Abstract
Regeneration of pulp-dentin complex in an infected necrotic tooth with an open apex is possible if the canal is effectively disinfected. The purpose of this case report is to add a regenerative endodontic case to the existing literature about using Platelet Rich Fibrin (PRF). A nine year old boy who accidently broke his immature maxillary central incisor tooth, developed pulpal necrosis with apical periodontitis. After the access cavity preparation, the canal was effectively irrigated with 20 ml of 5.25% sodium hypochlorite solution and 10ml of 0.2% chlorhexidine solution and dried with paper points. Triple antibiotic paste was placed inside the canal and left for 21 days. 12 ml of whole blood was drawn from the patient's right antecubital vein and centrifuged for 10 minutes to obtain the Choukroun's PRF. After the removal of the triple antibiotic paste, the PRF was placed into the canal till the level of cementoenamel junction and 3mm of grey MTA was placed directly over the PRF clot. The setting of MTA was confirmed 3 days later and the tooth was double sealed with GIC and Composite restoration. After 1 year the clinical examination revealed negative responses to percussion and palpation tests. The tooth responded positively to cold and electric pulp tests. Radiographic examination revealed continued thickening of the dentinal walls, root lengthening, regression of the periapical lesion and apical closure. On the basis of the results obtained in our case report we conclude that revitalization of necrotic infected immature tooth is possible under conditions of total canal disinfection and PRF is an ideal biomaterial for pulp-dentin complex regeneration.
Keywords: Open apex, revitalization, Platelet Rich Fibrin
INTRODUCTION
Regenerative endodontic procedures are biologically based procedures which deals with the regeneration of pulp like tissue, more idealistically the pulp-dentin complex, damaged coronal dentin such as that following a carious exposure or trauma; and regenerate resorbed root, cervical or apical dentin.[1] The mechanics behind the revitalization endodontic procedure is that, despite the tooth being necrotic, some pulp tissue can survive apically which under favorable conditions proliferate to aid in the process of regeneration.[2–4] Although there are no established standardized treatment protocols for endodontic regeneration, many of these cases have shown favorable results, with continued radiographic evidence of development of the dentin-pulp complex and an absence of clinical symptoms.[3,5–11]
The conventional method of revitalization procedure was done by inducing bleeding into the pulp canal by mechanically irritating the periapical tissues.[3] In necrotic teeth with open apices, some amount of pulp tissue along with Hertwigs Epithelial Root Sheath may survive apically and these tissues can proliferate once the inflammatory condition are reversed and the canal becomes totally disinfected.[3] The created blood clot acts as a matrix for the in growth of new tissues into the pulp canal. However, this procedure will cause discomfort for the patient while mechanically irritating the periapical tissues.
In the past two decades, an increased understanding of the physiological roles of platelets in wound healing and after tissue injury has led to the idea of using platelets as therapeutic tools. Platelet-Rich Plasma (PRP) consists of a limited volume of plasma enriched with platelets, which is obtained from the patient. The use of PRP as a potentially ideal scaffold for regenerative endodontic therapy has been documented in the literature.[12] However, the use of bovine thrombin for the activation of Platelet Rich Plasma (PRP) has been an issue of controversy which led to the development of the second generation Platelet concentrate known as Choukroun's Platelet Rich Fibrin (PRF) which is totally autologous in nature.[13,14]. PRF was developed in France by Choukroun et al, in 2001.[13] This technique is very simple and inexpensive. PRF contains platelets, growth factors, and cytokines that might enhance the healing potential of both soft and hard tissues.[14] Literature survey reveals that there is an absence of documentation regarding the application of PRF in the field of regenerative endodontics. The purpose of this case report is to add a regenerative endodontic case to the existing literature about using PRF.
CASE REPORT
A 9-year-old boy came with the chief complaint of broken upper front tooth (#8) along with discoloration [Figure 1a]. past dental history revealed trauma to his upper front tooth (#8). The medical history of the patient was non-contributory. Intraoral examination of his teeth revealed the presence of discolored tooth #8 along with Ellis class IV fracture. Tooth #8 was sensitive to both percussion and periapical palpation tests. It did not respond to CO2 ice and electric pulp test (EPT). Periodontal probing depth of the tooth #8 was within normal limit. Intraoral Periapical Radiographic examination of tooth #8 revealed an immature root and an open apex associated with periapical radiolucency [Figure 2a].
