Abstract
Patients and animals with renal injury exhibit increased urinary excretion of angiotensinogen. Although increased tubular synthesis of angiotensinogen contributes to the increased excretion, we do not know to what degree glomerular filtration of systemic angiotensinogen, especially through an abnormal glomerular filtration barrier, contributes to the increase in urinary levels. Here, we used multiphoton microscopy to visualize and quantify the glomerular permeability of angiotensinogen in the intact mouse and rat kidney. In healthy mice and Munich-Wistar-Frömter rats at the early stage of glomerulosclerosis, the glomerular sieving coefficient of systemically infused Atto565-labeled human angiotensinogen (Atto565-hAGT), which rodent renin cannot cleave, was only 25% of the glomerular sieving coefficient of albumin, and its urinary excretion was undetectable. In a more advanced phase of kidney disease, the glomerular permeability of Atto565-hAGT was slightly higher but still very low. Furthermore, unlike urinary albumin, the significantly higher urinary excretion of endogenous rat angiotensinogen did not correlate with either the Atto565-hAGT or Atto565-albumin glomerular sieving coefficients. These results strongly suggest that the vast majority of urinary angiotensinogen originates from the tubules rather than glomerular filtration.
The renin–angiotensin system (RAS) is one of the most important regulatory mechanisms of body fluid, electrolyte homeostasis, and BP.1–4 RAS in the kidney is independently regulated from RAS in the systemic circulation, and it has been implicated in the development of hypertension and kidney diseases. For example, renal epithelial cell-specific overexpression of human angiotensinogen (AGT) in human renin transgenic mice resulted in increased renal angiotensin II, hypertension, and renal fibrosis without any changes in circulating angiotensin II.5 Also, Dahl salt-sensitive rats, which show low plasma renin activity under high salt feeding, had higher renal angiotensin II content in the kidney compared with normal salt-fed control animals.6 Although all components that are necessary for angiotensin II production are expressed in the kidney,3 AGT is, currently, the only component that is noninvasively measurable in the urine of patients. The level of urinary AGT reflects the activity of the intrarenal RAS, and it is associated with pathologic states in several experimental7,8 and clinical studies.9,10 Although the major source of the circulating AGT is the liver, we and others have previously shown that AGT is produced in the proximal tubules through a de novo pathway.3,11 Intravenously injected human recombinant AGT (hAGT), which has little affinity for enzymatic cleavage by rodent renin because of high species specificity,12,13 was undetectable in the urine of angiotensin II-induced hypertensive rats,8 suggesting that the increase in urinary AGT is likely of tubular rather than glomerular (plasma) origin. However, the plasma level of AGT is much greater than the plasma level of urinary AGT, and therefore, the increase in urinary AGT in a kidney injury model could result from the damage of the glomerular filtration barrier (GFB) and AGT leakage into the urine.
Therefore, the present study was conducted to measure exactly how much AGT is filtered through the GFB and ultimately, excreted through the urine in normal circumstances and the glomerulosclerosis model of Munich-Wistar-Frömter (MWF) rats that shows significant GFB damage. We compared the glomerular permeability of AGT with the glomerular permeability of albumin, because albumin (66 kD) has a molecular mass similar to AGT (∼60 kD); also, the increase in its urinary excretion closely reflects the decline of renal function. Indirectly, these studies also addressed the currently highly debated issue of the glomerular filtration of albumin.14–19 To compare the glomerular processing of these two proteins, we employed intravital multiphoton fluorescence microscopy, which has been used by our laboratory15,20,21 and the laboratories of others,16–19 to directly and quantitatively visualize the glomerular permeability of macromolecules.
Results
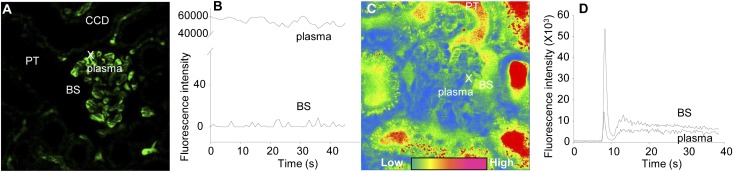
First, we performed intravital multiphoton imaging of the glomerular processing of 500 kD FITC-dextran and FITC-inulin in intact MWF rat kidneys to determine the low and high limits and the sensitivity of the glomerular permeability measurements. These two substances have very different filtration characteristics: zero permeability is expected for the very large molecule 500 kD dextran, whereas inulin is filtered freely. To measure glomerular permeability, the glomerular sieving coefficient (GSC) of substances was calculated by measuring the ratio of Bowman’s space/glomerular capillary plasma fluorescence. We employed 16-bit depth resolution to provide the necessary dynamic range (0–65,535 pixel intensity) for intensity measurements14,15 and calibrated the detector gain and offset in situ for every single glomerulus to minimize nonspecific background fluorescence. Figure 1A shows that, after the injection of 500 kD FITC-dextran into the cannulated carotid artery, the fluorescence was limited to the intravascular space (plasma) in glomerular and peritubular capillaries. No fluorescence was observed in any tubular segments, including the normally highly concentrated lumen of the collecting duct (Figure 1A). Supplemental Movie 1 shows the first 1 minute of time-lapse imaging of an MWF rat kidney after the injection of 500 kD FITC-dextran. As expected, the GSC of 500 kD FITC-dextran was extremely low (0.00015±0.00003, n=14 glomeruli from three different animals). The fluorescence intensity of 500 kD FITC-dextran was usually in the 0–10 range in the Bowman’s space when plasma fluorescence was slightly below saturation (Figure 1B). Therefore, throughout the paper, we give GSC values for macromolecules down to five decimal places, consistent with the sensitivity of these measurements and the approximate lowest limit of detection (e.g., 5/60,000=0.00008 shown in Figure 1B). In contrast to 500 kD FITC-dextran, a small bolus of FITC-inulin injected into the carotid artery quickly and freely cleared from the plasma through glomerular filtration (Supplemental Movie 2 shows the first 1 minute of FITC-inulin filtration). Figure 1, C and D (Supplemental Movie 2) shows that the fluorescence of FITC-inulin was higher in the Bowman’s space compared with the glomerular capillary plasma at all time points. Therefore, the GSC of FITC-inulin was artificially higher than one (2.12±0.16, n=8 from three different animals) because of the high level of light absorption by circulating red blood cells in the glomerular capillary and the recently discussed limitations in measuring the true plasma fluorescence with this technique.15 For GSC measurements, we carefully selected the time point after FITC-inulin injection and measured fluorescence intensities only when plasma and Bowman’s space fluorescence levels were stable (>20 s after injection) (Figure 1D).
