Abstract
Amongst the various routes of drug delivery, the field of ocular drug delivery is one of the most interesting and challenging endeavors facing the pharmaceutical scientist for past 10-20 years. As an isolated organ, eye is very difficult to study from a drug delivery point of view. Despite this limitation, improvements have been made with the objective of maintaining the drug in the biophase for an extended period. A major problem in ocular therapeutics is the attainment of an optimal drug concentration at the site of action. To achieve effective ophthalmic therapy, an adequate amount of active ingredient must be delivered and maintained within the eye. The most frequently used dosage forms, i.e., eye solution, eye ointments, eye gels, and eye suspensions are compromised in their effectiveness by several limitations leading to poor ocular bioavailability. Ophthalmic use of viscosity-enhancing agents, penetration enhancers, cyclodextrins, prodrug approaches, and ocular inserts, and the ready existing drug carrier systems along with their application to ophthalmic drug delivery are common to improve ocular bioavailability. Amongst these hydrogel (stimuli sensitive) systems are important, which undergo reversible volume and/or sol-gel phase transitions in response to physiological (temperature, pH and present of ions in organism fluids, enzyme substrate) or other external (electric current, light) stimuli. They help to increase in precorneal residence time of drug to a sufficient extent that an ocularly delivered drug can exhibit its maximum biological action. The concept of this innovative ophthalmic delivery approach is to decrease the systemic side effects and to create a more pronounced effect with lower doses of the drug. The present article describes the advantages and use stimuli sensitive of hydrogel systems in ophthalmic drug delivery.
Keywords: Hydrogel, in situ gel, instillation, ocular, stimuli sensitive
INTRODUCTION
The main aim of pharmacotherapeutics is the attainment of effective drug concentration at the intended site of action for a sufficient period of time to elicit the response. Amongst the various routes of drug delivery, the field of ocular drug delivery is one of the most interesting and challenging endeavors facing the pharmaceutical scientist. This is significantly improved over the past 10-20 years. As an isolated organ the eye is difficult to study from a drug point of view. It is very difficult to obtain eye tissue containing drugs from humans, so one is compelled to use animal tissue. Due to these human ocular disposition characteristics of virtually important drugs is unknown or incomplete. Despite these severe limitations significant improvements in ocular drug delivery have been made. The main objective of the improvement is to maintain the drug in the biophase for an extended period of time.
In the ophthalmic drug delivery systems, the physiological constraints imposed by the protective mechanism of the eye lead to the low absorption of drugs and results in a short duration of therapeutic action. A high frequency of the eye drops instillation is associated with patient's non compliance. After the instillation of eye drop into the eye cavity, the effective tear drainage and blinking action of eye results in a 10 times reduction in the drug concentration within 4-20 min.[1] Figure 1 explains the fate of the drugs after instillation into the eye. Due to the tear drainage, most of the administered dose passes via nasolacrimal duct into the gastro intestinal tract, leading to the side effects. Rapid elimination of the eye drops often results in a short duration of the therapeutic effect. The normal volume of tear in the eye is 7 μl, a non-blinking eye can accommodate a maximum of 30 μl[2] of the fluid where as a blinking eye can hold only 10 μl of fluid. Both normally and externally added solution are rapidly drained from eye. The usual single drop size of an instilled drug solution is up to 50 μl and thus most of the drug instilled as eye drop is lost.
Figure 1.
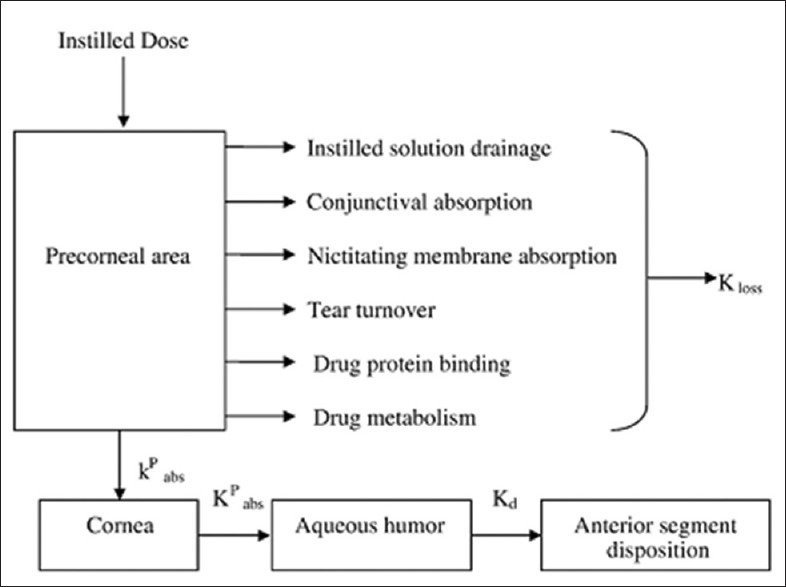
Fate of drugs absorbed by ophthalmic route
The following characteristics are generally required to optimize ocular drug delivery systems.