Figure 1a.
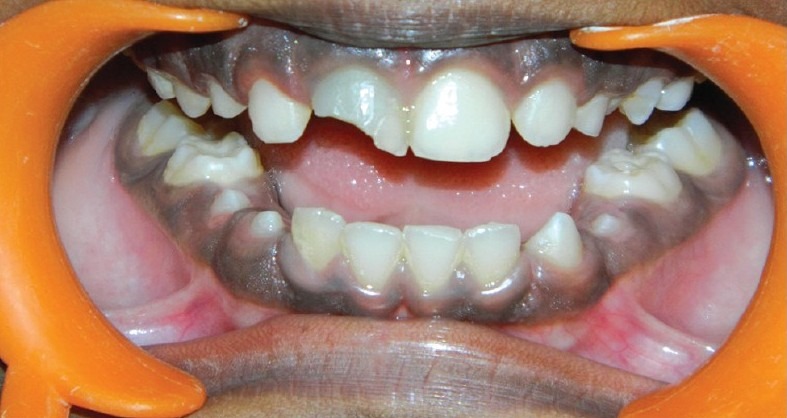
Broken upper front tooth (#8) along with discoloration
Figure 2a.
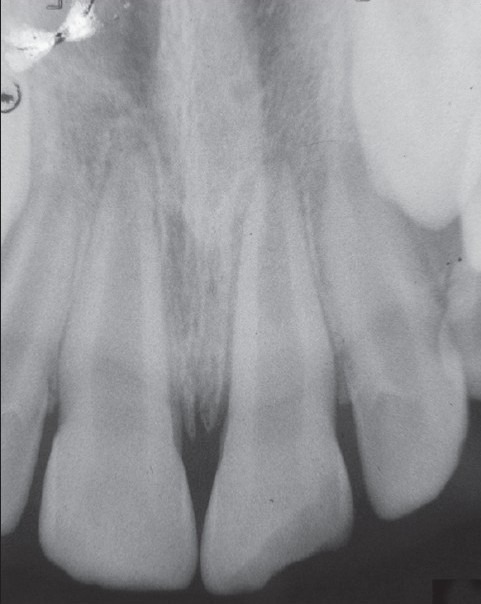
Immature root and an open apex associated with apical periodontitis
Further radiographic examination of the tooth revealed a 3 mm open apex along with thin dentinal walls that appeared prone to fracture. So a clinical decision of performing a regenerative endodontic treatment using Choukroun's Platelet Rich Fibrin was decided. A written informed consent was obtained from the patient's mother. Local anesthesia was achieved using Lignocaine (1:100000 adrenaline, DJ Lab, India). After the rubber dam application, access cavity preparation was done on the tooth #8. The canal was thoroughly irrigated with 20 ml of 5.25% sodium hypochlorite solution (Novo Dental Product, India) and nuetralised with saline. Following this, irrigation was done using 10ml of hexidine solution (0.2% Chlorhexidine, Vishal Dentocare, India) and dried with paper points (Dentsply Maillefer Ballaigues). A mixture of Ciprofloxacin (Cifran 500mg, Ranbaxy Lab, India), Metronidazole (Metrogyl 400mg, J.B.Chemicals and Pharmaceuticals, India), and Minocycline paste (Minoz 50 mg, Ranbaxy Lab, India) was prepared into a creamy consistency and introduced into the canal using a lentulospiral. A cotton pellet was placed and the cavity was temporarily sealed with cavit (Dental Products of India, India).
The patient returned after 21 days to the clinic and was asymptomatic. Local anesthesia was given, followed by rubber dam isolation; then the access cavity was reopened and thoroughly irrigated with sterile saline solution and dried with paper points. A 12ml sample of whole blood was drawn intravenously from the patient's right antecubital vein and centrifuged (REMI Model R-8c with 12×15ml swing out head) under 3000 rpm for 10 minutes to obtain the PRF which was jelly like in consistency. The PRF was condensed into the canal using a finger plugger (Dentsply Maillefer Ballaigues) till the level the cementoenamel junction. Grey MTA (ProRoot MTA; Dentsply) was placed directly over the PRF to a thickness of 3mm followed by a wet cotton pellet and cavit. The patient was recalled after 3 days and the setting of MTA was confirmed. The access cavity was then double sealed with GIC and Composite restoration [Figure 1b].