Figure 1.
Multiphoton imaging allows high sensitivity measurement of glomerular permeability. Calibration of the multiphoton GSC measurements using FITC conjugates of (A and B) an unfilterable (500 kD dextran) and (C and D) a freely filtered (inulin) substance. (A) After injection of 500 kD FITC-dextran into MWF rats, the fluorescence (green) was restricted to the intravascular space (plasma) in glomerular and peritubular capillaries. No fluorescence was visible in the Bowman’s space (BS), lumen of the proximal tubule (PT), or cortical collecting duct (CCD). The X shows the plasma region of interest that, together with its surrounding BS region, was used for the time-lapse intensity measurements shown in B. (C and D) In contrast, FITC-inulin was freely filtered from the capillary plasma into the BS and lumen of PT segments. (C) The pseudocolor intensity image and (D) time-lapse intensity plot show that FITC-inulin fluorescence was always higher in the BS compared with the capillary plasma. Supplemental Movies 1 and 2 show the same preparations and time periods.
AGT is the substrate of renin that cleaves off the small decapeptide angiotensin I. Fluorescent conjugation of endogenous AGT, therefore, may result in easily filterable fluorescent fragments. To prevent this possibility, we used fluorescently tagged Atto565 maleimide-conjugated hAGT (Atto565-hAGT) in these experiments. Renin has high species specificity, and therefore, murine and rat renin cannot cleave human AGT.12,13 In fact, the dose of hAGT administered in mice and rats did not change mean arterial BP throughout the 90-minute experimental period (mice: 92±2 mmHg at baseline and 88±1 mmHg 90 minutes after AGT; rats: 107±2 mmHg at baseline and 108±5 mmHg 90 minutes after AGT).
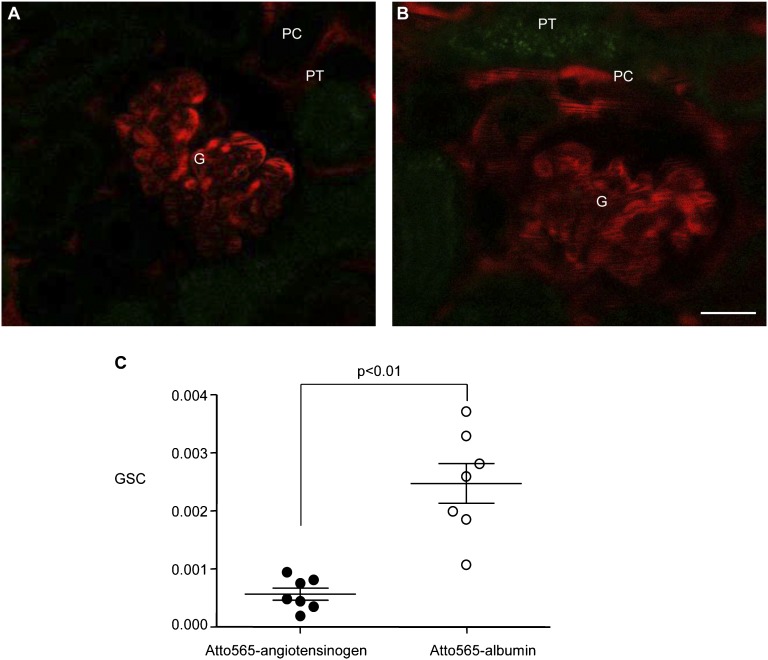
Multiphoton imaging of the intact kidney in vivo in control C57Bl/6 mice was performed to directly and quantitatively visualize the glomerular permeability of AGT and albumin by analyzing the GSC of Atto565-hAGT or Atto565-albumin in single glomeruli. The conjugated proteins were infused through the carotid artery at 60 μL/min for 2–3 minutes. Figure 2 shows that Atto565 fluorescence was detected only in glomerular and peritubular capillary plasma throughout these experiments. Tubular structures were devoid of labeling (Figure 2, A and B), indicating that there was negligible tubular uptake of Atto565-hAGT or Atto565-albumin in healthy mice. Supplemental Movie 3 shows the first 1 minute of time-lapse imaging of a C57Bl/6 mouse kidney after the injection of Atto565-albumin. Importantly, the GSCs of both Atto565-hAGT and Atto565-albumin were very low, and the GSC of Atto565-hAGT (0.00057±0.00010) was approximately one-fourth of the GSC of Atto565-albumin (0.00247±0.00034, n=7 each) (Figure 2C). To control for protein tagging, we used another conjugate, Atto565 NHS ester (Sigma). The GSC of Atto565 NHS ester-conjugated albumin was similar to the GSC of Atto565 maleimide-conjugated albumin (0.00226±0.00078, n=3).
Figure 2.
