are means ± SEM. #P<0.001.
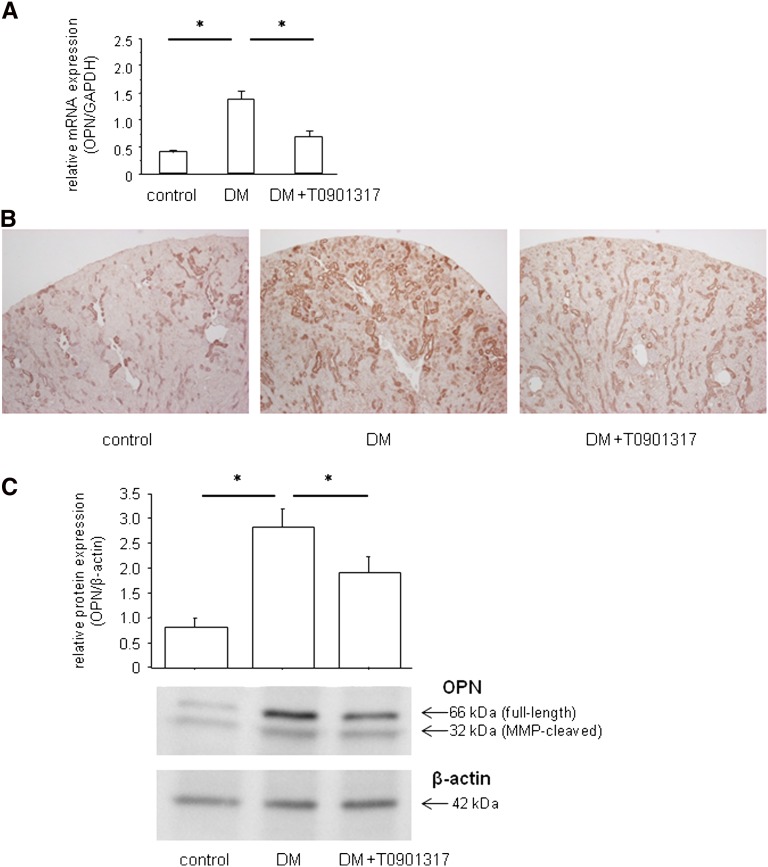
LXR Agonist Suppresses Diabetes-Induced OPN Expression in the Kidney
We detected OPN mRNA and protein expressions in the kidneys. At 8 weeks, renal OPN mRNA expression was significantly higher in the DM group than the control group (Figure 3A). Immunoperoxidase staining and Western blotting analysis also revealed that OPN protein expression in the kidney was significantly upregulated in the DM group compared with the control group (Figure 3, B and C). Treatment with T0901317 resulted in substantial suppression of the diabetes-induced OPN mRNA and protein expressions.
Figure 3.
LXR agonist suppresses diabetes-induced OPN mRNA and protein expressions in the kidney. (A) Total RNA was isolated from the kidney samples and analyzed for the OPN mRNA expression levels by qRT-PCR. Renal OPN mRNA expression is significantly increased in the DM group compared with the control group. The OPN expression was normalized by the GAPDH expression. Data are means ± SEM. *P<0.05. (B) Localization of renal OPN protein by immunohistochemistry. OPN protein is predominantly localized in the interstitium of the kidney in the DM group, and its expression is suppressed in the DM+T0901317 group compared with the DM group. Original magnification, ×400. (C) Western blotting analysis of OPN protein expression. Full-length OPN as well as the C-terminal fragment of MMP-cleaved OPN are significantly upregulated in the DM group compared with the control group, and they are suppressed in the DM+T0901317 group compared with the DM group. Quantification was performed by densitometry of three independently performed experiments, with normalization by β-actin. Data are means ± SEM. *P<0.05.
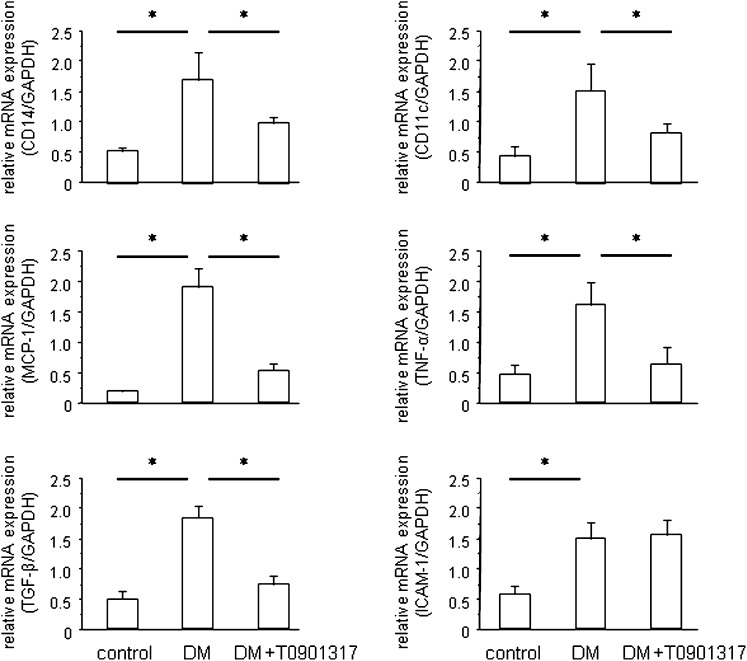
Macrophage and Inflammatory Gene Expressions in the Renal Cortex
Quantitative RT-PCR (qRT-PCR) analyses of the kidney tissues revealed that the expression levels of two macrophage marker genes, CD14 and CD11c, were increased in the DM group and that T0901317 treatment markedly reduced these gene expression levels (Figure 4). CD14 is a marker for all macrophages, whereas CD11c is specific for the proinflammatory (M1) subtype of macrophages. Similarly, diabetes induction increased the renal expressions of several proinflammatory and proatherogenic genes, including monocyte chemoattractant protein 1 (MCP-1), TNF-α, TGF-β, and intracellular adhesion molecule 1 (ICAM-1). Although T0901317 decreased the expressions of MCP-1, TNF-α, and TGF-β, it had no effect on ICAM-1 expression (Figure 4). Similar to OPN, MCP-1 is a key chemokine involved in macrophage recruitment, and it plays a significant role in diabetic nephropathy.26,27 TNF-α and TGF-β are also critical inflammatory cytokines involved in diabetic nephropathy.28,29 Collectively, these data indicate that the LXR agonist T0901317 inhibits diabetes-induced macrophage recruitment and inflammatory gene expression in the kidney.
