Abstract
Aim:
This study aimed at quantitatively evaluating the effect of colabased beverage on the calcium loss of enamel surface pre-treated with fluorideenriched casein phosphopeptideamorphous calcium phosphate (CPP-ACPF) and βeta-ricalcium phosphate (β-TCP) using energy dispersive X-ray analysis (EDX).
Materials and Methods:
24 enamel specimens were prepared from the buccal and palatal surfaces of extracted intact human premolars and were randomly assigned to study groups and control group. Specimens of Group II were pre-treated with CPP-ACPF and Group III treated with β-TCP twice daily for 4 for 28 days, followed by storage in artificial saliva. All specimens were evaluated for mineral (calcium and phosphorus) content (wt%) after pre-treatment using SEM-EDAX. The specimens were then placed in the acidic beverages for 4 days for 10 Mineral content was again measured using SEM-EDAX.
Results:
Statistical analysis using one-way ANOVA followed by Tukey's HSD test was applied to compare the re-mineralization and de-mineralization of the samples.
Conclusion:
The present study concluded that both the remineralizing agents tested were found to be effective in inhibiting the de-mineralization caused by colabased beverage. Among the remineralizing agents tested, β-TCP was found to be more effective than CPP-ACPF.
Keywords: Beta-ricalcium phosphate, colabased beverage, CPP-ACPF, de-mineralization, re-mineralization, scanning electron microscopy-energy dispersive X-ray analysis
INTRODUCTION
The tooth enamel is a microcrystalline porous structure that allows the access of ions into its deeper layers.[1] Tooth minerals are lost and regained constantly in normal oral environment. A key parameter considered in terms of re-mineralization is availability of inorganic constituents chiefly calcium. resence of calcium and phosphate ions during period of acid attack inhibits de-mineralization and re-mineralization.
De-mineralization in the oral cavity can be attributed mainly to dental caries, intrinsic and extrinsic erosive factors such as acidic foods, beverages, medicines etc. In recent decades researchers have shown a marked interest in studying the cause, process and management of both de-mineralization and re-mineralization of tooth structure. The basic mineral component of mature enamel is hydroxyapatite, Ca10(PO4)6(OH)2 For net remineralization to occur, adequate levels of calcium and phosphate ions must be available and this process is normally calcium phosphate limited. When the pH is neutral and there are sufficient Ca and P ions in the oral environment, the demineraliation process can be reversed i remineralization occurs
Erosive tooth wear or dental erosion has progressively gained more attention from the dental profession since the decline in dental caries.[2] Excessive consumption of acidic food beverages are one of the most common extrinsic factors that cause dental erosion.[3] Although, the de-mineralizing effect of acidic food beverages on human enamel has been well documented, the role of potentially protective agents that can inhibit this effect has not been investigated.
Recently, fluoridecontaining newer formulations such as fluorideenriched casein phosphopeptideamorphous calcium phosphate (CPP-ACPF) paste (GC Tooth Mousse Plus) and β-tricalcium phosphate (Clinpro™ Tooth Crème) have been introduced as advanced products for re-mineralization. The CPP-ACP has been shown to re-mineralize enamel subsurface lesions in situ when delivered in oral care products.[4] The level of fluoride is 900 ppm which approximates that of adult tooth pastes. CPP-ACPF binds to, plaque, hydroxyapatite localizing bio-available calcium, phosphate and fluoride.[2]
Has been considered as one possible means for enhancing levels of calcium in plaque and saliva.[5] Functionalized tricalcium phosphate (fTCP) mineral comprises of β-TCP and sodium lauryl sulfate (SLS)[6] with fluoride level of 950 ppm. The fTCP technology protects its bioavailable calcium with a fluoride-repelling surfactant (sodium lauryl sulfate)[7]
Several studies have been reported on the use of remineralizing agents in the management of de-mineralization arising from dental caries. However, there are only a few reports on the effect of acidic food beverages on enamel and even fewer on role of remineralizing agents in preventing the loss of minerals from the tooth structure previously treated with remineralizing agents.
Hence, the aim of this study is to evaluate the effect acidic food beverage on the calcium loss of enamel surface pre-treated with various remineralizing agents.
