Abstract
Background:
Many polymeric systems have been used to fabricate ocular inserts for improve ocular bioavailability and retention to drug of which matrix systems have shown advantages of reduce dosing frequency and increased corneal residence time. The objective of the present investigation was to prepare and evaluate ocular inserts of gatifloxacin.
Materials and Methods:
Ocular insert was made from an aqueous dispersion of gatifloxacin, sodium alginate, polyvinyl alcohol, and glycerin by solvent casting method. Ocular insert (5.5 mm) was cross-linked by CaCl2 and was coated with Eudragit RL-100 or Eudragit RS-100. The ocular inserts were characterized for thickness; uniformity of weight, drug content uniformity, % moisture absorption or moisture loss, and surface pH. The in vitro diffusion studies were carried out by putting insert on Millipore membrane filter (0.8 μm) fixed between donor and receptor compartment of an all glass modified Franz diffusion cell.
Results:
The thickness and drug content of ocular insert were found in the range of 0.11 ± 0.003 to 0.24 ± 0.010 mm and 0.718 ± 0.002 to 0.867 ± 0.007 mg, respectively. The surface pH, % moisture absorption or moisture loss and weight variation values were obtained in satisfactory range. The cross-linked ocular insert coated with Eudragit RL-100 shows maximum drug permeation i.e. 89.53 % ± 0.43 at 11 h. The stability studies suggest that all ocular insert remained stable, showed lesser degradation rate and maximum shelf life.
Conclusion:
Ocular inserts of gatifloxacin were prepared successfully by using solvent casting method for sustained drug delivery. The cross-linked and Eudragit RL-100 coated ocular insert of gatifloxacin provides better in vitro drug release and sustained upto 11 h.
Keywords: Cross-linked, gatifloxacin, ocular insert, polyvinyl alcohol, sodium alginate
INTRODUCTION
The eye as a portal for drug delivery is generally used for local therapy against systemic therapy in order to avoid the risk of eye damage from high blood concentrations of the drug, which is not intended. After the introduction of quinolones/fluoroquinolones, ocular preparation of these antimicrobial agents became available to control various eye infections caused by Staphylococcus aureus, Streptococcus pneumoniae, Hemophilus species, and others.[1] Most ocular treatments like eye drops and suspensions call for the topical administration of ophthalmologically active drugs to the tissues around the ocular cavity. These dosage forms are easy to instill but suffer from the inherent drawback that the majority of the medication they contain is immediately diluted in the tear film as soon as the eye drop solution is instilled into the cul-de-sac and is rapidly drained away from the precorneal cavity by constant tear flow and nasolacrimal drainage. Therefore, only a very small fraction of the instilled dose is absorbed by the target tissue for this reason, concentrated solutions and frequent dosing are required for the instillation to achieve an adequate level of therapeutic effect.[2] For this reason, standard treatment of severe bacterial keratitis requires administration of eye drops at frequent intervals (every 15 to 60 min for 48 to 72 h) often containing fortified (more concentrated than commercially available solutions) solutions of fluoroquinolones.[3,4] However, this regimen is not only disruptive to the patient and usually necessitates hospitalization, but it has also been associated with in vitro toxicity to the corneal epithelium.[5,6] This problem can be overcome by increasing the contact time of the vehicle on the ocular surface and slow down drug elimination from the dosage form. Ocular insert seems promising which improves the efficiencies of the therapy by prolonging the contact time with improved patient compliance. Ocular insert offers many advantages over conventional dosage forms, like increased ocular residence, possibility of releasing drugs at slow and constant rate, accurate dosing, and exclusion of preservative and increased shelf life.[7–9]
Gatifloxacin[1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy- 7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid, sesquihydrate] is soluble in water. Gatifloxacin 0.3% and 0.5% ophthalmic solutions showed better effect than ofloxacin 0.3% in methicillin-resistant Staphylococcus aureus (MRSA) corneal infections in rabbits.[10] In vitro transcorneal permeation of gatifloxacin from aqueous drops has been reported.[11] The mucoadhesive gellan system of gatifloxacin was studied for antibacterial activity with combination of sodium carboxymethylcellulose and sodium alginate.[12] The as ion-activated in situ gelling system of gatifloxacin ophthalmic solution (0.3%) using sodium alginate (Kelton®) as a gelling agent in combination with HPMC-E50LV as a viscosity enhancing agent has been reported. The formulation showed gelation in the cul-de-sac upon instillation as drops in the eye. The gel formed in vitro showed sustained drug release for 8 h. The formulation was nonirritating and showed prolonged precorneal retention in rabbit eye. Prolonged precorneal retention in the conjunctival sac and slow drug release from the formulation could impart improved in vivo ocular bioavailability.[13] Gellan gum (Gelrite)-based in situ gelling system of gatifloxacin (0.27%) for ophthalmic drug delivery has been published.[14] Nanoparticle of gatifloxacin based on chitosan and sodium alginate was prepared and evaluated for prolonged ocular drug delivery. The nanoparticles provided fast release during the first hour followed by a more gradual drug release during a 24 h period following non-Fickian diffusion kinetics.[15] Taking above information in view, the purpose of the current study was to prepare and evaluate ocular insert of gatifloxacin for enhancing intra-corneal delivery of ophthalmic medicament.