Figure 4.
LXR activation suppresses diabetes-induced renal inflammation and macrophage infiltration. qRT-PCR analysis of the expressions of two macrophage markers, CD14 and CD11c, reveals that T0901317 inhibits diabetes-induced macrophage infiltration into the kidney. Similarly, T0901317 suppresses the MCP-1, TNF-α, and TGF-β mRNA levels in the kidney. The mRNA levels were normalized by the GAPDH mRNA level. Data are means ± SEM. *P<0.05.
LXR Agonist Inhibits OPN Expression in Proximal Tubular Epithelial Cells
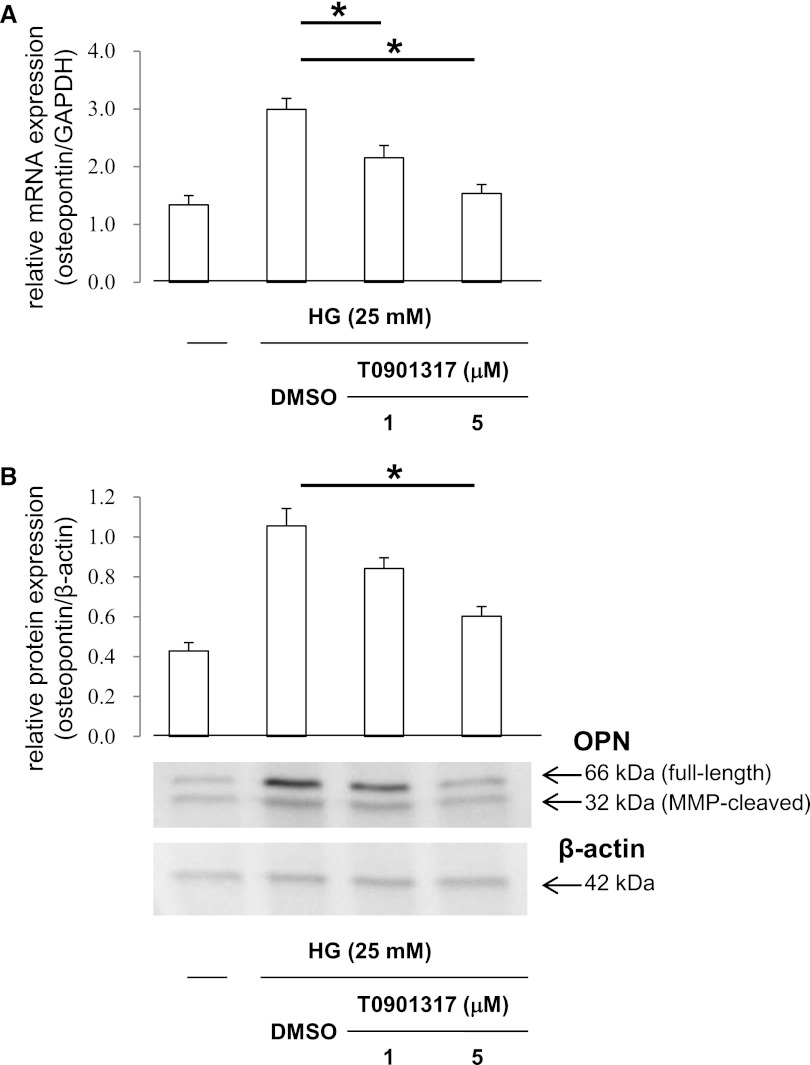
Our in vivo studies showed that diabetes-induced OPN expression in the kidney interstitium was suppressed by T0901317 treatment. Based on these data, we analyzed whether T0901317 modulates OPN expression in mProx24 cells, a mouse proximal tubular epithelial cell line. qRT-PCR experiments showed that high glucose-induced OPN mRNA expression was dose-dependently suppressed by T0901317 (Figure 5A), indicating that OPN suppression in mProx24 cells by the LXR ligand occurs at the gene expression level. Similarly, pretreatment of mProx24 cells with T0901317 resulted in dose-dependent inhibition of OPN protein expression (Figure 5B).
Figure 5.
LXR agonist suppresses HG-induced OPN expression in proximal tubular epithelial cells. mProx24 cells, a mouse renal proximal tubular epithelial cell line, were pretreated for 24 h with vehicle (DMSO) or the indicated concentrations of the synthetic LXR agonist T0901317. (A) After stimulation for 24 h with high-glucose (HG) medium (25 mM), total RNA was isolated from mProx24 cells and analyzed for the OPN mRNA expression levels by qRT-PCR. Quantification was performed by densitometry, with normalization by the cohybridized GAPDH mRNA expression levels. Data are means ± SEM. *P<0.05. (B) After 24 h, OPN protein expression was analyzed by Western blotting. Full-length OPN as well as the C-terminal fragment of MMP-cleaved OPN are significantly upregulated by HG stimulation, and they are suppressed by pretreatment with T0901317. Quantification was performed by densitometry of three independently performed experiments, with normalization by β-actin. Data are means ± SEM. *P<0.05.