A good corneal penetration.
A prolonged contact time with corneal tissue.
Simplicity of installation for the patient.
A non-irritative and comfortable form (the viscous solution should not provoke lachrymation and reflex blinking).
Appropriate rheological properties and concentration of viscolyzer[3]
Target delivery within the ocular globes so as to prevent the loss to other ocular sites.
The preceding summary demonstrates that the formulator faces many constraints and prerequisites when developing a modified-release topical ophthalmic drug. In addition to the traditional requirements of oral drugs for safety, efficacy, and stability, ophthalmic products must exhibit additional properties. The regulatory demands for new ophthalmic chemical entities are, most of the time, outweighed by the development efforts and costs compared to the size of the ophthalmic market.
Ophthalmic therapy can be improved by increasing the corneal residence time of drugs. The existing ocular drug delivery systems are thus fair and inefficient. Most of the conventional ophthalmic dosage forms, i.e., eye solution, eye ointments, eye gels, and eye suspensions are comprised in their effectiveness by several limitations leading to poor ocular bioavailability[4] and the limitations are-
-
They have poor bioavailability because of -
- Rapid precorneal elimination
- Conjunctival absorption
- Solution drainage by gravity
- Induced lachrymation
- Normal tear turnover[4]
Frequent administration is needed, which would increase the risk of drug toxicity and side effects[5]
Systemic absorption of the drug and additives drained through nasolacrimal duct may result in undesirable side effects.
The amount of drug delivered during external application may vary the drop size of commercial ocular medication is not uniform and those delivered is generally not correct.
Presence of viscous vehicle can cause blurred vision[6]
To overcome the drawbacks of conventional ophthalmic dosage form, novel ophthalmic dosage forms such as niosomes, liposomes, pharmacosomes, nanoparticles, contact lenses, and ocular inserts are developed. These dosage forms also comprises of certain limitations as-
They cause initial discomfort due to their movement around the eye, especially in case of elderly people. Many patients sometimes lose it without noticing it.
Occasional inadvertent loss during sleeps or while rubbing the eye.
Interference with vision and a difficult placement
All these disadvantages can be overcome by developing a specific dosage form for ophthalmic drug delivery known as hydrogels systems. Previous studies on rabbits by Robinson et al.[7] established that the rate of drainage from the eye of an instilled solution is markedly reduced as the viscosity of the solution is increased.
Hydrogels can be defined as polymers endowed with the ability to swell in water or aqueous solvents and induce a sol-gel transition. They resemble natural living tissue more than any other class of synthetic biomaterials due to their high water content; furthermore, the high water content of the materials contributes to their biocompatibility. In this regard, the phase-change polymers, which may trigger drug release in response to external stimuli, are the most investigated. Hydrogels providing such “sensor” properties are referred as “Stimuli-sensitive hydrogels” or smart hydrogels.
STIMULI-SENSITIVE HYDROGELS
“Smart” hydrogels, or stimuli-sensitive hydrogels, are very different from inert hydrogels in that they can “sense” changes in environmental properties such as pH and temperature and respond by increasing or decreasing their degree of swelling. The volume-changing behavior of “smart” hydrogels is particularly useful in drug delivery applications as drug release can be triggered upon environmental changes. These “intelligent” or “smart” polymers play important role in drug delivery since they may dictate not only where a drug is delivered, but also when and with which interval it is released. The stimuli that induce various responses of the hydrogels systems include physical (temperature) or chemical (pH, ions) ones.
There are many mechanisms have been employed to cause reversible sol-gel phase transition by different stimuli in physiological environmental conditions of human body: The stimuli that induce various responses of the hydrogel systems include-
Physical stimuli like: Change in temperature, electric fields, light, pressure, sound, and magnetic fields.