Extremely low levels of plasma AGT and albumin are filtered in normal mouse glomeruli. Quantitative imaging of the glomerular permeability of (A) Atto565-hAGT (red) and (B) Atto565-albumin in normal C57Bl/6 mice in vivo. (C) The GSC was calculated as the ratio of Atto565 fluorescence in the Bowman’s space versus the ratio of Atto565 fluorescence in glomerular capillary plasma. The GSC of Atto565-hAGT (solid circles) was significantly smaller than the GSC of Atto565-albumin (open circles; n=7 each). The representative multiphoton images were taken 3 minutes after the injection of (A) Atto565-hAGT or (B) Atto565-albumin into the carotid artery. In both A and B, intense Atto565 fluorescence is visible in the glomerular (G) and peritubular capillary (PC) plasma in contrast to extremely low fluorescence in the Bowman’s space and proximal tubule (PT). Dark objects within capillaries are circulating red blood cells.
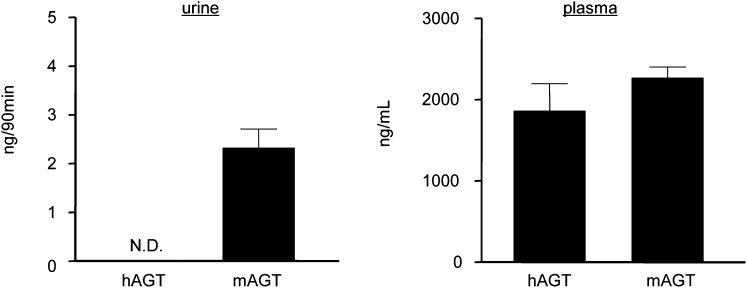
To further confirm the low glomerular permeability of AGT, urinary excretion of hAGT was measured using ELISA. Urine samples were collected from the same mice while they were undergoing in vivo multiphoton imaging for GSC measurements. The systemically administered hAGT was below the limits of detection in the urine (Figure 3), which is in contrast to the low but measurable levels of the endogenous murine AGT (mAGT). The plasma levels of the exogenous Atto565-hAGT and endogenous mAGT were similar (Figure 3).
Figure 3.
The majority of urinary AGT is of tubular rather than glomerular origin in mice. Urine (left) and plasma (right) AGT levels in the same mice measured by ELISA 90 minutes after hAGT injection. hAGT was undetectable in the urine in contrast to the endogenous mAGT. After the injection of hAGT, the plasma levels of hAGT and endogenous mAGT were similar (n=7 each). N.D., undetectable.
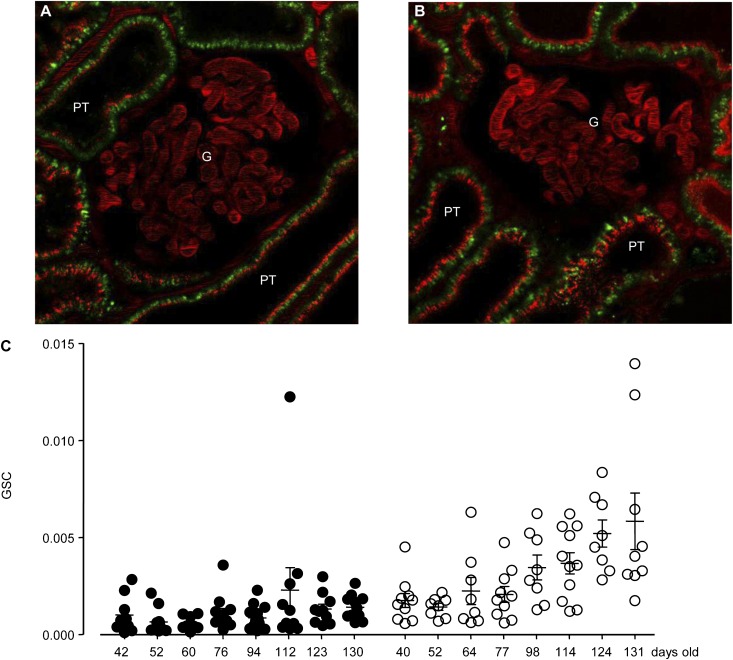
Although GSC measurements in healthy control mice indicated extremely low glomerular permeability of plasma AGT, AGT filtration may be increased in pathologic conditions because of GFB damage. To test this possibility, similar GSC measurements were performed in MWF rats, which is a well-established model of glomerulosclerosis, increased glomerular permeability, RAS activation, and hypertension that develop with aging.22 To control for glomerulus and nephron heterogeneity in this progressive model, at least eight randomly selected superficial glomeruli were imaged for GSC measurements. We used MWF rats at different stages of disease (age range was 40–130 days old; n=8 for each protein). The average GSCs of both Atto565-hAGT and Atto565-albumin were higher in MWF rats compared with control mice and increased with aging (Figure 4). During multiphoton imaging, GSC values did not change significantly over time; GSC of Atto565-hAGT was 0.00092±0.00017 and 0.00104±0.00019, whereas GSC of Atto565-albumin was 0.00217±0.00058 and 0.00388±0.00093 measured 3–5 and 60 minutes after infusion, respectively (n=7 each). Supplemental Movie 4 shows the first 1 minute of time-lapse imaging of a young MWF rat kidney after the injection of Atto565-hAGT.
Figure 4.
The glomerular permeability of AGT and albumin is higher in glomerulosclerosis and increases with aging. Quantitative imaging of the glomerular permeability of (A) Atto565-hAGT and (B) Atto565-albumin in vivo in MWF rats, a model of glomerulosclerosis. In addition to the glomerular (G) capillary plasma, intense Atto565 fluorescence (red) was visible in cells of the proximal tubule (PT), indicating tubular uptake. Autofluorescence is shown in green. (C) Age-dependent changes in GSC of Atto565-hAGT (solid circles) or Atto565-albumin (open circles). GSCs were analyzed from at least eight glomeruli in each rat. GSC of Atto565-hAGT in young rats (42 days old; 0.0010±0.0003) tended to be lower than GSC of Atto565-albumin at similar age (40 days old; 0.0018±0.0004). This trend became more apparent with aging (Atto565-hAGT GSC=0.0014±0.0002 at 130 days old versus Atto565-albumin GSC=0.0058±0.0015 at 130 days old).