MATERIALS AND METHODS
Enamel specimens were prepared from 12 freshly extracted human premolars by sectioning from buccal and lingual surfaces using low speed diamond discs. Each enamel slab was carefully shaped into a dimension of 4 × 4 × 2 mm. Teeth were disinfected and handled according to the recommendations and guidelines laid down by OSHA and CDC.[8,9]
Artificial saliva was prepared in the central research lab, A. B Shetty Memorial Institute of Dental Sciences, Mangalore with components as follows with pH 6.57
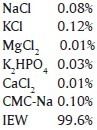
All the specimens were randomly divided into groups of specimens each and subjected to surface treatment as follows:
Group I (Control Group) – No surface treatment was done
Group II – Specimens were treated with CPP-ACPF for 4 twice daily for 28 days
Group III – Specimens were treated with β-TCP for 4 once daily for 28 days
The specimens were treated with remineralizing pastes once in the morning and once at night and were rinsed after each application and then stored in the artificial saliva.
After the samples were remineralized for 28 days, they were immersed in 5 ml colabased beverage for 10 for 4 days. After immersing in the cola solution, the samples were not rinsed and were directly stored in the artificial saliva.
Specimens were stored in artificial saliva throughout the study, both after surface application of the test materials and exposure to acidic food beverages. All the specimens were subjected to analysis using scanning electron microscopy (SEM)energy dispersive X-ray analysis (EDAX) (JEOL, JSM-840 A, Tokyo, Japan).
Statistical analysis of the data was done using oneway ANOVA (Analysis of variance) test, Tukey HSD Test SPSS (Statistical Package for Social Science) software version 15 was used.
RESULTS
SEM-EDAX analysis was used to determine calcium and phosphorus content in demineralized and re-mineralized enamel in each group [Figures 1 and 2].
Figure 1.
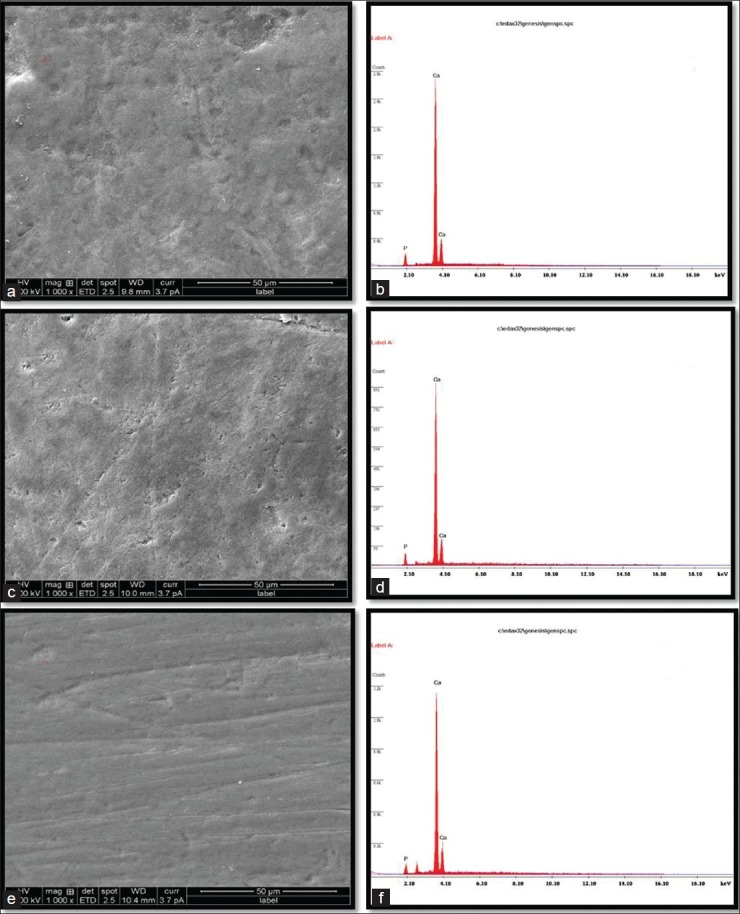
(a-b) Structural analysis by SEM and elemental analysis by EDX of sound enamel surface (Control)1000. (c-d) Structural analysis by SEM and elemental analysis by EDX, of enamel surface after remineralization with CPP-ACPF-1000. (e-f) Structural analysis by SEM, and elemental analysis by EDX, of enamel surface after remineralization with β-TCP1000
Figure 2.