Figure 1b.
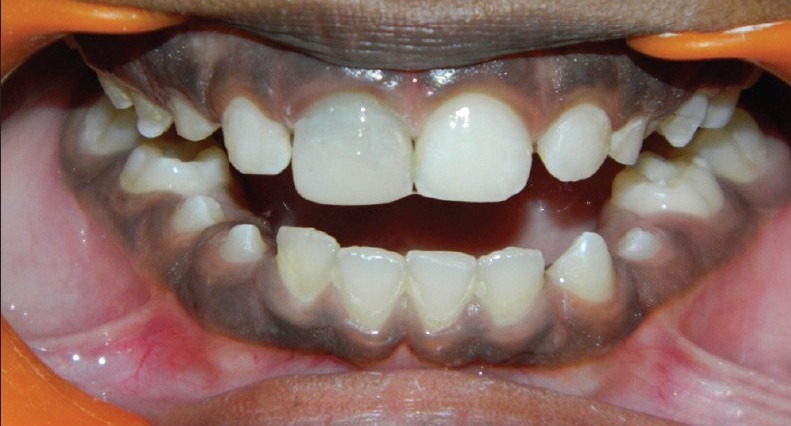
Double sealed with GIC and Composite restoration
The patient returned to the clinic after 3 months, 6 months, 9 months and 1 year for review and was asymptomatic; the tooth #8 showed negative response to percussion and palpation tests and responded positive to CO2 ice or an electric pulp tester (EPT). Radiograph revealed continued thickening of the dentinal walls, root lengthening, regression of the periapical lesion and apical closure [Figure 2b]. The patient is still under review.
Figure 2b.
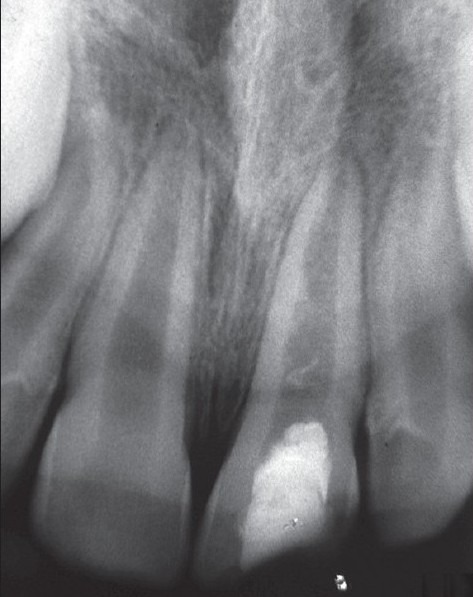
After 1 year there is continued thickening of the dentinal walls, root lengthening, and regression of the periapical lesion and apical closure
DISCUSSION
Banchs and Trope documented a case report where revascularization of immature permanent teeth with apical periodontitis was made possible by inducing blood clot into the pulp canal by mechanically irritating the periapical tissues which they attributed to the total disinfection of the canal.[3] However, this procedure might cause discomfort for the patient. So there was a quest for a better approach in regenerative endodontics which led to the introduction of Platelet Rich Plasma in the revitalization procedures.[12]
PRP can either be activated by bovine thrombin or calcium sulfate.[15] However, there is an ongoing debate regarding the bovine thrombin which is used in the activation of PRP. Bovine thrombin has been reported to cause adverse reactions like hemorrhage, thrombosis and substantial immune reactions like Systemic Lupus Erythematosus (SLE).[16] Factor V and thrombin inhibitors may develop following exposure to bovine thrombin preparations.[17] Calcium sulfate is also used in the activation of PRP with equal efficacy as that of bovine thrombin with no adverse effects.[15] Nevertheless, the process of preparation of PRP is time consuming and difficult compared to PRF.[18] Though, platelet-rich plasma has shown clinical success in enhancing endodontic regeneration for periapical inflammatory lesion, the long-term predictability remains questionable, and its anticipated benefits are moderate.[14]
PRF has been shown to have several advantages over traditionally prepared platelet-rich plasma. Its chief advantages include ease of preparation and lack of biochemical handling of blood, which makes this preparation strictly autologous.[13,14,19] Recently, studies have demonstrated that the PRF has a very significant slow sustained release of many key growth factors like PDGF and TGF β for at least 1 week and up to 28 days, which means that PRF could release growth factors with its own biological scaffold for wound healing process.[13,14,20] This leads to the idea of using PRF as a capping agent for reparative dentin formation or as a biomaterial for pulp regeneration.[14] Unlike the PRP, PRF by Choukroun's technique does not dissolve quickly after application; instead the strong fibrin matrix is slowly remodeled in a similar way to a natural blood clot.[13,14,20]