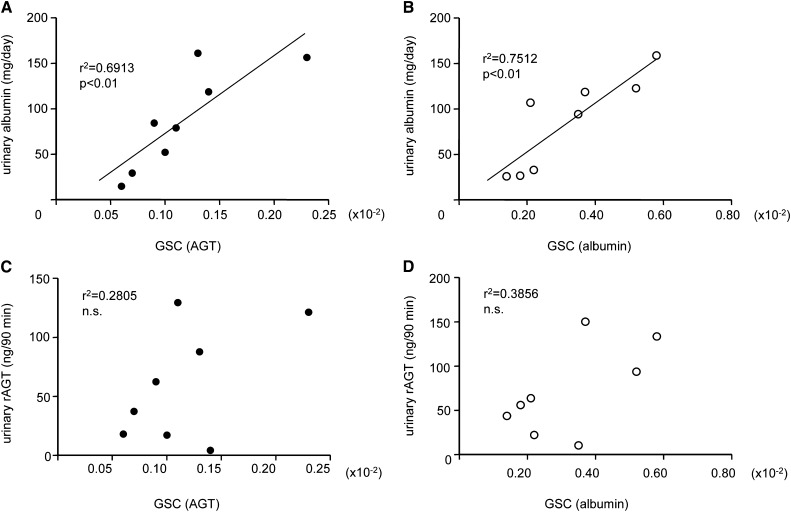
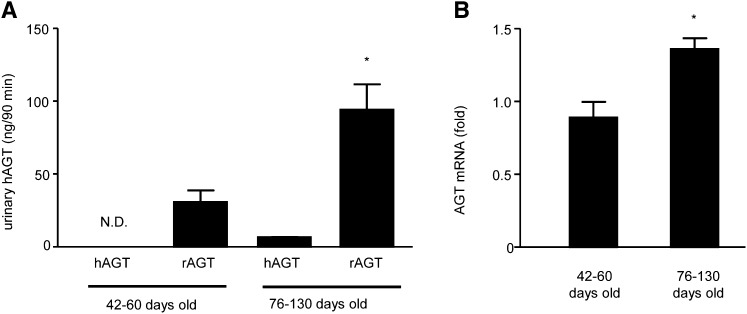
Glomerular heterogeneity was evident from the age-dependent progressive increases in the SD of albumin GSC values in MWF rats. Importantly, GSC measurements in MWF rats (Figure 4) indicated that the glomerular permeability of Atto565-hAGT was lower than the glomerular permeability of Atto565-albumin at all time points, similar to the findings in healthy mice (Figure 2). The development of albuminuria in aging MWF rats was confirmed by urinary albumin measurements (30±5 mg/d at 40–64 days of age, n=6; 120±10 mg/d at 76–130 days of age, n=10; P<0.001). The albuminuria strongly correlated with the GSC of either Atto565-hAGT or Atto565-albumin (Figure 5, A and B). However, the excretion of endogenous rat AGT (rAGT) did not correlate with either Atto565-hAGT or Atto565-albumin GSC, indicating that urinary rAGT is of tubular rather than glomerular origin (Figure 5, C and D). ELISA measurements failed to detect the systemically infused Atto565-hAGT in the urine of the young (42, 52, and 60 days old) rats (Figure 6A). Urinary excretion of Atto565-hAGT was detectable only in rats 76–130 days of age. Importantly, the urinary excretion of endogenous rAGT was about 15 times higher than the urinary excretion of hAGT in these animals (76–130 days of age) (Figure 6A). The expression of AGT mRNA in the kidney (as a marker of de novo AGT synthesis) was greater in the older MWF rats (76–130 days of age) than the younger MWF rats (42–60 days of age) (Figure 6B).
Figure 5.
The majority of urinary AGT is of tubular rather than glomerular origin in MWF rats. Correlation between glomerular permeability of (A and C) Atto565-hAGT or (B and D) Atto565-albumin and (A and B) urinary albumin or (C and D) rAGT (C and D). The albuminuria strongly correlated with the GSC of each protein in (A) Atto565-hAGT– and (B) Atto565-albumin–injected MWF rats. (C and D) However, there was no significant correlation between rAGT excretion and the GSC of either protein.
Figure 6.
Synthesis and urinary excretion of tubular AGT increases in aging MWF rats. Urinary excretion of (A) hAGT and rAGT and (B) mRNA level of endogenous AGT in the MWF rat kidney. Urinary hAGT excretion was below the limits of detection in three young rats. (A) hAGT was detectable in older animals; however, the excretion level was much smaller than the level of rAGT. The expression of AGT mRNA was significantly higher in the kidneys of older animals compared with younger rats (*P<0.05, n=5 each). N.D., undetectable.
In contrast to healthy control mice (Figure 2, A and B), Atto565-derived fluorescence was observed in cells of the proximal tubules of MWF rats of all ages after injection of either Atto565-hAGT or Atto565-albumin (Figure 4, A and B). However, in this study, we did not quantitatively analyze tubular uptake of the dye-conjugated proteins for three reasons. (1) Fluorescence intensity is strongly influenced by the depth of tubules, and the depth of the S1 segment may not be the same in different glomeruli. (2) Atto565 fluorescence in the proximal tubular S1 segments was heterogeneous, most likely because of the differences in single nephron GSC. (3) Atto565 fluorescence of whole-kidney homogenates separated by SDS-PAGE confirmed that the majority of the dye-conjugated proteins in the kidney has a small size (∼15 kD) compared with the intact proteins (Supplemental Figure 1). Plasma fluorescence at the time of kidney sample collection (90 minutes after Atto565-protein infusion) was still clearly visible. However, it is hard to distinguish whether the small protein fragments were generated in the plasma and filtered in the glomeruli or whether the full-length proteins passed through the GFB and were degraded intracellularly in the proximal tubules.