LXR Ligand Suppresses OPN Transcription
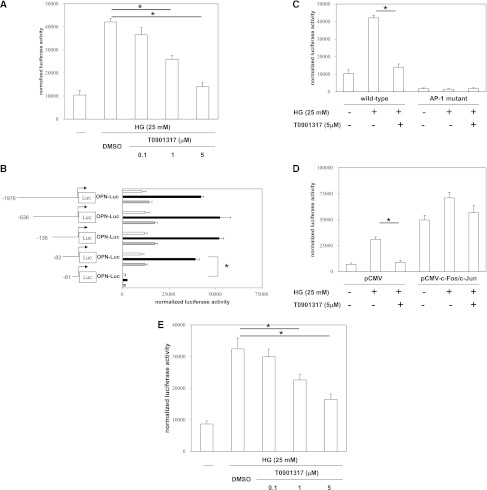
To further analyze the effects of the LXR ligand on OPN transcription, we transiently transfected mProx24 cells with a 2-kb OPN promoter fragment. High-glucose stimulation resulted in significant induction of OPN promoter activity, which was suppressed by T0901317 (Figure 6A). The inhibition of OPN promoter activity by T0901317 was dose-dependent, and it paralleled the inhibition of OPN mRNA and protein expressions. These findings suggest that the LXR ligand suppresses high glucose-induced OPN expression by inhibiting OPN gene transcription. To confirm the specificity of T0901317 for LXRα and LXRβ, we performed small interfering RNA (siRNA) experiments (Supplemental Figure 2A). High glucose-induced OPN mRNA expression was suppressed by T0901317 in mProx24 cells transfected with a scrambled siRNA, and this suppression was canceled by both LXRα siRNA and LXRβ siRNA (Supplemental Figure 2B). These findings suggest that inhibition of OPN promoter activity by T0901317 is mediated by both LXRα and LXRβ.
Figure 6.
LXR agonist inhibits OPN promoter activity by negative interference with AP-1 signaling pathways. mProx24 cells were transiently transfected with a full-length OPN promoter construct. After the transfection, the cells were pretreated for 24 h with vehicle (DMSO) or the indicated concentrations of the synthetic LXR agonist T0901317 and stimulated for 24 h with high glucose (HG) medium (25 mM) as indicated. Data are expressed as the normalized luciferase activities. Data are means ± SEM. *P<0.05. (A) The transfected mProx24 cells were pretreated for 24 h with T0901317 and stimulated for 24 h with HG medium as indicated. The transfection efficiency was adjusted by normalization of the firefly luciferase activities by the Renilla luciferase activities generated by cotransfection with 10 ng pRL-CMV. (B) OPN promoter elements encoded by nucleotides −83 to −61 convey the transcriptional activity in mProx24 cells. mProx24 cells were transiently transfected with the indicated 5′-deletion constructs of the OPN promoter. The transfected mProx24 cells were left untreated (white bars) or incubated with vehicle (DMSO; black bars) or T0901317 (5 μmol/L; gray bars) for 24 h before stimulation with HG medium. (C) mProx24 cells were transiently transfected with the full-length wild-type or AP-1–mutated OPN promoter. (D) mProx24 cells were transfected with the OPN promoter construct alone or cotransfected with the OPN promoter construct and the empty pCMV vector (400 ng) or pCMV-c-Fos (200 ng) and pCMV-c-Jun (200 ng) expression vectors. (E) mProx24 cells were transfected with an AP-1–driven heterologous promoter.
Proximal OPN Promoter Confers Transcriptional Regulation of OPN in Proximal Tubular Epithelial Cells
To identify the promoter elements that regulate high glucose-induced OPN gene transcription and mediate transcriptional suppression of OPN in mProx24 cells by the LXR agonist, we used a series of 5′-deletion constructs (Figure 6B). Although the high glucose-induced transcriptional activity of the −83OPN-Luc promoter construct was completely suppressed by T0901317, the −61OPN-Luc construct was not inducible by high-glucose stimulation and exhibited transcriptional activity that was only slightly above the background signal. These findings indicate that the basal and high glucose-induced OPN promoter activity in mProx24 cells is dependent on transcription factor-binding sites located between −83 and −61 relative to the transcription initiation site.
LXR Agonist Suppresses OPN Promoter Activity by Negatively Interfering with AP-1–Dependent Transactivation of the OPN Promoter
Based on our previous study showing an important role of the AP-1 site located at −76 from the transcription initiation site for regulation of the OPN promoter in macrophages,25 we generated a site-directed mutation in this AP-1 consensus element. Transient transfection of mProx24 cells with the OPN wild-type promoter construct and treatment with the LXR ligand T0901317 resulted in almost complete inhibition of OPN transcription (Figure 6C). In marked contrast, the OPN promoter construct bearing the mutated AP-1 site exhibited low basal activity, and it was not induced by high glucose. To further confirm an important role for this AP-1 site in the inhibition of OPN promoter activity by T0901317, we cotransfected mProx24 cells with the wild-type OPN reporter construct and eukaryotic expression vectors for c-Fos and c-Jun (Figure 6D). Overexpression of c-Fos and c-Jun resulted in complete loss of the ability of T0901317 to inhibit the high glucose-induced OPN promoter activity. To further corroborate the observation that the LXR ligand suppresses AP-1–dependent transactivation, we performed transfection experiments using a heterologous promoter driven by multiple AP-1 response elements. The high glucose-induced transcriptional activity of this AP-1–driven reporter construct was dose-dependently inhibited by T0901317 (Figure 6E). Taken together, these findings suggest that the suppression of the high glucose-induced OPN promoter activity by the LXR ligand is mediated through negative interference with c-Fos/c-Jun acting on the proximal OPN promoter.