Chemical stimuli like: Change in pH and ion activation from biological fluid.
Biological/biochemical (bimolecular) stimuli[8] like: Change in glucose level
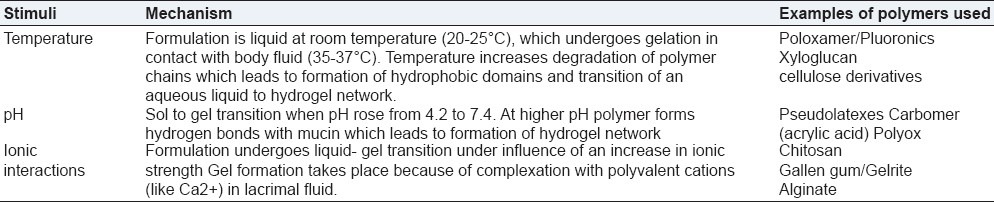
In ophthalmic drug delivery three types of stimuli-sensitive hydrogels - temperature sensitive, pH sensitive, and ion-sensitive hydrogels are mainly used. Table 1 explains the properties and mechanism of three types of hydrogels in brief. Details are discussed further.
Table 1.
Three types of stimuli-sensitive hydrogels with mechanism
Temperature-sensitive hydrogels
Temperature-sensitive hydrogels are probably the most commonly studied class of environment-sensitive polymer systems in drug delivery research. These hydrogels are able to swell or de-swell as a result of changing in the temperature of the surrounding fluid. For convenience, temperature-sensitive hydrogels are classified into negatively thermosensitive, positively thermosensitive, and thermally reversible gels.[9]
Negative temperature-sensitive hydrogels have a lower critical solution temperature (LCST) and contract upon heating above the LCST. Copolymers of (Nisopropylacrylamide) (PNIAAm) are usually used for negative temperature release. Hydrogels show an on off drug release with on at a low temperature and off at high temperature allowing pulsatile drug release. LCST systems are mainly relevant for controlled release of drugs, and of proteins in particular. Thermosensitive polymers may be fixed on liposome membranes; in that case liposomes exhibit control of their content release.[10]
Positive temperature-sensitive hydrogel has an upper critical solution temperature (UCST), such hydrogel contracts upon cooling below the UCST. Polymer networks of poly (acrylic acid) (PAA) and polyacrylamide (PAAm) or poly (acrylamide-co-butyl methacrylate) have positive temperature dependence of swelling.
The most commonly used thermoreversible gels are these prepared from poly (ethylene oxide)-b-poly (provpylene oxide)-b-poly (ethylene oxide) (Pluronics®, Tetronics®, poloxamer)[11] Polymer solution is a free-flowing liquid at ambient temperature and gels at body temperature, such a system would be easy to administer into desired body cavity. In some cases, if lowering the amount of thermogelling polymer is necessary, it may be blended with a pH-sensitive reversibly gelling polymer. Recently, new series of biodegradable triblock copolymers were designed. The polymers consisting of poly (ethylene glycol)-poly-(DLlactic acid-co-glycolic acid)-poly(ethylen glycol) (PEGPLGA- PEG)[12] or PLGA-PEG-PLGA[13] were investigated for sustained injectable drug delivery systems. Some natural polymers like xyloglucan may also form thermoreversible gels.[14]
Some of the earliest work with temperature-sensitive hydrogels was done by the group of Tanaka[15] PNIPAAm is the best example of a negative temperature-sensitive hydrogel. Hitotsu et al.[16] worked with crosslinked PNIPAAm and determined that the LCST of the PNIPAAm gels was 34.38°C. They also found that the LCST could be increased by mixing small amounts of ionic copolymers in the gels. Beltran et al.[17] also worked with PNIPAAm gels containing ionic comonomers. They observed results similar to those achieved by Tanaka.
Hoffman[18] proposed the application of PNIPAAm and its copolymers for temperature-modulated drug release by bulk squeezing and surface regulation. In the bulk squeezing system, the drug that is distributed evenly inside the matrix is squeezed out of the system due to the de-swelling of the hydrogel as a result of increasing the temperature of the environment above the volume phase transition temperature. In the surface regulation system, the swelling ratio of the skin layer is increased as the temperature of the system is lowered below the volume phase transition temperature, and hence, the drug molecules will be able to diffuse through the skin layer.