Unlike previous case reports,[3,5–10] we used PRF as a scaffolding material in an infected necrotic immature tooth for pulpal regeneration and tooth revitalization as it satisfies many criteria of an ideal physical scaffold. Another advantage of using PRF as a scaffold is that it has a trimolecular or equilateral fibrin branch junction which makes its architecture flexible and can support cytokine enmeshment and cellular migration.[13] 20 ml of 5.25% sodium hypochlorite was used to irrigate the canal. Care was taken to ensure that the irrigating needle was loose in the canal and that the NaOCl irrigation was performed very slowly. Triple antibiotic paste was used for disinfection of the canal because this particular combination is effective in addressing the diverse flora present in the root canal.[21] Sato et al, investigated this drug combination in vitro and found it to be very effective in the sterilization of carious lesions, necrotic pulps, infected root dentin and periapical lesions. This drug combination is also effective in killing the bacteria in the deep layers of root canal dentin.[21]
Directly over the PRF clot the MTA was packed and condensed to obtain a tight coronal seal as it is hydrophilic and needs moisture to set, which is a favorable property when there is potential for moisture contamination in the clinical setting, and also MTA by itself provides signaling molecules for the growth of the stem cells.[22,23] The positive response to cold test and EPT testing in our case report can be attributed to the placement of MTA slightly below the level of CEJ. If we had got negative response to the vitality testing it could have been due to the thickness of MTA which halts the growth of the new tissue ahead of it and also if placement of MTA is near CEJ the chances of eliciting a positive response is high.[12,24] Nevertheless, even after the closure of the apex (apexogenesis), thickening of the root dentin walls (maturogenesis) and regression of the apical periodontitis; if the response to vitality testing is still negative it does not mean that the tooth is non-vital because the very fact that the root maturation has taken place, it means that there is pulp like tissue in growth into the root canal with functional odontoblasts.[3] We double sealed the access cavity preparation with GIC and composite restoration. On radiographic examination, we could elucidate that there was continued thickening of the dentinal walls, root lengthening, regression of the periapical lesion and apical closure in 1 year which can be due to the use of PRF. Based on the clinical and radiographic examination we can only say with certainty that the pulp space had returned to a vital state. Based on research in avulsed teeth and on a recent study on infected teeth, it is more likely that the tissue in the pulp space is more similar to periodontal ligament than to pulp tissue. It appears that there is about a 30% chance of pulp tissue re-entering the pulp space.[2,25]
The potential theory behind the success of the presented case could be attributed to a study conducted by Huang et al, who concluded that the PRF causes proliferation of human Dental Pulp Cells and increases the protein expression of osteoprotegerin (OPG) and alkaline phosphatase (ALP) activity. Some amounts of human dental pulp cells present in the apical papilla usually remain vital even in case of a large periapical lesion. After the regression of the inflammation and under the influence of Hertwigs Epithelial Root Sheath these Dental Pulp Cells differentiate into odontoblasts like cells.[3,11] OPG and ALP expression are generally regarded as markers of odontoblastic differentiation.[14] As there was no bleeding in the root canal before placing the PRF we conclude that whatever tissue was produced in the canal could be attributed to the presence of PRF.