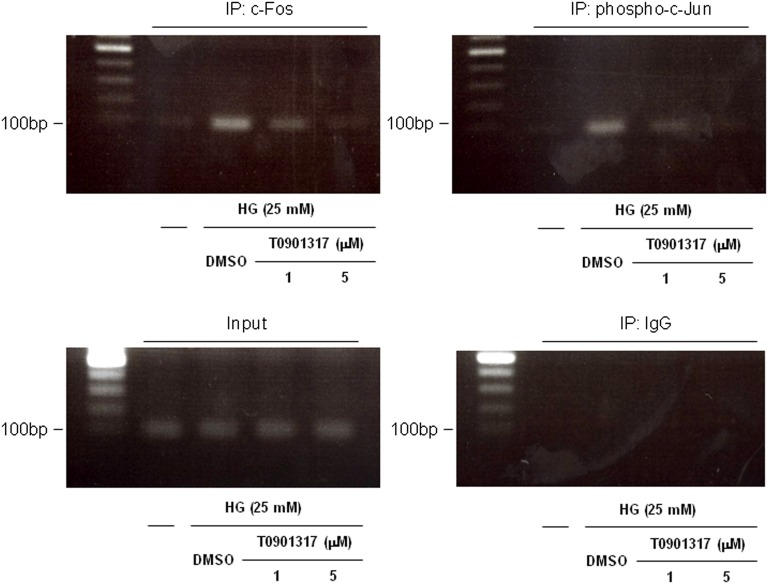
LXR Ligand Inhibits AP-1 Binding to the Proximal OPN Promoter
Chromatin immunoprecipitation (ChIP) assays using a primer pair covering the AP-1 site at −76 in the OPN promoter were performed to confirm that the LXR ligand interferes with c-Fos and phospho-c-Jun binding to the endogenous OPN promoter. As shown in Figure 7, high-glucose stimulation of mProx24 cells resulted in binding of c-Fos and phospho-c-Jun to the AP-1 site at −76 relative to the endogenous OPN promoter, an effect that was inhibited by T0901317. Therefore, the suppression of the high glucose-induced binding to the AP-1 site at −76 by the LXR ligand reflects, at least in part, the inhibition of c-Fos and c-Jun expressions.
Figure 7.
LXR agonist inhibits AP-1 binding to the proximal OPN promoter. mProx24 cells were pretreated for 24 h with vehicle (DMSO) or 1 or 5 μmol/L T0901317 and stimulated with HG medium (25 mM) for 24 h. ChIP assays were performed using anti–c-Fos and anti–phospho-c-Jun antibodies. Total extract (input) and rabbit IgG were used as controls. The ethidium bromide-stained agarose gels shown are representative of three independently performed experiments.
Discussion
LXR signaling pathways have recently been proposed as potential targets for therapeutic interventions in cardiovascular diseases.21,30 However, the effects of LXR agonists on diabetic nephropathy have not been elucidated. In the present study, we have shown that the LXR agonist T0901317 ameliorated albuminuria, glomerular mesangial expansion, and interstitial fibrosis without affecting blood glucose and triglyceride levels in STZ-induced diabetic mice. T0901317 treatment markedly decreased the expression of OPN, a key proinflammatory cytokine in diabetic nephropathy, macrophage infiltration, and expressions of inflammatory genes, including MCP-1, TNF-α, and TGF-β, in the diabetic kidney. Furthermore, in vitro experiments using renal proximal tubular epithelial cells revealed that high glucose induced OPN expression and that T0901317 inhibited this OPN expression. This inhibition was mediated by inhibition of AP-1–dependent transcriptional activation of the OPN promoter. These observations further support an important role for LXR agonists in suppressing the inflammatory responses in diabetic kidneys and preventing the development of nephropathy.
Accumulating evidence has shown that a chronic low-grade state of inflammation plays an important role in the pathogenesis of diabetic nephropathy.31,32 OPN is a proinflammatory cytokine implicated in the chemoattraction of monocytes and development of diabetic nephropathy,16,17 and its levels are significantly elevated in patients with diabetic nephropathy.9,33 OPN transcription is induced in response to high glucose,34 raising the possibility that increased OPN secretion in diabetes may play a direct causal role in the development of diabetic nephropathy. For many years, it has been well known that OPN is upregulated in the interstitium, especially the proximal tubules, in the diabetic kidney.12–14 Moreover, OPN deficiency prevents the development of diabetic nephropathy in murine models.19,20 We recently reported that a peroxisome proliferator-activated receptor δ agonist, GW0742, attenuates diabetic nephropathy by suppressing inflammatory mediators, including OPN and MCP-1.35 Therefore, OPN is regarded as a key molecule in the pathogenesis of diabetic nephropathy, and it may be a potential therapeutic target.
LXRs were first discovered as orphan receptors, and subsequently, they were identified as nuclear receptor targets of the cholesterol metabolites oxysterols. There are two LXRs encoded by distinct genes, namely LXRα, which is most highly expressed in the liver, adipose tissue, kidney, adrenal tissue, and macrophages, and LXRβ, which is ubiquitously expressed.36 LXRα mRNA is widely expressed in the kidney and present in every nephron segment, including the glomeruli, and its activation by T0901317 mediates cholesterol efflux through ATP-binding cassette transporter A1 in cultured glomerular mesangial cells.37 Meanwhile, T0901317 increases the expression of stearoyl-CoA desaturase-1 through increased SREBP-1c in the proximal straight tubules.38 These observations suggest that LXRs may play a role in regulating lipid metabolism and maintaining proximal tubule function. Although atheroprotective effects of LXR agonists have been reported,22,24 there are no reports regarding the effects of LXR agonists on diabetic nephropathy.