Poloxamers are thermo reversible gels that seem to fulfill the aforementioned conditions. Poloxamers are a broad group of compounds that were introduced in the early 1950s as food additives and for pharmaceutical preparations. These water-soluble surfactants are triblock co-polymers[19] prepared from poly (ethylene oxide)-b-poly (propylene oxide)-b-poly (ethylene oxide) commercially available as Pluronic® are the most commonly used thermosetting polymers and could be applicable for the development of effective ophthalmic drug delivery.[20] Depending on the ratio and the distribution along the chain of the hydrophobic and hydrophilic subunits, several molecular weights are available, leading to different gelation properties Pluronic F127, which gives colorless and transparent gels, is the most commonly used in pharmaceutical technology. Poloxamers were employed as solubilizers and proposed as artificial tears. Pluronic F 127 is no more damaging to the mouse or rabbit cornea than a physiological saline.[21] The Poloxamers are reported to be well tolerated and non-toxic even though large amounts (25-30%) of polymers are required to obtained a suitable gel.
At concentrations of 20% w/v and higher aqueous solutions of Poloxamer-407 remain as a liquid at low temperatures [<15°C] and yield a highly viscous semisolid gel upon instillation into the cul-de-sac. At low temperatures, the Poloxamer forms micellar subunits in solution, and swelling gives rise to large micellar subunits and the creation of cross-linked networks. The result of this phenomenon is a sharp increase in viscosity upon heating.[22] Miller et al. examined a temperature-sensitive solution of poloxamer used to deliver the miotic pilocarpine.[23] In order to reduce the concentration of polymer and/or to achieve a phase transition temperature higher than room temperature (25°C) and gelling at precorneal temperature (35°C), the combining Pluronic® analogs or the addition of further polymer, e.g. PEG,[24] PAA,[25] methylcellulose (MC), HPMC, CMC[26] is often necessary.
Attwood et al. has reported enhancement of the miotic response following sustained release of Pilocarpine from the 1% w/w xyloglucan gel. In order to develop a thermosetting gel with a suitable phase transition temperature, Wei et al. combined poloxamer (Pluronic F 127 and F 68) and sodium hyaluronan. Gamma scintigraphy demonstrated that the clearance of an optimized formulation containing 21% F127 and 10% F68 was significantly delayed with respect to a phosphate buffer solution. A three-fold increase of the corneal residence time was achieved in the rabbits.
Three principal mechanisms have been proposed to explain the liquid-gel phase transition after an increase in temperature, including:
Gradual desolvation of the polymer,
Increased micellar aggregation, and
The increased entanglement of the polymeric network.
Despite all the promising results obtained with thermo reversible gels, there remains an important drawback associated with their use; the risk of gelation before administration by increase in ambient temperature during packing or storage.
pH-sensitive hydrogels
These hydrogels respond to changes in pH of the external environment. These gels have ionic groups (which are readily ionizable side groups) attached to impart peculiar characteristics. Some of the pH sensitive polymers used in hydrogels’ preparations are polymethyl methacrylate (PMMA), polyacrylamide (PAAm), polyacrylic acid (PAA), poly dimethylaminoethylmethacrylate (PDEAEMA) and polyethylene glycol. These polymers though in nature are hydrophobic but swells in water depending upon the pH prevalent in the external environment. Any change in pH of the biological environment causes changes in the swelling behavior, for example, the hydrogel of caffeine is prepared with poly- mer PDEAEMA at pH below 6.6. As the polymer shows high swellability but when pH changes to higher side, the polymer showed shrinkage leading to drug release. The other pH-sensitive hydrogels are copolymer of PMMA and polyhydroxyethyl methyl acrylate (PHEMA), which are anionic copolymers, swell high in neutral or high pH but do not swell in acidic medium. It was also observed that pH and ionic strength determines kinetics of swelling of PHEMA and guar gum (Peppas and Peppas, 1990; Das et al., 2006).[27,28]
Cellulose acetate phthalate (CAP) latex, cross linked acrylic, and derivatives such as carbomer are used. Cellulose acetate derivatives are the only polymer known to have a buffer capacity that is low enough to gel effectively in the cul-de-sac of the eye. The pH change of about 2.8 units after instillation of the native formulation (pH 4.4) into the tear film leads to an almost instantaneous transformation of the highly fluid latex into viscous gel.