Discussion
AGT is a high-molecular weight protein substrate of the RAS that circulates in much higher concentration than angiotensin peptides, and its urinary excretion is increased during the development of renal disease.10 Although the augmentation of the intrarenal tubular AGT synthesis is well established, the contribution (glomerular filtration) of systemic AGT to urinary AGT has not been clarified. The present study applied the state of the art imaging technique multiphoton microscopy to directly and quantitatively visualize the glomerular permeability of AGT in the intact mouse and rat kidney in vivo. The GSC of Atto565-labeled and systemically infused hAGT was detectable but extremely low (even lower than the GSC of albumin), and its urinary excretion was undetectable in healthy mice and MWF rats at the early stage of glomerulosclerosis. The glomerular permeability of Atto565-hAGT was slightly increased but still very low in a more advanced phase of kidney disease. The albuminuria in MWF rats strongly and positively correlated with the GSC of either Atto565-hAGT or Atto565-albumin, indicating that the albuminuria in this pathologic model reflects damage of the GFB. In contrast, the urinary excretion of endogenous rAGT increased significantly, and it did not correlate with either Atto565-hAGT or Atto565-albumin GSC. All together, the results of the present study indicate that, under both physiologic and pathologic conditions (at least in the MWF rat model of glomerulosclerosis), the vast majority of urinary AGT is of tubular rather than glomerular origin.
The GSC values of Atto565-hAGT in both rats and mice were approximately 25%–50% of the GSC values of Atto565-albumin. The molecular masses of these proteins are almost the same (albumin = 66 kD and AGT = 56.8 kD)23, but the Stokes–Einstein radius of hAGT was found to be 4.29 nm and the isoelectric point was pI = 4.724 compared with 3.5 nm for albumin and pI = 4.5.25 Thus, hAGT is significantly larger than albumin, but it is not close to the value of 5.4 nm of IgG.25 Therefore, other explanations are needed to account for the fourfold difference in sieving coefficients. Likewise, the charge barrier in the GFB and the endothelial glycocalyx are also not likely to be responsible, because both AGT and albumin are negatively charged (more so in albumin).24,26,27 Polymerization of the injected AGT28 and/or binding to other plasma proteins could have caused lower glomerular permeability. However, electrophoresis of the infused solution and the animals’ sera showed that the size of the majority of labeled proteins was around 60 kD (Supplemental Figure 2). This finding suggests that both proteins were monomers in the plasma. A very recent report by Weyer et al.29 found small amounts of low-molecular weight fragments of injected labeled albumin in the plasma of mice. Because these small fragments can be readily filtered in the kidney, they may help explain the higher albumin GSC results. Thus, if we assume that the true GSC for albumin is the same as the GSC for AGT (i.e., 0.00057), it would only take a fragmented fraction of labeled albumin of 0.19% to explain the fourfold difference between the two proteins (because of the fact that such fragments will have a sieving coefficient of 100%). Such a minute dissociation of a labeled protein is not unlikely. However, the presence of small filterable albumin fragments in the plasma would be difficult to detect in, for example, dialysis experiments, and they were not measured in the present study.
The albumin GSC values that were measured in the present study in healthy mice and younger MWF rats (below 0.003) are in agreement with most of the data in the literature25 and much smaller than those values reported recently by others using the same multiphoton imaging technique.16–18 In those studies, the Molitoris group reported that the glomeruli under normal and/or early diabetic states filtered nephrotic levels of albumin (GSC around 0.03) and that the proximal tubular uptake regulated the level of urinary albumin excretion.16–18 Several possible reasons for this difference have been discussed in detail elsewhere, including animal maintenance, imaging technical issues, and detection of scattered, nonspecific fluorescence in the Bowman’s space, which may be the most important factor.14,15,19,30 The small fractions of labeled albumin in the plasma (detailed above) may be yet another reason for the physiologically unrealistic high sieving values presented by other groups. As reported in the work by Weyer et al.,29 low-molecular weight albumin fragments amounting to 2% of the total albumin in serum would cause the albumin GSC to be grossly overestimated (0.0206 instead of 0.0006).
The present studies used 16-bit depth resolution of fluorescence intensities, in situ calibration, and optimal use of the detector gain and offset, which seem to be very important technical requirements to accurately measure the GSC of macromolecules using multiphoton microscopy. Figure 1 showed the approximate lowest limit (in the 0.0001 range) of our GSC measurements using a high-molecular mass (500 kD) dextran. Compared with this low limit, significantly higher (4- to 16-fold) but still extremely low GSC values were measured for AGT and albumin, indicating the minute but detectable amount of glomerular filtration of these macromolecules.