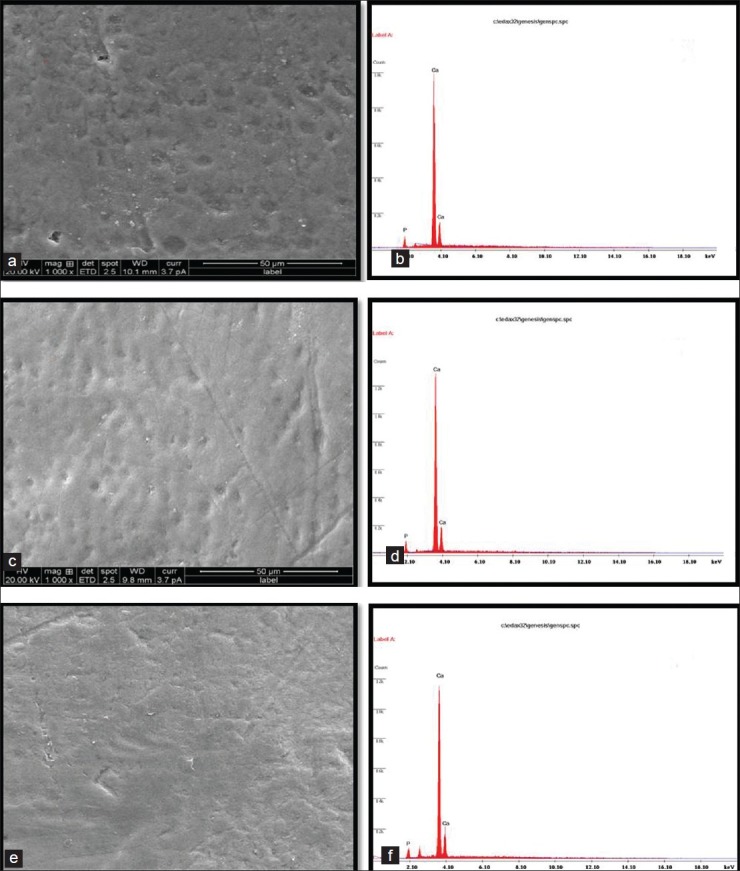
(a-b) Structural analysis by SEM and elemental analysis by EDX of enamel surface after demineralization with colabased beverage1000. (Control) (c-d) Structural analysis by SEM, and elemental analysis by EDX, after demineralization with colabased beverage, pre-treated with CPP-ACPF-1000. (e-f) Structural analysis by SEM, and elemental analysis by EDX, after demineralization with colabased beverage, pre-treated with β-TCP-1000
Figures 1a and b represents the surface and elemental representation of a section of sound enamel respectively.
Figure 1c represents the enamel surface after treatment with CPP-ACPF, showing occlusion of the enamel surface with calcium and phosphorus minerals which has been supported by EDAX graph [Figure 1d].
Figure 1e indicates enamel surface after treatment with βTCP which shows better results than CPP-ACPF which is justified by EDAX graph [Figure 1f]
Figures 2a and 2b represents the surface and elemental representation of a section of sound enamel surface after demineralization with colabased beverage respectively showing more mineral dissolution which is depicted in Figure 2b
Figure 2c indicates CPP-ACPF pre-treated enamel surface after demineralization with cola based beverage. Dissolution of enamel surface is visible but to a lesser exten compared to normal enamel surface which is justified by EDAX graph [Figure 2d]
Figure 2e indicates βTCP pre-treated enamel surface after demineralization with colabased beverage. The image shows that βTCP pre-treated enamel surface dissolves to much lesser exten after demineralization when compared to CPP-ACPF pre-treated enamel surface as supported by EDAX graph [Figure 2f]
Statistical analysis was done using oneway ANOVA test to compare the Ca/P ratios of specimens after re-mineralization [Table 1], and Tukey's HSD test to compare the Ca/P ratios of specimens after de-mineralization [Table 2] Ca/P ratios for Remineralized and Demineralized Enamel Samples of individual sample [Table 3], Mineral Content of Remineralized and Demineralized Enamel Samples [Table 4]
Table 1.
Intergroup comparison of the remineralization potential of the study groups using One-Way ANOVA
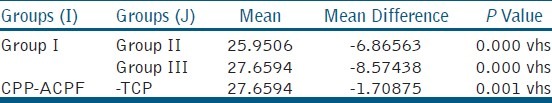
Table 2.
Tukey's hsd test showing the comparison of demineralization within the subgroups

Table 3.
