Abstract
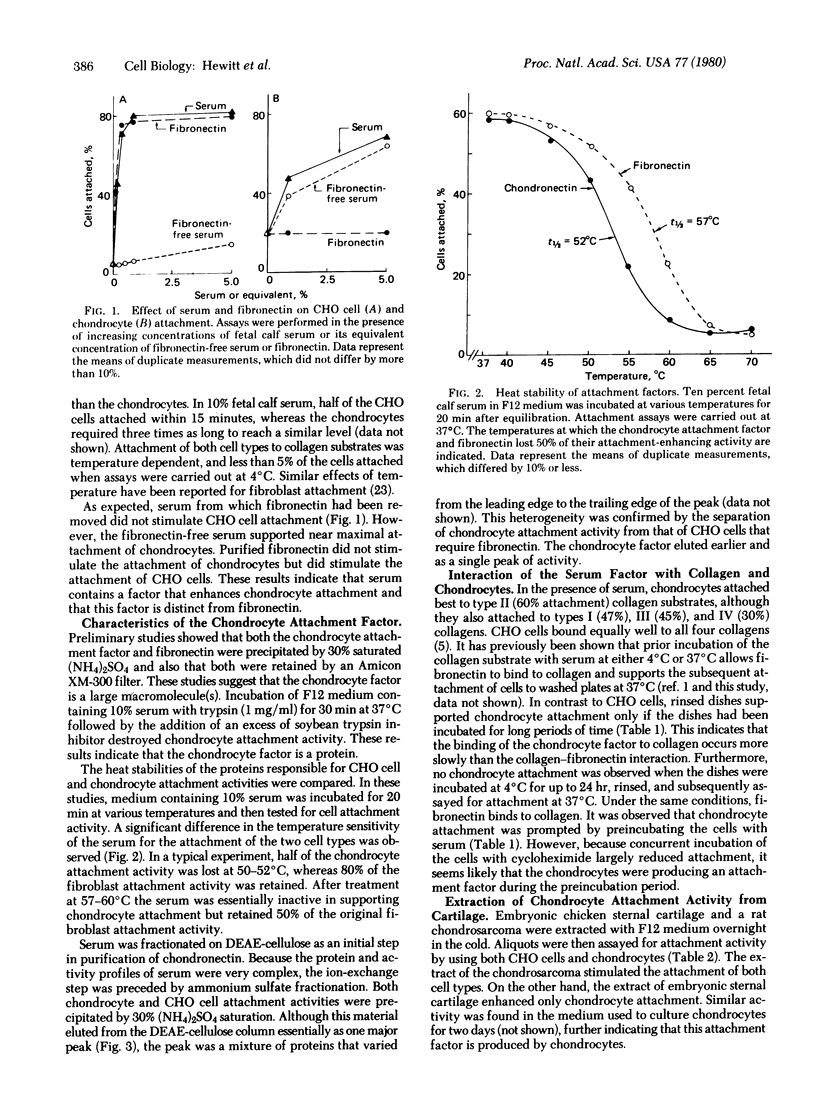
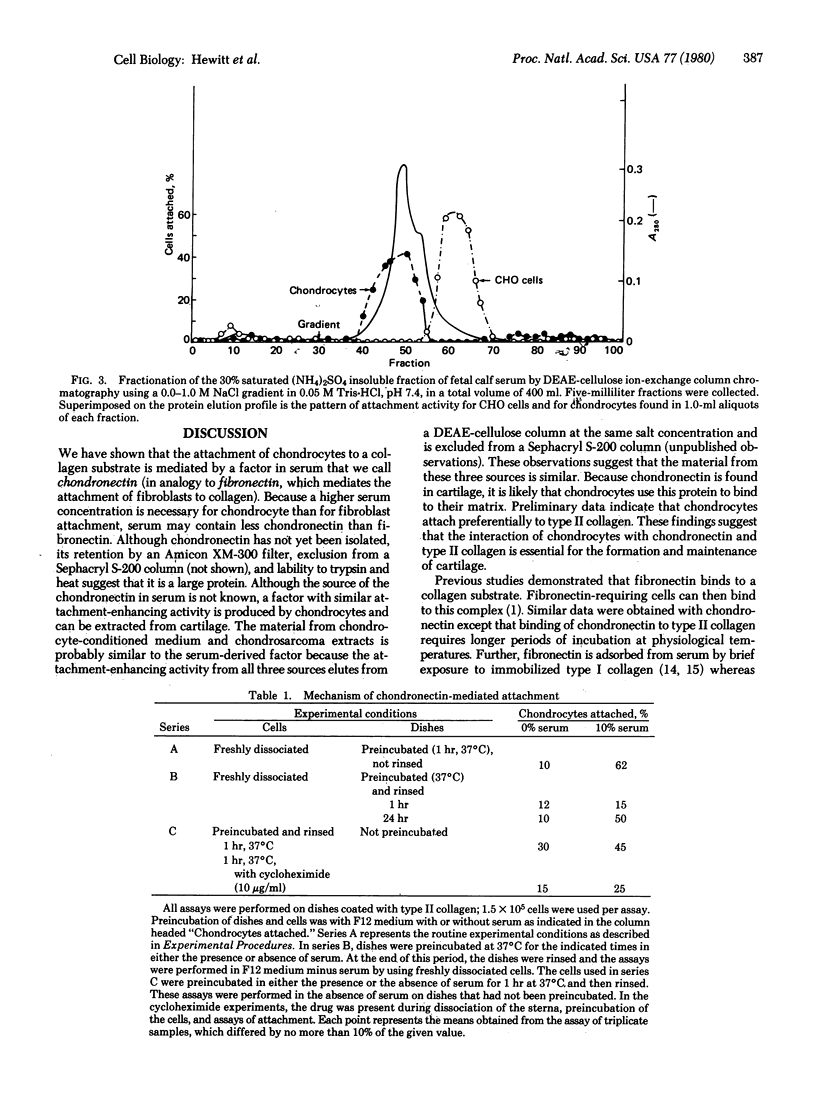
The attachment of chondrocytes to collagen substrates is stimulated by serum but not by fibronectin. The active material in serum was partially purified and was shown to be a protein by its sensitivity to trypsin and heat and its chromatographic properties. This factor, which we have named chondronectin, is distinct from fibronectin and does not stimulate fibroblast attachment. Because material with similar attachment-enhancing activity is produced by chondrocytes and is extractable from cartilage, chondronectin may be a chondrocyte-specific attachment protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Chen L. B. Alteration in cell surface LETS protein during myogenesis. Cell. 1977 Mar;10(3):393–400. doi: 10.1016/0092-8674(77)90026-5. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Maitland N., Gallimore P. H., McDougall J. K. Detection of the large external transformation-sensitive protein on some epithelial cells. Exp Cell Res. 1977 Apr;106(1):39–46. doi: 10.1016/0014-4827(77)90238-5. [DOI] [PubMed] [Google Scholar]
- Dessau W., Sasse J., Timpl R., Jilek F., von der Mark K. Synthesis and extracellular deposition of fibronectin in chondrocyte cultures. Response to the removal of extracellular cartilage matrix. J Cell Biol. 1978 Nov;79(2 Pt 1):342–355. doi: 10.1083/jcb.79.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRMANN R. L., GEY G. O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956 Jun;16(6):1375–1403. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Hauschka S. D., Konigsberg I. R. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966 Jan;55(1):119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Immunologic detection of retina cognin on the surface of embryonic cells. Exp Cell Res. 1979 Mar 15;119(2):191–204. doi: 10.1016/0014-4827(79)90348-3. [DOI] [PubMed] [Google Scholar]