The significant level of light absorption by the high density of circulating red blood cells in the glomerular capillary was recently shown by our laboratory.15 This limiting factor when performing intravital multiphoton microscopy has been recognized in other vascular beds as well.31 Consistent with those measurements and the limitations in measuring the true plasma fluorescence with this technique,15 artificially high GSC values (more than two compared with the expected value of one) were measured for the freely filtered FITC-inulin (Figure 1, C and D). These data suggest that the true plasma fluorescence is more than twofold higher than detected and more importantly, that our AGT and albumin GSC data are overestimates. Therefore, the true GSC of AGT may be even lower than measured (i.e., in the 0.0003 range). It should also be noted that the Molitoris group recently reported a very different GSC value (one, which was physiologically expected) for FITC-inulin in their multiphoton measurements.18 The fact that red blood cells did not affect their detection of plasma fluorescence levels18 suggests very different conditions of glomerular hemodynamics, under which their measurements were performed. In fact, the very low and irregular speed of glomerular capillary red blood cells (determined by line scans) in their studies18 is a sign of poor renal perfusion. Red blood cell velocity in glomerular capillaries measured by multiphoton imaging is high and regular when physiologic conditions are properly maintained.14,15,20 Several studies suggested before14,15,19 that poor animal conditions (among other imaging-related factors) have been responsible for the high albumin GSC values measured by the Molitoris group.16–18
Also, the present study used two different species, C57Bl/6 mice and MWF rats, as well as two different fluorescence dyes, Atto565-maleimide and Atto565-NHS ester, for fluorescent labeling. Our current experimental conditions provided GSCs for albumin in the range of 0.0010–0.0037 from C57Bl/6 mice and 0.0007–0.0045 from 40-day-old MWF rats. These values are much closer to the GSC of 0.0006 previously reported by using a micropuncture technique in Sprague Dawley rats.32 Importantly, Atto565-hAGT or albumin fluorescence in the present multiphoton studies was limited to the glomerular and peritubular capillary plasma in healthy control mice and was absent in tubular structures (Figure 2). This finding is in stark contrast with the imaging data from MWF rats (Figure 4), which showed significant tubular uptake of both proteins. The conclusion from these studies is that imaging data from pathologic animal models cannot be used for the interpretation of normal glomerular and tubular processes and functions.
A recently published work by Matsusaka et al.33 using kidney- or liver-specific and dual AGT null mice provided additional support that, in the normal kidney, only a minor but detectable fraction of plasma AGT is filtered. However, disruption of the GFB led to the leakage of massive amounts of AGT protein into the tubular lumen, which was then reabsorbed by proximal tubular cells.33 In terms of urinary AGT excretion, the present study found robust elevations in endogenous, intrarenally produced AGT in the urine in aging MWF rats in contrast to the small increase in AGT of systemic (filtered) origin (Figure 6). Consistent with our data and the intrarenal tubular origin of urinary AGT, the work by Matsusaka et al.33 found that the urinary AGT to creatinine ratio was significantly lower in kidney and dual AGT knockout than control mice, but the ratio in liver AGT knockout mice was not.33 Also, it should be mentioned that MWF rats used in the present study have high RAS activity and are hypertensive22 in contrast to the NEP25 model of GFB damage (low renin and normotensive) used in the work by Matsusaka et al.33 Therefore, in the presently used MWF rat model, RAS activity (angiotensin II levels) was likely higher, which is known to cause strong stimulation of local intrarenal (tubular) AGT generation de novo.3 Also, the work by Pohl et al.34 reported that the small amount of filtered AGT was retrieved in early proximal segments through a megalin-dependent pathway and that 95% of it stayed (or was being degraded) in the cells, especially in lysosomes. The work by Pohl et al.34 also found AGT mRNA expression that is necessary for de novo production in proximal S3 straight segments. Our present findings are consistent with this previous report, and they support the view that AGT is produced in the tubules through de novo pathways and secreted into the urine during renal injury.35 Urinary AGT does not seem to reflect damage to the GFB (Figure 5, C and D).
Multiphoton fluorescence microscopy is a powerful experimental tool to directly and quantitatively visualize physiologic processes, including the function of the GFB in the intact kidney in vivo. We successfully visualized individual glomeruli in the intact C57Bl/6 mouse kidney and analyzed the GSC of AGT and albumin in the present study. This finding is an important technical advance, and the present study shows that the in vivo imaging of surface glomeruli and glomerular function are not limited to the use of the pathologic MWF rat model. However, this method has certain limitations for analyzing GSC. (1) A part of the fluorescently conjugated protein could be nonspecifically cleaved into small fragments and filtered by the glomeruli. This process would result in the overestimation of GSC. (2) Only a small fraction of the glomeruli can be analyzed in the kidney. Therefore, the GSC values in the present study may not represent all glomeruli, although the GSCs of Atto565-hAGT and Atto565-albumin strongly correlated with albuminuria in MWF rats (Figure 5, A and B). It is known that the pathologic changes in glomeruli are observed relatively earlier in juxtamedullary glomeruli compared with superficial glomeruli.36 Therefore, the GSC values of AGT and albumin in juxtamedullary glomeruli might be higher than the values measured in this study.
In conclusion, the glomerular permeability of injected exogenous AGT and albumin is extremely low in the healthy glomerulus. The filtration of AGT increases only slightly in kidney disease because of GFB damage, whereas much more robust elevations in endogenous, intrarenally produced AGT can be detected in the urine. Overall, the new findings strongly support the view that increases in urinary AGT reflect de novo production of AGT within the kidney and can be used as a marker of the activation of the intrarenal RAS.
Concise Methods
Fluorophores and Labeling
FITC-inulin and 500 kD FITC-dextran (anionic) were purchased from Sigma (St. Louis, MO) and Invitrogen (Carlsbad, CA), respectively. Recombinant full-length hAGT was a gift from IBL Japan. Mouse and rat serum albumin (Sigma) and hAGT were conjugated to Atto-565 maleimide (Sigma; Atto565-albumin and Atto565-hAGT, respectively) according to the manufacturer’s instructions. The samples were dialyzed by using 20,000 molecular weight cutoff dialysis tubing in a cold room for 48 hours. The dialyzed albumin was stored at −80°C until use. Just before every experiment, the dye-conjugated albumin and hAGT solutions were rinsed by adding isotonic saline in a Nanosep centrifugal device with an Omega 10-kD membrane filter (Pall, Port Washington, NY).