Ca/P ratios for remineralized and demineralized enamel samples
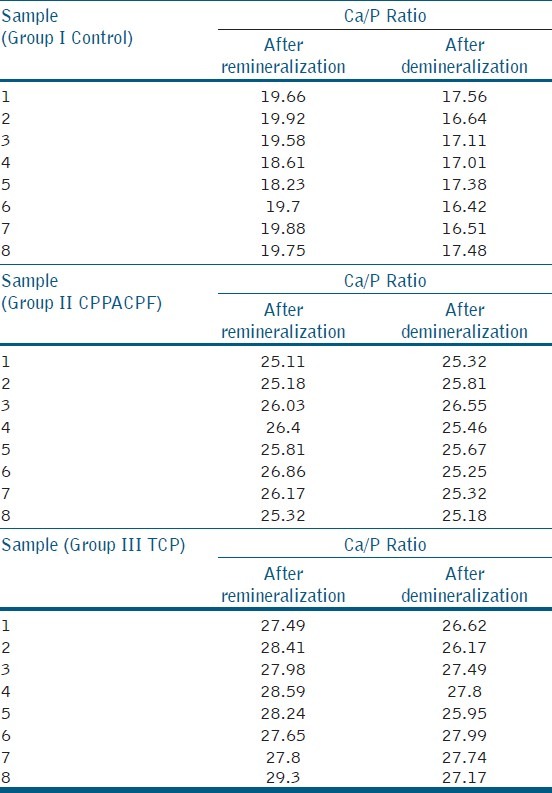
Table 4.
Mineral content of remineralized and demineralized enamel samples
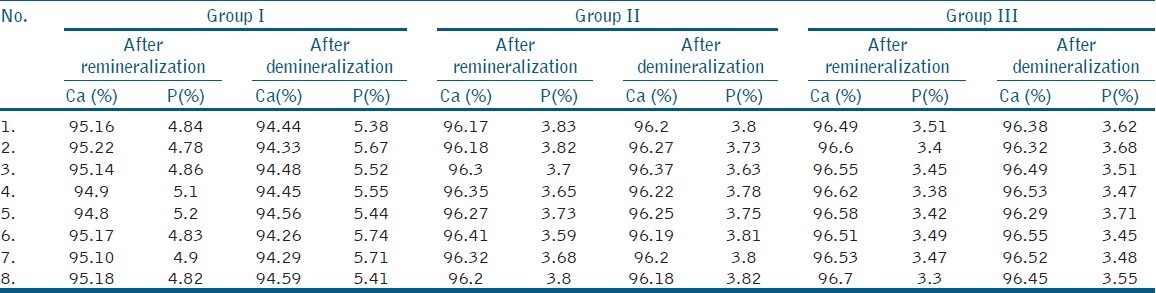
When the means of all the groups were compared β-TCP showed more re-mineralization potential than CPP-ACPF
When the means of all the groups after demineralization were compared, the least Ca/P value was observed in ontrol group and highest with β-TCP.
DISCUSSION
Dental erosion has reportedly shown an increased incidence in adolescents, especially after consumption of acidic beverages such as carbonated soft drinks.[2] Recently, new remineralizing materials, β-TCP and CPP-ACPF paste containing fluoride, introduced to the market. None of the studies studied the protective antierosive effect of these materials on permanent teeth.
Colabased beverage was used in this study to induce artificial erosive effect as in other studies[2,3,10] since it is commonly consumed acidic beverages amongst the youngsters. The cola drink was changed every cycle to ensure it was carbonated. The containers containing the cola were hermetically sealed as removal of gas from the drink increases its pH and decreases its potential of dissolving hydroxyapatite.[2] The specimens were incubated in artificial saliva to simulate the oral conditions.
Energy dispersive X-rays analysis (EDAX) has been used for elemental analysis at the ultrastructural level. It is one of the latest microanalytical techniques that are used in conjunction with scanning electron microscope (SEM) wherein SEM does the structural analysis and elemental analysis is done by EDAX.[4,11] The EDAX x-ray detector measures the number of emitted x-rays versus their energy. Spectrum of energy versus relative counts of detected x-ray is obtained and evaluated for qualitative and quantitative determinations of the elements present in the specimen using a computerbased program.[4]
The result of this study showed that both the remineralizing agents were able to provide protective effect against erosive enamel loss. Specimens pre-treated with β-TCP showed better protective effect than the specimens pre-treated with CP-ACPF
β-TCPbased tooth crème is one of the recently introduced remineralizing agents; hence, reports on clinical trials or efficacy reviews for comparison are unavailable in published literature. According to the manufacturer, as β-TCP is a precursor to hydroxyapatite formation, it is biocompatible, bioactive and manifests lattice defects that allow for crystal modification. β-TCP is created with a milling technique fusing β-TCP and sodium lauryl sulfate, ensuring that the calcium oxides not interact with fluoride, which could render both calcium and fluoride ineffective. When TCP comes into contact with the tooth surface which is moistened by saliva, the protective barrier breaks down, making the calcium, phosphate, and fluoride ions available to the teeth.[12]
CPP-ACPF, a fluorideenriched CPP-ACP formulation also showed resistance to demineralization following β-TCP. This can be attributed to the formation of stabilized amorphous calcium fluoride phosphate phase. This is in agreement with several other studies that proved combined effect of CPP-ACP and fluoride in enhancing re-mineralization and also improving acidresisting effect.[2,13]
CPP-ACP both localizes and increases bioavailability of calcium and phosphate ions and enhances uniform subsurface mineralization of enamel surface.[2] Incorporation of fluoride with CPP-ACP co-localizes calcium and phosphate ions with fluoride ions at the tooth surface as CPP-ACPF nanocomplexes. This increased concentration of calcium, phosphate and fluoride ions on the tooth surface leads to the diffusion of ions into the enamel and the sub-surface lesion fluid, resulting in higher levels of remineralization and fluoride incorporation into the mineral phase.[14]
The results of the present study showed among the remineralizing agents used β-TCP was found to be highly effective and showed better preventive effect.