Because the LXR ligand suppressed OPN expression and the OPN promoter analyses did not reveal the presence of any putative LXR response elements, the inhibition of the high glucose-induced OPN transcription by the LXR ligand probably involves an indirect mechanism through the regulation of other transcription factors that support OPN transcription. In this study, we identified an AP-1 consensus site in the OPN promoter located between −80 and −71 that supports the basal and induced transcriptional activity of the OPN promoter in proximal tubular epithelial cells. Using ChIP assays, we showed that the LXR agonist inhibited c-Fos and phospho-c-Jun binding to this AP-1 site in the proximal OPN promoter. The ability of the LXR ligand to suppress the OPN promoter activity was lost in cells overexpressing c-Fos and c-Jun. These findings, combined with our observation that the LXR ligand inhibited the transactivation of a heterologous AP-1–driven promoter, support the notion that LXR ligands suppress OPN expression by interfering with AP-1–dependent transactivation of the proximal OPN promoter.
Recent studies have suggested that OPN plays an important role in not only the interstitium but also the glomeruli. In the Ins2Akita mouse model of type 1 diabetic nephropathy, OPN immunostaining was increased in podocytes, and OPN knockout mice were protected against diabetes-induced albuminuria and mesangial expansion.19 Furthermore, OPN deletion decreased albuminuria and the mesangial area in both type 1 (Ins2Akita) and type 2 (db/db) diabetic mouse models, and recombinant OPN upregulated TGF-β expression and signaling in cultured mouse mesangial cells.20 In the present study, glomerular hypertrophy, mesangial matrix expansion, and macrophage infiltration were observed in the diabetic glomeruli, and these changes were ameliorated by administration of T0901317. These findings suggest that LXR agonists may have beneficial effects in not only the interstitium but also the glomerular cells. Because LXRα and LXRβ are present in not only the interstitium but also the glomeruli,37,39 T0901317 may suppress OPN expression and macrophage infiltration in the glomeruli and subsequently, alter glomerular hypertrophy and albuminuria. However, OPN expression was predominantly localized in the proximal tubular epithelial cells and to a lesser extent, the glomeruli of the diabetic kidneys. Therefore, we focused on the regulation of OPN expression in proximal tubular epithelial cells by an LXR agonist in this study, and additional investigations are needed.
There are several limitations to this study. The first limitation is the selectivity of T0901317 as an LXR agonist. Recent studies showed that T0901317 can activate not only LXRs but also other nuclear hormone receptors, including pregnane X receptor, farnesoid X receptor, and retinoic acid receptor-related orphan receptor-α/γ.40–42 We showed that activation of both LXRα and LXRβ by T0901317 inhibited OPN expression in the present study. However, it remains possible that activation of other nuclear hormone receptors could suppress the OPN expression. Therefore, it is expected that highly selective LXR agonists will be developed.
The second limitation is that T0901317 has lipogenic effects. In this study, although the gene expressions of lipogenic enzymes were slightly increased after administration of T0901317, lipid accumulation in renal tissues and serum triglyceride elevation were not observed. There are several speculations of why T0901317 did not induce lipogenic activities. One speculation is that insulin deficiency may strongly suppress the lipogenesis and lipid accumulation in the kidney because of two reasons: we used STZ to induce diabetes, and insulin was defective in this model. Another speculation involves the LXR downregulation by diabetes induction (Supplemental Figure 1C). These findings are consistent with a previous study analyzing the expressions of LXRs in other type 1 diabetes models.43 This downregulation of LXRs may be involved in weakening the lipogenic effects of T0901317. A third speculation is the amount of T0901317. Many investigators used T0901317 at 50 mg/kg per day and observed lipogenic effects in previous studies.44–48 To avoid severe hepatic lipogenesis, we used a comparatively low dose (10 mg/kg per day) in this study. Recently, T0901317 and a new steroidal LXR agonist, DMHCA, were reported to decrease the activity of intestinal and renal sodium gradient-dependent phosphate transporters, and the gene expressions of lipogenic enzymes with DMHCA were lower than those gene expressions after activation by T0901317.49 Additional studies are required to address whether the generation of selective LXR modulators will circumvent lipogenesis and provide a novel strategy for treating diabetic nephropathy.
In conclusion, the present data show that the LXR agonist T0901317 shows renoprotective effects through its anti-inflammatory activity by inhibiting OPN expression and macrophage infiltration in the diabetic kidney. Because OPN is a key component in the inflammatory response and recruitment of monocytes/macrophages into the diabetic kidney, inhibition of OPN expression by LXR agonists could be a therapeutic target in human diabetic nephropathy.
Concise Methods
Experimental Protocol
Male C57BL/6J mice were purchased from Charles River (Yokohama, Japan). At 8 weeks of age, the mice were divided into three groups: nondiabetic control mice (control; n=8), STZ-induced diabetic mice (DM; n=8), and diabetic mice treated with the LXR agonist T0901317 (DM+T0901317; n=8). T0901317 was purchased from Sigma-Aldrich (Tokyo, Japan). Diabetes was induced by peritoneal injection of 200 mg/kg STZ (Sigma-Aldrich) in citrate buffer (pH 4.5). Blood glucose was measured by the glucose oxidase method at 3 and 7 days after the STZ injection, and only mice with blood glucose concentrations of >16 mmol/L were used in the study. The mice in the control group were injected with citrate buffer. The mice in the DM+T0901317 group received 10 mg/kg per day T0901317 by gavage for 8 weeks. All mice had free access to a standard diet and tap water. All procedures were performed according to the Guidelines for Animal Experiments at Okayama University Medical School, Japanese Government Animal Protection and Management Law (Number 105), and Japanese Government Notification on Feeding and Safekeeping of Animals (Number 6). The mice were euthanized at 8 weeks after diabetes induction. The kidneys were removed, weighed, and fixed in 10% formalin for PAM staining and Masson’s trichrome staining. Parts of the remaining tissues were embedded in optimal cutting temperature compound (Sakura Finetechnical, Tokyo, Japan) and frozen immediately in acetone cooled on dry ice. Other tissues were snap-frozen in liquid nitrogen and stored at −80°C.