Animals
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Southern California. Male and female 5- to 6-week-old C57Bl/6 mice and male 40- to 130-day-old MWF rats were used for experiments from our established animal colonies at the University of Southern California (breeding pairs were originally received from Scott Thomson, University of California, San Diego, CA). Baseline 24-hour urine was collected in metabolic cages before multiphoton microscopy.
Surgical Procedures and Multiphoton Imaging
The animals, which had free access to food and water (no fasting), were weighed and then anesthetized with ketamine/xylazine (80+10 mg/kg, respectively) or isoflurane (4%) for mice and Inactin (100 mg/kg) for rats. After tracheotomy, the femoral and carotid artery (for BP measurement and drug injection), jugular vein (for maintenance fluid), and bladder (for urine collection) were cannulated, and the left kidney was exteriorized through a small flank incision. Body fluid losses were compensated by infusion of 0.9% NaCl containing 2% BSA at a rate of 1.0 μL/min for mice and 10 μL/min for rats. Bolus injections of 500 kD FITC-dextran (6 mg/kg) and FITC-inulin (65 mg/kg) were given into the cannulated carotid artery. The dye-conjugated protein (hAGT: 400 μg/kg in mice and 100 μg/kg in rats; albumin: 6.7 mg/kg in mice and 1.7 mg/kg in rats) was infused through the carotid artery at a rate of 60 μL/min for 2–3 minutes; this infusion rate does not affect heart rate and renal perfusion pressure. Continuous BP measurements were performed before and during imaging through the cannulated femoral artery by a pressure transducer and the BP-1 Blood Pressure Monitor (World Precision Instruments Inc., Sarasota, FL). Intravital multiphoton microscopy was performed using a Leica TCS SP5 multiphoton confocal fluorescence imaging system powered by a Chameleon Ultra-II MP laser at 860 nm (Coherent Inc., Santa Clara, CA) and a DMI 6000 inverted microscope’s external nondescanned detectors with a FITC/TRITC filter (Leica Microsystems) and previously published techniques.20 Fluorescence intensity was measured with 16-bit depth resolution to provide the necessary dynamic range for intensity measurements.14,15 The microscope stage and animals were warmed using a heating pad. The microscope objective was a 63× glycerol immersion lens with a 1.4 numerical aperture.
GSC Measurement
GSC was evaluated from one glomerulus in each mouse and at least eight superficial glomeruli in each rat shortly after the injection of the Atto565-labeled proteins. We confirmed that the range of Atto565-hAGT or Atto565-albumin fluorescence in the Bowman’s space and glomerular capillary plasma did not change significantly during the imaging period and was nonsaturating.
We calculated GSC as the ratio of fluorescence in the Bowman’s space versus the ratio of fluorescence in glomerular capillary plasma. Fluorescence intensity in the capillary was measured in regions of interest where plasma fluorescence was the brightest and at time points when the plasma level of fluorophores was stable. Fluorescence intensity in the Bowman’s space was analyzed at a focus with no glomerular capillaries immediately above or below the focal plane. The normal physiologic oscillations in Bowman’s space Atto565 fluorescence (which was described previously)14,15,20 were confirmed in each glomerulus before GSC imaging as an indication that the signal was specific for GFB function. Fluorescence excitation (laser power) and detector gain were set to the absolute minimum and were kept constant throughout imaging. Detector offset (background fluorescence level) was calibrated in situ in each glomeruli to reduce nonspecific fluorescence by applying different offset values in steps over a low optimal range (usually at −15%, −20%, −25%, and −30%, representing reductions in detector current) before and after dye injections. Using images that were obtained with the corresponding offset values before and after dye injection, background subtraction was performed for the analyzed regions of interest in the Bowman’s space and plasma. The detector offset was determined to be optimal if the setting allowed the detection of physiologic fluorescence oscillations14,15,20 and provided constant GSC values. We often found that increasing the offset above this low optimal level resulted in artificially high GSC values because of the high level of light scattering by fast moving red blood cells (nonspecific out of focus fluorescence in the Bowman’s space)15,19 after dye injection.
Ninety minutes after Atto565-protein injection, the kidneys were perfused by saline, isolated, snap frozen by liquid nitrogen, and stored at −80°C until use.
Plasma, Kidney, and Urinary Protein Content Measurement
The AGT levels in urine and plasma were measured by highly specific human, mouse, and rat AGT ELISAs for hAGT, mAGT, or rAGT, respectively (detection limit: 0.3 ng/ml).10,37,38 Urinary albumin was measured by ELISA (Nephrat II; Exocell, Philadelphia, PA). Plasma samples or the whole-kidney tissue were homogenized in a buffer containing 20 mM Tris⋅HCl, 1 mM EGTA, pH 7.0, and a protease inhibitor cocktail (BD Bioscience, San Jose, CA); 40 µg protein was separated on a 4%–20% SDS-PAGE and transferred onto polyvinylidene difluoride membrane. Blots with embedded Atto565 fluorescence were visualized with Odyssey Infrared Imaging System, Western Blot Analysis (LI-COR Biosciences).
Real-Time RT-PCR
The mRNA expression of β-actin and AGT were analyzed by real-time RT-PCR using a LightCycler FastStart DNA Master SYBR Green I kit or TaqMan Gene Expression Assay kits (Applied Biosystems, Foster City, CA). The primer sequences of β-actin and AGT were β-actin forward, 5′-GCCAGGATAGAGCCACCAATC-3′, reverse, 5′-ACTGCCCTGGCTCCTAGCA-3′, and AGT forward, 5′-TTGTGTGAGGAGGGCTGTAT-3′, reverse, 5′-TGCTGAGAGTGTAGGTCCTG-3′. The data were expressed as the relative differences between rats 42–60 days of age and rats 76–130 days of age after normalization to β-actin expression.