CONCLUSION
Under the parameters of this study following conclusions were made
Both the remineralizing agents tested were found to be effective in inhibiting the demineralization caused by cola-based beverage.
Among the remineralizing agents used in the study β-TCP was found to be more effective than CPP-ACPF
Energy dispersive X-ray analysis was found to be an efficient way to quantitatively access the changes in mineral content during in vitro studies
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Nikiforuk G. The Nature of Tooth Substance. The Caries Process-Morphological and Chemical Events. In: Nikiforuk G, editor. Understanding Dental Caries; Basic and Clinical Aspects: Etiology and Mechanisms. Vol. 1. Canada: Basel: S Karger; 1985. pp. 261–89. [Google Scholar]
- 2.Badr SB, Ibrahim MA. Protective effect of three different fluoride pretreatments on artificially induced dental erosion in primary and permanent teeth. J Am Sci. 2010;6:442–51. [Google Scholar]
- 3.Tantbirojn D, Huang A, Ericson MD, Poolthong S. Change in the surface hardness of enamel by a cola drink and a CPP-ACP paste. J Dent. 2008;36:74–9. doi: 10.1016/j.jdent.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Hegde MN, Shetty S, Pardal D. Remineralization of enamel sub-surface lesion using Casein Phosphopeptide Amorphus Calcium Phosphate (CPP-ACP) J Conserv Dent. 2007;10:19–25. [Google Scholar]
- 5.Contemporary technologies for re-mineralization therapies: A review. Laurence J Walsh Int Dent SA. 2009;11:6–16. [Google Scholar]
- 6.Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Spectroscopic evaluation of native, milled, and functionalized β-TCP seeding into dental enamel lesions. J Mater Sci. 2009;44:5013–6. [Google Scholar]
- 7.Karlinsey RL, Mackey AC, Walker ER, Amaechi BT, Karthikeyan R, Najibfard K, et al. Remineralization potential of 5,000 ppm fluoride dentrifices evaluated in a pH cycling model. J Dent Oral Hyg. 2010;2:1–6. [Google Scholar]
- 8.Pantera EA, Jr, Schuster GS. Sterilization of extracted human teeth. J Dent Educ. 1990;54:283–5. [PubMed] [Google Scholar]
- 9.Centers for disease control and prevention. Guidelines for infection control in dental health-care settings-2003. MMWR Recomm Rep. 2003;52:1–66. [PubMed] [Google Scholar]
- 10.Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of Cola drinks and Orange juices. J Dent Res. 2006;85:226–30. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 11.Arends J, Bosch JJ. Demineralization and re-mineralization evaluation techniques. J Dent Res. 1992;71(special issue):924–8. doi: 10.1177/002203459207100S27. [DOI] [PubMed] [Google Scholar]
- 12.Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Surfactant-modified β-TCP: Structure, properties, and in vitro remineralization of subsurface enamel lesions. J Mater Sci Mater Med. 2010;21:2009–20. doi: 10.1007/s10856-010-4064-y. [DOI] [PubMed] [Google Scholar]
- 13.Lennon AM, Pfeffer M, Buchalla K, Becker K. Effect of a casein/calcium phosphate containing tooth cream and fluoride on enamel erosion in vitro. Caries Res. 2006;40:154–8. doi: 10.1159/000091063. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, et al. Fluoride and Casein phosphopeptide – Amorphous calcium phosphate. J Dent Res. 2008;87:344–8. doi: 10.1177/154405910808700420. [DOI] [PubMed] [Google Scholar]