The gamma scintigraphy technique was used to monitor the ocular residence time of an ophthalmic preparation based on cellulose acetate phthalate (CAP) dispersion. The gelled system constitutes a micro-reservoir of high viscosity. First preliminary investigations of pH-sensitive latexes for ophthalmic administration began in early 1980s and have been extensively studied by Boye.[29] He proposed the preparation of latexes containing Pilocarpine with CAP.
Cellulose acetate phthalate latex is a polymer with potentially useful properties for sustained drug delivery to the eye because latex is a free running solution at a pH of 4.4, which undergoes coagulation when the pH is raised by the tear fluid to pH. 7.4. The use of pH-sensitive latex Nanoparticles has been described by Gurny.[30] But the low pH of the preparation can elicit discomfort in some patients. The poly acrylic acid and its lightly cross-linked commercial forms (Polycarbophil and Carbopol) exhibit the strongest muco-adhesion. In the pioneering paper, Hui and Robinson[31] demonstrated that the use of acrylates for ocular delivery of progesterone was based not only on viscosifying but also on bioadhesion properties. Carbomer (Carbopol) a cross-linked acrylic acid polymer (PAA) also shows pH induced phase transition as the pH is raised above its pKa of about 5.5. Different grades of Carbopol are available. The manufacturer states that Carbopol 934 gel has the lowest cross-linking density, while Carbopol 981 intermediate and Carbopol 940 have the highest.
However, the amount of PAA required to form stiff gel upon instillation in the eye is not easily neutralized by the buffering action of tear fluid. Combination PAA with a suitable viscosity enhancing polymer, e.g. HPMC or MC allows a reduction in the PAA concentration without comprising the in situ gelling properties. The formulation containing Carbopol® 940 and Methocel E50LV (HPMC) afforded sustained release of ofloxacin over an 8-h period.[32] Polycarbophil-based in situ gelling systems were developed by Robinson and Miynek.[33]
Ion-sensitive hydrogels
Ion-sensitive polymers belong to the mainly used in situ gelling materials for ocular drug delivery. Gelling of the instilled solution is also triggered by change in ionic strength. It is assumed that the rate at which electrolytes from the tear fluid is absorbed by the polymer will depend on the osmotic gradient across the surface of the gel. It is therefore likely that the osmolality of the solution might have an influence on the rate of the sol-gel transition occurring in the eye. One example is Gelrite, an anionic extra cellular polysaccharide, low acetyl Gellan gum secreted by pseudomonas elodea. Gelrite formulations in aqueous solutions form a clear gel in the presence of the mono or divalent cations typically found in the tear fluids. The electrolyte of the tear fluid and especially Na+, Ca++, and Mg++ cations are particularly suited to initiate gelation of the polymer when instilled as a liquid solution in to the cul-de-sac. Gelrite has been the most widely studied and seems to be preferred compared to the pH sensitive or temperature setting systems. The polymeric concentration is much lower compared to previously described systems.[34] Slightly viscous gellan gum solutions in low concentrations (<1%) show markedly increase in apparent viscosity, when introduced into presence of a physiological level of cations, without requiring more ions than 10–25% of those in tear fluid.[35] The precorneal contact times for drugs can thus be extended up to 20-h.[36] Gellan-containing formulations of pilocarpine HCl allowed reduction of drug concentration from 2% to 0.5% obtaining the same bioavailability.
Rozier et al.,[37] found an improvement in the ocular absorption of timolol in albino rabbits when absorption of timolol in albino rabbits when administered in Gelrite when compared with an equiviscous solution of hydroxyl-ethyl cellulose. Sanzgiri et al.,[38] compared various systems of Methyl prednisolone (MP); esters of MP with Gelrite eye drops, Gellan-MP film, and Gellan film with dispersed MP. Gellan eye drops provided better performance because they afforded the advantage of faster gelation over a high surface area in eye, whereas the results obtained with the Gellan-MP film seemed to indicate that the gelation at the surface of the film occurred very slowly, and the surface of release was not controlled.