Metabolic Data
We measured the body weight, BP, HbA1c, 24 h UAE, and creatinine clearance at 0, 4, and 8 weeks. BP was measured using the tail-cuff method (Softron, Tokyo, Japan). HbA1c was measured using a high-pressure liquid chromatography method, and serum creatinine was measured using an enzymatic method. Urine was collected for 24 h with each mouse individually housed in a metabolic cage and provided food and water ad libitum. The urinary albumin concentration was measured as previously described.50
Light Microscopy
Sections (4 μm thick) cut from 10% formalin-fixed, paraffin-embedded kidney samples were subjected to PAM staining and Masson’s trichrome staining. To evaluate the glomerular size, we examined 20 randomly selected glomeruli in the cortex per animal under high magnification (×400) at 8 weeks after the induction of diabetes. The areas of the glomerular tuft and mesangial matrix index (MMI) were measured using Lumina Vision software (Mitani Corporation, Tokyo, Japan). The MMI was defined as the PAM-positive area in the tuft area, and it was calculated using the following formula: MMI = PAM-positive area/tuft area. The results were expressed as means ± SE (per micrometer squared for the tuft area and arbitrary units for the MMI). The Masson’s trichrome-stained sections were assessed for the proportion of fibrosis in the kidney tissues using a computer image analysis system as described above.
Immunoperoxidase Staining
Immunoperoxidase staining was performed as previously described.50 Briefly, fresh-frozen sections were cut to 4-μm thickness using a cryostat. To evaluate the macrophage infiltration, we applied a rat anti-mouse monocyte/macrophage (F4/80) monoclonal antibody (ab6640; Abcam, Tokyo, Japan) followed by a biotin-labeled goat anti-rat IgG antibody (ab6844; Abcam). The avidin–biotin coupling reaction was performed on the sections using a Vectastain Elite Kit (Vector Laboratories, Burlingame, CA). We examined 20 glomeruli per animal and counted the number of F4/80-positive cells. The mean numbers of positive cells per glomerulus and interstitial tissue (per millimeter squared) were used for the estimation. To evaluate OPN expression, a rabbit anti-OPN polyclonal antibody (ab8448; Abcam) was applied, and it was followed by a biotin-labeled donkey anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA).
PCR Assays for Gene Expression
RNA from the renal cortex was isolated after 8 weeks of treatment using an RNeasy Mini Kit (Qiagen, Valencia, CA). Single-stranded cDNA was synthesized from the extracted RNA using a GeneAmp RNA PCR Core Kit (Applied Biosystems, Foster City, CA). To evaluate the mRNA expressions of OPN, CD14, CD11c, MCP-1, TNF-α, TGF-β, and ICAM-1 in the renal cortex, qRT-PCR was performed using a StepOnePlus Real-Time PCR System (Applied Biosystems) and SYBR Premix Ex Taq II (Takara Bio Inc., Otsu, Japan). The primers were purchased from Takara Bio Inc. and are listed in Table 2. Each sample was analyzed in triplicate and normalized by the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression.
Table 2.
Primers used for qRT-PCR
| Gene | Sense | Antisense |
|---|---|---|
| OPN | TACGACCATGAGATTGGCAGTGA | TATAGGATCTGGGTGCAGGCTGTAA |
| CD14 | GGGCTGCTCAAACTTTCAGAATCTA | AATTGAAAGCGCTGGACCAATC |
| CD11c | AGGTCTGCTGCTGCTGGCTA | GAAGTTCTCACTGGGCAACCTG |
| MCP-1 | GCATCCACGTGTTGGCTCA | CTCCAGCCTACTCATTGGGATCA |
| TNF-α | GTTCTATGGCCCAGACCCTCAC | GGCACCACTAGTTGGTTGTCTTTG |
| TGF-β | GTGTGGAGCAACATGTGGAACTCTA | TTGGTTCAGCCACTGCCGTA |
| ICAM-1 | CAATTCACACTGAATGCCAGCTC | CAAGCAGTCCGTCTCGTCCA |
| GAPDH | TGTGTCCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
Western Blot Analysis
Western blotting was performed as previously described.35 Briefly, the proteins were eluted, resolved by SDS-PAGE, and transferred to nitrocellulose membranes. After blocking in 20 mM Tris⋅HCl (pH 7.6) containing 150 mM NaCl, 0.1% Tween-20, and 5% (wt/vol) nonfat dried milk, the membranes were incubated with an anti-OPN antibody (ab8448; Abcam). This antibody recognizes full-length OPN (which appears at 66 kD on Western blotting) as well as the C-terminal fragment of matrix metalloproteinase (MMP) -cleaved OPN (which appears at 32 kD on Western blotting).51 The membranes were hybridized with an anti–β-actin antibody (ab6276; Abcam) to monitor equivalent loading in different lanes. All experiments were repeated at least three times.
Cell Culture and Treatment
mProx24 cells, a mouse renal proximal tubular epithelial cell line, were cultured in DMEM supplemented with 5.5 mM d-glucose, 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM l-glutamine. For ligand treatment, the cells were serum-starved by culture in medium containing 0.5% FBS for 24 h. After pretreatment with T0901317 for 24 h, the cells were stimulated with 25 mM d-glucose (high glucose) for 24 h. Individual experiments were repeated at least three times with different lots or preparations of cells. OPN mRNA and protein expressions in mProx24 cells were measured by qRT-PCR and Western blotting, respectively, as described above.