- Hopper K. E., Adelmann B. C., Gentner G., Gay S. Recongnition by guinea-pig peritoneal exudate cells of conformationally different states of the collagen molecule. Immunology. 1976 Feb;30(2):249–259. [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Cell attachment to collagen: the requirement for energy. J Cell Physiol. 1975 Oct;86(2 Pt 1):231–236. doi: 10.1002/jcp.1040860206. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Lewis C. A., Pratt R. M., Pennypacker J. P., Hassell J. R. Inhibition of limb chondrogenesis in vitro by vitamin A: alterations in cell surface characteristics. Dev Biol. 1978 May;64(1):31–47. doi: 10.1016/0012-1606(78)90058-1. [DOI] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol. 1974 Jun;38(2):249–270. doi: 10.1016/0012-1606(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Muir L. W., Bornstein P., Ross R. A presumptive subunit of elastic fiber microfibrils secreted by arterial smooth-muscle cells in culture. Eur J Biochem. 1976 Apr 15;64(1):105–114. doi: 10.1111/j.1432-1033.1976.tb10278.x. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Stingl G., Kleinman H. K., Martin G. R., Katz S. I. Epidermal cells adhere preferentially to type IV (basement membrane) collagen. J Cell Biol. 1979 Jan;80(1):197–202. doi: 10.1083/jcb.80.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Smith B. D., Martin G. R., Miller E. J., Dorfman A., Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975 Jan;166(1):181–186. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E., Westermark B., Ponten J. A common cell-type specific surface antigen in cultured human glial cells and fibroblasts: loss in malignant cells. J Exp Med. 1976 Jan 1;143(1):64–72. doi: 10.1084/jem.143.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]