Statistical Analyses
Values are presented as direct values or mean ± SEM. Statistical comparisons of the differences in the GSC of Atto565-hAGT and Atto565-albumin were performed by t test. Correlation was obtained by Pearson linear correlation analysis. A value of P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to IBL Japan for providing recombinant hAGT.
This work was supported in part by a grant-in-aid for scientific research from the Kagawa University International Foundation (to D.N.), Kagawa University International Foundation Grant DK64324, and American Diabetes Association 1-11-BS-121 Grant (to J.P.-P.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012010078/-/DCSupplemental.
References
- 1.Gonzalez-Villalobos RA, Billet S, Kim C, Satou R, Fuchs S, Bernstein KE, Navar LG: Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol 22: 449–459, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG: Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol 298: F150–F157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobori H, Nangaku M, Navar LG, Nishiyama A: The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, Hamada M, Kishida M, Hitomi H, Shirahashi N, Kobori H, Imanishi M: Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant 26: 170–177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG: Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol 293: F938–F945, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobori H, Nishiyama A, Abe Y, Navar LG: Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobori H, Harrison-Bernard LM, Navar LG: Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG: Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension 41: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A: Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558–1565, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG: Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study). J Hypertens 28: 1422–1428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terada Y, Tomita K, Nonoguchi H, Marumo F: PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int 43: 1251–1259, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Ganten D, Wagner J, Zeh K, Bader M, Michel JB, Paul M, Zimmermann F, Ruf P, Hilgenfeldt U, Ganten U, Kaling M, Bachmann S, Fukamizu A, Mullins JJ, Murakami K: Species specificity of renin kinetics in transgenic rats harboring the human renin and angiotensinogen genes. Proc Natl Acad Sci U S A 89: 7806–7810, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G, Merrill DC, Thompson MW, Robillard JE, Sigmund CD: Functional expression of the human angiotensinogen gene in transgenic mice. J Biol Chem 269: 32497–32502, 1994 [PubMed] [Google Scholar]
- 14.Peti-Peterdi J: Independent two-photon measurements of albumin GSC give low values. Am J Physiol Renal Physiol 296: F1255–F1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peti-Peterdi J, Sipos A: A high-powered view of the filtration barrier. J Am Soc Nephrol 21: 1835–1841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D: Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20: 489–494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Sandoval RM, Wagner MC, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, Wean SE, Clendenon SS, Molitoris BA: Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol 23: 447–457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanner GA: Glomerular sieving coefficient of serum albumin in the rat: A two-photon microscopy study. Am J Physiol Renal Physiol 296: F1258–F1265, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J: Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol 291: F495–F502, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Peti-Peterdi J, Burford JL, Hackl MJ: The first decade of using multiphoton microscopy for high-power kidney imaging. Am J Physiol Renal Physiol 302: F227–F233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remuzzi A, Puntorieri S, Battaglia C, Bertani T, Remuzzi G: Angiotensin converting enzyme inhibition ameliorates glomerular filtration of macromolecules and water and lessens glomerular injury in the rat. J Clin Invest 85: 541–549, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tewksbury DA, Frome WL, Dumas ML: Characterization of human angiotensinogen. J Biol Chem 253: 3817–3820, 1978 [PubMed] [Google Scholar]
- 24.Auzan C, Genain C, Corvol P, Menard J, Chrambach A: Evaluation of the capacity of gel electrophoresis, steady-state and transient state electrofocusing to resolve native human des-angiotensin-I-angiotensinogen from angiotensinogen. Electrophoresis 6: 201–206, 1985 [Google Scholar]
- 25.Haraldsson B, Nyström J, Deen WM: Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Hirsch AT, Opsahl JA, Lunzer MM, Katz SA: Active renin and angiotensinogen in cardiac interstitial fluid after myocardial infarction. Am J Physiol 276: H1818–H1826, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Mera K, Takeo K, Izumi M, Maruyama T, Nagai R, Otagiri M: Effect of reactive-aldehydes on the modification and dysfunction of human serum albumin. J Pharm Sci 99: 1614–1625, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Stanley P, Serpell LC, Stein PE: Polymerization of human angiotensinogen: Insights into its structural mechanism and functional significance. Biochem J 400: 169–178, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weyer K, Nielsen R, Christensen EI, Birn H: Generation of urinary albumin fragments does not require proximal tubular uptake. J Am Soc Nephrol 23: 591–596, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen EI, Birn H, Rippe B, Maunsbach AB: Controversies in nephrology: Renal albumin handling, facts, and artifacts! Kidney Int 72: 1192–1194, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Haiss F, Jolivet R, Wyss MT, Reichold J, Braham NB, Scheffold F, Krafft MP, Weber B: Improved in vivo two-photon imaging after blood replacement by perfluorocarbon. J Physiol 587: 3153–3158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tojo A, Endou H: Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol 263: F601–F606, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I: Liver Angiotensinogen Is the Primary Source of Renal Angiotensin II. J Am Soc Nephrol 23: 1181–1189, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F: Intrarenal renin angiotensin system revisited: Role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 285: 41935–41946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingelfinger JR, Pratt RE, Ellison K, Dzau VJ: Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J Clin Invest 78: 1311–1315, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ihara G, Kiyomoto H, Kobori H, Nagai Y, Ohashi N, Hitomi H, Nakano D, Pelisch N, Hara T, Mori T, Ito S, Kohno M, Nishiyama A: Regression of superficial glomerular podocyte injury in type 2 diabetic rats with overt albuminuria: Effect of angiotensin II blockade. J Hypertens 28: 2289–2298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H: Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol 293: F956–F960, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, Hagiwara Y, Miyashita K, Navar LG: Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol 294: F1257–F1263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.