Plasmids and Transient Transfections
The OPN promoter constructs, AP-1 luciferase reporter construct, and c-Fos and c-Jun expression vectors were used as previously described.25,34 The AP-1 site at −76 in the 2-kb OPN promoter construct was mutated from −80CCTCATGAC−71 to −80CGGCGGGAC−71 as previously described.25 mProx24 cells were transfected with 1 μg DNA using a Neon Transfection System (Invitrogen, Carlsbad, CA). At 8 h after transfection, the cells were cultured in DMEM supplemented with 0.5% FBS in the presence of specified concentrations of T0901317 for 12 h before high-glucose stimulation. The luciferase activity was assayed after 24 h of stimulation using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI) as previously described.25 All experiments were repeated at least three times and in triplicate with different cell preparations.
ChIP Assays
ChIP assays were performed using a ChIP-IT Express (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. Soluble chromatin was prepared from mProx24 cells treated with 1 or 5 μmol/L T0901317 for 24 h followed by stimulation with high glucose (25 mM) for 24 h. Chromatin was immunoprecipitated with antibodies (2 mg) directed against c-Fos (sc-8047; Santa Cruz Biotechnology, Santa Cruz, CA) and phospho-c-Jun (sc-822; Santa Cruz Biotechnology). The final DNA extracts were amplified by PCR using a primer pair that covered the AP-1 consensus sequence at −76 in the OPN promoter to yield a 151-bp PCR product (forward, 5′-ACCACAAAACCAGAGGAGGA-3′ and reverse, 5′-TTCAGTGTGAGCTGCTGGTG-3′).
Statistical Analyses
All values are presented as means ± SEM. The statistical significance of differences between groups was analyzed using one-way ANOVA followed by Scheffé’s test. Values of P<0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Noriko Yamamoto and Yoshiko Hada for technical support.
This study was supported in part by Grants-in-Aid for Young Scientists (B) 21790813 and 23790942 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to D.O.) and a Grant-in-Aid for Diabetic Nephropathy from the Ministry of Health, Labour, and Welfare of Japan. This work also received support from the Takeda Science Foundation, the Naito Foundation, and the Ryobi Teien Memory Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012010022/-/DCSupplemental
References
- 1.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H: Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 287: 860–864, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Denhardt DT, Giachelli CM, Rittling SR: Role of osteopontin in cellular signaling and toxicant injury. Annu Rev Pharmacol Toxicol 41: 723–749, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Giachelli CM, Steitz S: Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol 19: 615–622, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM: Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest 92: 1686–1696, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA: Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest 112: 1318–1331, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui Y, Rittling SR, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, Sugawara T, Morimoto J, Kimura C, Kon S, Denhardt D, Kitabatake A, Uede T: Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 23: 1029–1034, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ohmori R, Momiyama Y, Taniguchi H, Takahashi R, Kusuhara M, Nakamura H, Ohsuzu F: Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis 170: 333–337, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Minoretti P, Falcone C, Calcagnino M, Emanuele E, Buzzi MP, Coen E, Geroldi D: Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J 27: 802–807, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi H, Igarashi M, Hirata A, Tsuchiya H, Sugiyama K, Morita Y, Jimbu Y, Ohnuma H, Daimon M, Tominaga M, Kato T: Progression of diabetic nephropathy enhances the plasma osteopontin level in type 2 diabetic patients. Endocr J 51: 499–504, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Takemoto M, Yokote K, Yamazaki M, Ridall AL, Butler WT, Matsumoto T, Tamura K, Saito Y, Mori S: Enhanced expression of osteopontin by high glucose. Involvement of osteopontin in diabetic macroangiopathy. Ann N Y Acad Sci 902: 357–363, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Nakamachi T, Nomiyama T, Gizard F, Heywood EB, Jones KL, Zhao Y, Fuentes L, Takebayashi K, Aso Y, Staels B, Inukai T, Bruemmer D: PPARalpha agonists suppress osteopontin expression in macrophages and decrease plasma levels in patients with type 2 diabetes. Diabetes 56: 1662–1670, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Fischer JW, Tschöpe C, Reinecke A, Giachelli CM, Unger T: Upregulation of osteopontin expression in renal cortex of streptozotocin-induced diabetic rats is mediated by bradykinin. Diabetes 47: 1512–1518, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Kelly DJ, Wilkinson-Berka JL, Ricardo SD, Cox AJ, Gilbert RE: Progression of tubulointerstitial injury by osteopontin-induced macrophage recruitment in advanced diabetic nephropathy of transgenic (mRen-2)27 rats. Nephrol Dial Transplant 17: 985–991, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Li C, Yang CW, Park CW, Ahn HJ, Kim WY, Yoon KH, Suh SH, Lim SW, Cha JH, Kim YS, Kim J, Chang YS, Bang BK: Long-term treatment with ramipril attenuates renal osteopontin expression in diabetic rats. Kidney Int 63: 454–463, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH: Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney Int 65: 116–128, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Susztak K, Böttinger E, Novetsky A, Liang D, Zhu Y, Ciccone E, Wu D, Dunn S, McCue P, Sharma K: Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes 53: 784–794, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Hsieh TJ, Chen R, Zhang SL, Liu F, Brezniceanu ML, Whiteside CI, Fantus IG, Ingelfinger JR, Hamet P, Chan JS: Upregulation of osteopontin gene expression in diabetic rat proximal tubular cells revealed by microarray profiling. Kidney Int 69: 1005–1015, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Usui HK, Shikata K, Sasaki M, Okada S, Matsuda M, Shikata Y, Ogawa D, Kido Y, Nagase R, Yozai K, Ohga S, Tone A, Wada J, Takeya M, Horiuchi S, Kodama T, Makino H: Macrophage scavenger receptor-a-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation. Diabetes 56: 363–372, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lorenzen J, Shah R, Biser A, Staicu SA, Niranjan T, Garcia AM, Gruenwald A, Thomas DB, Shatat IF, Supe K, Woroniecki RP, Susztak K: The role of osteopontin in the development of albuminuria. J Am Soc Nephrol 19: 884–890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholas SB, Liu J, Kim J, Ren Y, Collins AR, Nguyen L, Hsueh WA: Critical role for osteopontin in diabetic nephropathy. Kidney Int 77: 588–600, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Tontonoz P, Mangelsdorf DJ: Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol 17: 985–993, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P: Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9: 213–219, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P: Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem 278: 10443–10449, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P: Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A 99: 7604–7609, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa D, Stone JF, Takata Y, Blaschke F, Chu VH, Towler DA, Law RE, Hsueh WA, Bruemmer D: Liver x receptor agonists inhibit cytokine-induced osteopontin expression in macrophages through interference with activator protein-1 signaling pathways. Circ Res 96: e59–e67, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH: Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int 69: 73–80, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH: Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia 50: 471–480, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Navarro-González JF, Mora-Fernández C: The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19: 433–442, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Sharma K, Ziyadeh FN: Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes 44: 1139–1146, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Lund EG, Menke JG, Sparrow CP: Liver X receptor agonists as potential therapeutic agents for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol 23: 1169–1177, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH: Diabetic nephropathy is associated with low-grade inflammation in Type 1 diabetic patients. Diabetologia 46: 1402–1407, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Nelson CL, Karschimkus CS, Dragicevic G, Packham DK, Wilson AM, O’Neal D, Becker GJ, Best JD, Jenkins AJ: Systemic and vascular inflammation is elevated in early IgA and type 1 diabetic nephropathies and relates to vascular disease risk factors and renal function. Nephrol Dial Transplant 20: 2420–2426, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Yan X, Sano M, Lu L, Wang W, Zhang Q, Zhang R, Wang L, Chen Q, Fukuda K, Shen W: Plasma concentrations of osteopontin, but not thrombin-cleaved osteopontin, are associated with the presence and severity of nephropathy and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 9: 70, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bidder M, Shao JS, Charlton-Kachigian N, Loewy AP, Semenkovich CF, Towler DA: Osteopontin transcription in aortic vascular smooth muscle cells is controlled by glucose-regulated upstream stimulatory factor and activator protein-1 activities. J Biol Chem 277: 44485–44496, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Matsushita Y, Ogawa D, Wada J, Yamamoto N, Shikata K, Sato C, Tachibana H, Toyota N, Makino H: Activation of peroxisome proliferator-activated receptor delta inhibits streptozotocin-induced diabetic nephropathy through anti-inflammatory mechanisms in mice. Diabetes 60: 960–968, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calkin AC, Tontonoz P: Liver x receptor signaling pathways and atherosclerosis. Arterioscler Thromb Vasc Biol 30: 1513–1518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Zhang Y, Wang N, Davis L, Yang G, Wang X, Zhu Y, Breyer MD, Guan Y: Liver X receptor-alpha mediates cholesterol efflux in glomerular mesangial cells. Am J Physiol Renal Physiol 287: F886–F895, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Zhang X, Chen L, Wu J, Su D, Lu WJ, Hwang MT, Yang G, Li S, Wei M, Davis L, Breyer MD, Guan Y: Liver X receptor agonist TO-901317 upregulates SCD1 expression in renal proximal straight tubule. Am J Physiol Renal Physiol 290: F1065–F1073, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Morello F, de Boer RA, Steffensen KR, Gnecchi M, Chisholm JW, Boomsma F, Anderson LM, Lawn RM, Gustafsson JA, Lopez-Ilasaca M, Pratt RE, Dzau VJ: Liver X receptors alpha and beta regulate renin expression in vivo. J Clin Invest 115: 1913–1922, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitro N, Vargas L, Romeo R, Koder A, Saez E: T0901317 is a potent PXR ligand: Implications for the biology ascribed to LXR. FEBS Lett 581: 1721–1726, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Houck KA, Borchert KM, Hepler CD, Thomas JS, Bramlett KS, Michael LF, Burris TP: T0901317 is a dual LXR/FXR agonist. Mol Genet Metab 83: 184–187, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez RD, Burris TP, Griffin PR: The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Mol Pharmacol 77: 228–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M: Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 55: 2502–2509, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P: Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem 277: 11019–11025, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Ide T, Shimano H, Yoshikawa T, Yahagi N, Amemiya-Kudo M, Matsuzaka T, Nakakuki M, Yatoh S, Iizuka Y, Tomita S, Ohashi K, Takahashi A, Sone H, Gotoda T, Osuga J, Ishibashi S, Yamada N: Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. II. LXRs suppress lipid degradation gene promoters through inhibition of PPAR signaling. Mol Endocrinol 17: 1255–1267, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Chisholm JW, Hong J, Mills SA, Lawn RM: The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J Lipid Res 44: 2039–2048, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Miao B, Zondlo S, Gibbs S, Cromley D, Hosagrahara VP, Kirchgessner TG, Billheimer J, Mukherjee R: Raising HDL cholesterol without inducing hepatic steatosis and hypertriglyceridemia by a selective LXR modulator. J Lipid Res 45: 1410–1417, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Cha JY, Repa JJ: The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem 282: 743–751, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Caldas YA, Giral H, Cortázar MA, Sutherland E, Okamura K, Blaine J, Sorribas V, Koepsell H, Levi M: Liver X receptor-activating ligands modulate renal and intestinal sodium-phosphate transporters. Kidney Int 80: 535–544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kido Y, Nagase R, Wada J, Shikata Y, Makino H: Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes 52: 2586–2593, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L: Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 276: 28261–28267, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.