Mourice and Srinivas[39] measured a two fold increase in the permeation of the fluorescein in humans when using Gellan gum compared to isotonic buffer solution. The ability of gel formation at physiological Ca2+ levels was used in case of alginic acid as well. Presence of this polymer significantly extended the duration of the pressure reducing effect of pilocarpine to 10-h and carteolol to 8-h, allowing only once a day administration in case of carteolol. Cohen et al. demonstrated that an aqueous solution of sodium alginate could gel in the eye, without addition of external calcium ions or other bivalent/polyvalent cations. The extent of alginate gelation and consequently the release of Pilocarpine were found to be dependent upon the percentage of Glucuronic Acid residues in the polymer backbone. Alginates with G content more than 65%, such as Manugel DMB, instantaneously formed gels upon their addition to STF.
In vitro release studies indicated the slow release of Pilocarpine over a period of 24 hours. Recently some other natural polymers believed to be able to form in situ gels by interacting with the lachrymal fluid have been evaluated as potential adjuvant in ophthalmic formulation. This includes carageenans, xyloglucans, and some alginates that are rich in guluronic acid residues. K-carrageenan forms rigid, brittle gels in reply of small amount of K+, I-carrageenan forms elastic gels mainly in the presence of Ca2+. Gelation of the low-methoxy pectins can be caused by divalent cations, especially Ca2+. Likewise, alginic acid undergoes gelation in presence of divalent/polyvalent cations, e.g., Ca2+ due to the interaction with glucuronic acid blocks in alginate chains. Sodium alginate consists chiefly of the sodium salt of alginic acid, a linear glycuronan polymer consisting of a mixture of β- (1, 4) Dmannosyluronic acid and α- (1, 4)-LGulosyluronic acid residues. Silver et al. compared the commercial product Timoptic XE 0.5% with a timolol mealeate gelforming solution with xanthan gum as the gelling polymer (Timolol GFS 0.5% Alcon Research). The xanthan gum preparation was developed for once-daily dosing. The reported data indicated equivalent efficacy in the reduction intraocular pressure (a maintained reduction during long term use) and consequently therapeutic equivalence.[40] Keipert reported that the increase in therapeutic effects (i.e., miosis) in rabbits could be due to a permeation enhancing effect of gellan gum comparable to EDTA. Apart from its in situ gelling property, Gellan gum diminishes drainage after instillation. The commercial product Timoptol XE preparation containing Gelrite remains for a longer period at the eye surface when compared to conventional timolol maleate eye drops. This resulted in an enhanced drug transfer sufficient enough to obtain an intro ocular pressure reduction after a once-daily topical instillation.[41] Divalent ions were found superior to monovalent in promoting the gelation of the polysaccharides. However, the concentration of sodium in tears (2.6 g/L) is quite sufficient to induce gelation. Because the presence of lachrymal fluid is necessary to induce gel formation, accidental gelation during storage does not occur as with thermo reversible gels.
Hence, stimuli-sensitive hydrogels are better alternative for ophthalmic drug delivery of pharmaceuticals; they show the following advantages:
Prolonged drug release
Reduced systemic side effects
Reduced number of applications
Better patient compliance
CONCLUSION
The main efforts in ocular drug delivery during the past two decades has been on the design of systems to prolong the residence time of topically applied drugs in conjunctival sac. The most widely developed drug delivery system is represented by the polymeric hydrogels. Hydrogels generally offer a moderate improvement of ocular drug bioavailability despite their favorable bioadhesive properties. One of the disadvantages is that hydrogel may result in blurred vision as well as foreign body sensation to patients. Stimuli activated gel-forming systems seem to be preferred as they can be administered in drop form and create significantly less problems with vision. Moreover, they provide good sustained release properties. Thus, the fascinating properties of the stimuli-sensitive polymers seem promising in many future applications and offer possible use as the next generation of materials in biological, biomedical, and pharmaceutical products, because as with non-viscous eye drops, accurate and precise sustained release properties with little or no eye irritation is possible. However, there is still a basic need for more details in this area.