On the basis of the results obtained in our case report we conclude that revitalization of necrotic infected immature tooth is possible under conditions of total canal disinfection and PRF is an ideal biomaterial for pulp-dentin complex regeneration. The only disadvantage we faced with PRF was its manipulation to place inside the canal. Clinical trials are necessary to compare the effect of PRP, PRF and Induced bleeding in the revitalization of tooth with necrotic pulp and open apex on a long term basis.
Footnotes
Source of Support: Nil
Conflict of Interest: (If present, give more details): NA.
REFERENCES
- 1.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: A review of current status and a call for action. J Endod. 2007;33:377–90. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Trope M. Treatment of the immature teeth with non-vital pulps and apical periodontitis. Dent Clin North Am. 2010 Apr;31:313–24. doi: 10.1016/j.cden.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J Endod. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Ritter AL, Ritter AV, Murrah V, Sigurdsson A, Trope M. Pulp revascularization of replanted immature dog teeth after treatment with minocycline and doxycycline assessed by laser Doppler flowmetry, radiography, and histology. Dent Traumatol. 2004;20:75–84. doi: 10.1111/j.1600-4469.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 5.Chueh LH, Ho YC, Kuo, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod. 2009;35:160–4. doi: 10.1016/j.joen.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Chueh LH, Huang GT. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: A paradigm shift. J Endod. 2006;32:1205–13. doi: 10.1016/j.joen.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185–7. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 8.Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: Case report and review of the literature. Pediatr Dent. 2007;29:47–50. [PubMed] [Google Scholar]
- 9.Petrino JA. Revascularization of necrotic pulp of immature teeth with apical periodontitis. Northwest Dent. 2007;86:33–5. [PubMed] [Google Scholar]
- 10.Cotti E, Mereu M, Lusso D. Regenerative treatment of an immature, traumatized tooth with apical periodontitis: Report of a case. J Endod. 2008;34:611–6. doi: 10.1016/j.joen.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: A pilot clinical study. J Endod. 2008;34:919–25. doi: 10.1016/j.joen.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. J Endod. 2011;37:265–8. doi: 10.1016/j.joen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod. 2010;36:1628–32. doi: 10.1016/j.joen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Intini G, Andreana S, Intini FE, Buhite RJ, Bobek LA. Calcium sulfate and platelet-rich plasma make a novel osteo inductive biomaterial for bone regeneration. J Transl Med. 2007;5:13. doi: 10.1186/1479-5876-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronstein BN. Bovine thrombin and systemic autoimmunity. Am J Pathol. 2003;162:1389. doi: 10.1016/s0002-9440(10)63935-1. author reply 1389-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bomgaars L, Carberry K, Fraser C, West A, Teruya J. Development of factor V and thrombin inhibitors in children following bovine thrombin exposure during cardiac surgery: A report of three cases. Congenit Heart Dis. 2010;5:303–8. doi: 10.1111/j.1747-0803.2009.00344.x. [DOI] [PubMed] [Google Scholar]
- 18.Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: Evolution of a second-generation platelet concentrate. Indian J Dent Res. 2008;19:42–6. doi: 10.4103/0970-9290.38931. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell SM. Safety issues associated with platelet-rich fibrin method. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:587. doi: 10.1016/j.tripleo.2007.03.017. author reply 587-93. [DOI] [PubMed] [Google Scholar]
- 20.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate.Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Windley W, 3rd, Teixeira F, Levin L, Sigurdsson A, Trope M. Disinfection of immature teeth with a triple antibiotic paste. J Endod. 2005;31:439–43. doi: 10.1097/01.don.0000148143.80283.ea. [DOI] [PubMed] [Google Scholar]
- 22.Torabinejad M, Parirokh M. Mineral trioxide aggregate: A comprehensive literature review--part II: Leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Gancedo-Caravia L, Garcia-Barbero E. Influence of humidity and setting time on the push-out strength of mineral trioxide aggregate obturations. J Endod. 2006;32:894–6. doi: 10.1016/j.joen.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Johns DA, Vidyanath S. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. J Endod. 2011;37:743. doi: 10.1016/j.joen.2011.03.018. author reply 743-4. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Thibodeau B, Trope M, Trope M, Lin LM, Huang GT. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36:56–63. doi: 10.1016/j.joen.2009.09.039. [DOI] [PubMed] [Google Scholar]