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Saettone MS, Bucci P. Kinetics of topically applied drugs. In: Maurice DM, Saettone MS, Bucci P, Speiser P, editors. Ophthalmic drug delivery, biopharmaceutical, technological and clinical aspects, fidia research series. Vol. 11. Padova: Livinia press; 1987. pp. 19–26. [Google Scholar]
- 2.Alfonso R, Gennaro . Remington pharmaceutical sciences. 18th ed. Pennsylvania: Mack Publishing Company; 1990. Ophthalmic Preparation; p. 1581. [Google Scholar]
- 3.Keister JC, Cooper ER, Missel PJ, Lang JC, Hager DF. Limits on optimizing ocular drug delivery. J Pharm Sci. 1991;80:50–3. doi: 10.1002/jps.2600800113. [DOI] [PubMed] [Google Scholar]
- 4.Shyamala B, Lakshmi PK, Harish GG. Topical ocular drug delivery-A Review. Indian J Pharm Sci. 2005;67:404–8. [Google Scholar]
- 5.Monem AS, Ali FM, Ismail MW. Prolonged effect of liposomes encapsulating pilocarpine HCL in normal and glaucomatons rabbits. Int J Pharm. 2000;198:29–38. doi: 10.1016/s0378-5173(99)00348-8. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Lobel E. A novel in situ forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Controlled Release. 1997;44:201–8. [Google Scholar]
- 7.Patton TF, Robinson JR. Ocular evaluation of polyvinyl alcohol vehicle in rabbits. J Pharm Sci. 1975;64:1312–6. doi: 10.1002/jps.2600640811. [DOI] [PubMed] [Google Scholar]
- 8.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–39. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 9.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 10.Kono K. Thermosensitive polymer-modified liposomes. Adv Drug Deliv Rev. 2001;53:307–19. doi: 10.1016/s0169-409x(01)00204-6. [DOI] [PubMed] [Google Scholar]
- 11.Bromberg LE, Ron ES. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv Drug Deliv Rev. 1998;31:197–221. doi: 10.1016/s0169-409x(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 12.Jeong B, Choi YK, Bae YH, Zentner G, Kim SW. New biodegradable polymers for injectable drug delivery systems. J Control Release. 1999;62:109–14. doi: 10.1016/s0168-3659(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Choi S, Koh JJ, Lee M, Ko KS, Kim SW. Controlled release of insulin from injectable biodegradable triblock copolymer. Pharm Res. 2001;18:548–50. doi: 10.1023/a:1011074915438. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki S, Suisha F, Kawasaki A, Shirakawa M, Yamatoya K, Attwood D. Thermally reversible xyloglucan gels as vehicles for rectal drug delivery. J Control Release. 1998;56:75–83. doi: 10.1016/s0168-3659(98)00079-0. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T. Collapse of gels and the critical endpoint. Phys Rev Lett. 1978;40:820–3. [Google Scholar]
- 16.Hirotsu S, Hirokawa Y, Tanaka T. Volume-phase transitions of ionized N-isopropyl acrylamide. J Chem Phys. 1987;87:1392–5. [Google Scholar]
- 17.Beltran S, Baker JP, Hooper HH, Blanch HW, Prausnitz JM. Swelling equilibria for weakly ionizable, temperature sensitive hydrogels. Macromolecules. 1991;24:549–51. [Google Scholar]
- 18.Hoffman AS. Applications of thermally reversible polymers and hydrogels in therapeutics and diagnostics. J Control Release. 1987;6:297–305. [PubMed] [Google Scholar]
- 19.Alexandridis P, Hatton TA. Poly (ethylene oxide) Poly (propylene oxide)-poly (ethylene oxide) block co-polymer surfactants in aqueous solutions and at interface thermodynamic, structure, dynamic, and modeling. Colloids Surf A Physicochem Eng Asp. 1995;96:1–46. [Google Scholar]
- 20.Wei G, Xu H, Ding PT, Li SM, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: The rheological and gamma scintigraphic studies. J Control Release. 2002;83:65–74. doi: 10.1016/s0168-3659(02)00175-x. [DOI] [PubMed] [Google Scholar]
- 21.Furrer P, Plazonnet B, Mayer JM, Gurny R. Application of in vivo convocal microscopy to the objective evaluation of ocular irritation induced by surfactant. Int J Pharm Sci. 2002;207:89–98. doi: 10.1016/s0378-5173(00)00540-8. [DOI] [PubMed] [Google Scholar]
- 22.Chen Chow PC, Frank SG. In vitro release of Lidocaine from Pluronic F 127. Int. J. Pharmaceutics. 1981;8:89–99. [PubMed] [Google Scholar]
- 23.Miller SC, Donovan MD. Effect of Poloxamer 407 gel on the miotic activities of pilocarpine nitrate in rabbit. Int J Pharm Sci. 1982;12:147–52. [Google Scholar]
- 24.Edsman K, Carlfors J, Petersson R. Rheological evaluation of poloxamers as an in situ gel for ophthalmic use. Eur J Pharm Sci. 1998;6:105–12. doi: 10.1016/s0928-0987(97)00075-4. [DOI] [PubMed] [Google Scholar]
- 25.Ling HR, Sung KC. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J Control Release. 2000;69:379–88. doi: 10.1016/s0168-3659(00)00329-1. [DOI] [PubMed] [Google Scholar]
- 26.El-Kamel AH. In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate. Int J Pharm. 2002;241:47–55. doi: 10.1016/s0378-5173(02)00234-x. [DOI] [PubMed] [Google Scholar]
- 27.Peppas LB, Peppas NA. Dynamic and equilibrium behavior of pH sensitive hydrogels containing 2-hydroxy ethyl methacrylates. Biomaterials. 1990;11:635–44. doi: 10.1016/0142-9612(90)90021-h. [DOI] [PubMed] [Google Scholar]
- 28.Das A, Wadhwa S, Srivastava AK. Cross-linked guar gum hydrogels discs for colon-specific delivery of ibuprofen: Formulation and in-vitro evaluation. Drug Deliv. 2006;13:139–42. doi: 10.1080/10717540500313455. [DOI] [PubMed] [Google Scholar]
- 29.Boye T, Gurny R, Ibrahim H. Ocular therapy with nanoparticulate systems for controlled drug delivery. J Control Release. 1985;2:353–61. [Google Scholar]
- 30.Gurny R. Preliminary study of prolonged acting drug delivery system for the treatment of glaucoma. Pharma Acta Helv. 1981;56:130–2. [PubMed] [Google Scholar]
- 31.Hui HW, Robinson JR. Ocular drug delivery of progesterone using of bioadhesion polymer. Int J Pharm Sci. 1985;26:203–13. [Google Scholar]
- 32.Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Control Release. 2001;73:205–11. doi: 10.1016/s0168-3659(01)00279-6. [DOI] [PubMed] [Google Scholar]
- 33.Robinson JR, Miynek GM. Bioadhesive and phase change polymers for ocular drug delivery. Adv Drug Del Rev. 1995;16:147–52. [Google Scholar]
- 34.Bhaskaran S, Lakshmi PK, Harish CG. Topical ocular drug delivery: A review. Indian J Pharm Sci. 2005;64:404–8. [Google Scholar]
- 35.Paulsson MH, Hägerström H, Edsman K. Rheological studies of the gelation of deacetylated gellan gum (Gelrite®) in physiological conditions. Eur J Pharm Sci. 1999;9:99–105. doi: 10.1016/s0928-0987(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 36.Carlfors J, Edsman K, Petersson RJ, Jörnving K. Rheological evaluation of Gelrite® in situ gels for ophthalmic use. Eur J Pharm Sci. 1998;6:113–9. doi: 10.1016/s0928-0987(97)00074-2. [DOI] [PubMed] [Google Scholar]
- 37.Rozier A, Mazuel C, Grove J, Plazonnet B. Gelrite: A novel ion activated in situ gelling effect bioavailability of timolol. Int J Pharm. 1989;57:163–8. [Google Scholar]
- 38.Sanzgiri YD, Maschi S, Crescenzi V, Callengaro L, Topp EM, Stella VJ. Gellan based system for ophthalmic sustained delivery of methyl prednisolone. J Controlled Release. 1993;26:195–201. [Google Scholar]
- 39.Maurice DM, Srinivas SP. Use of flurometry in assessing the efficacy of a cation-sensitive gel as an ophthalmic vehicle: Comparision with scintigraphy. J Pharm Sci. 1992;81:615–9. doi: 10.1002/jps.2600810704. [DOI] [PubMed] [Google Scholar]
- 40.Schenkar HI, Silver LH. Long term intraocular pressure lowering efficiency and safety of timolol maleate gel forming solution 0.5% compared with Timoptic XE 0.5% in a 12 month study. Am J Ophthalmol. 2000;13:145–50. doi: 10.1016/s0002-9394(00)00458-x. [DOI] [PubMed] [Google Scholar]
- 41.Shedden A, Laurence J, Tipping R Timoptic-XE 5% Study Group. Efficacy and tolerability of timolol maleate gel forming solution in adults with open angle glaucoma or ocular hypertension: A six month double masked multicenter study. Clin Ther. 2001;23:440–50. doi: 10.1016/s0149-2918(01)80048-5. [DOI] [PubMed] [Google Scholar]