MATERIALS AND METHODS
Materials
Gatifloxacin sesquihydrate was obtained from Helios Pharma Ltd. Baddi, as a gift. Sodium alginate, Eudragit RL-100 (acrylic acid and methacrylic acid ester copolymer containing 10% trimethyl ammonium methacrylate chloride and Eudragit RS- 100 (acrylic acid and methacrylic acid ester copolymer containing 5% trimethyl ammonium methacrylate chloride) were obtained from Jubilant Organosys, Noida as a gift sample. Polyvinyl alcohol and glycerin were obtained from Central Drug House, New Delhi and S.D. Fine chemicals Ltd, New Delhi, respectively. Millipore Filter Paper (0.8 μm) was procured from Himedia Lab. Pvt. Ltd. Mumbai. All other chemicals were of analytical grade.
Preparation of ocular insert
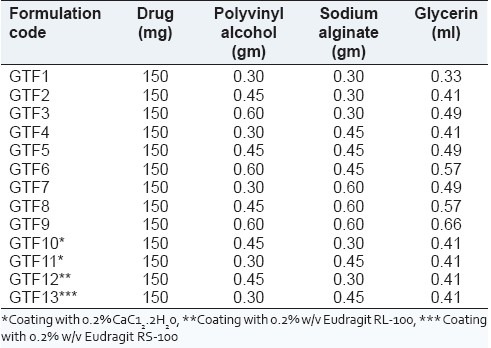
All the ocular inserts (GTF 1 to GTF 9) of gatifloxacin sesquihydrate were prepared by using the solvent casting method with combination of polymers (sodium alginate, polyvinyl alcohol), and glycerine used as a plasticizer for the uniform strength of insert, as per composition given in Table 1. The required amount of polymers were dissolved in two different beakers in required quantity of distilled water (30 ml) and stirred on magnetic stirrer until completely dissolved than both solutions of polymers are mixed together and then glycerine (20%w/w of dry polymer) was added as plasticizer to the solution under stirring condition. The weighed amount of drug (150 mg) was added to the above solution and stirred for 5 h to get uniform dispersion. The solution of polymers was sonicated for 30 min to remove the air bubbles. After proper mixing, the casting solution is poured in clean glass Petri dish and covered with an inverted funnel to allow slow and uniform evaporation at room temperature for 48 h. The dried films thus obtained were cut into circular pieces of definite size (5.5 mm diameter). The ocular inserts were then wrapped in aluminium foil and were stored in an airtight container (desiccators).[16] Ocular inserts (GTF2 and GTF4) were cross-linked by CaCl2 (0.2% w/v) by dipping and drying five times. Similarly, the cross-linked insert (GTF9 and GTF10) were coated with different grade of Eudragit polymer (0.2% w/v) in isopropyl alcohol and acetone by the dip and dry method [Table 1]. The polymers used were Eudragit RL-100 (GTF12) and Eudragit RS-100 (GTF13). The weight gain of ocular insert after coating with polymer was found to be in range 23.4–29.6%.
Table 1.
Composition of gatifloxacin ocular inserts
Physiochemical evaluation of ocular inserts
Thickness uniformity
Insert thickness (5.5 mm diameter) was measured at five different points using micrometer screw gauge (Mitutoya Co., Japan) and mean insert thickness was noted (n = 3).[17]
Uniformity of weight
Ocular inserts were randomly selected and weighed individually using calibrated digital balance. The average weight of prepared ocular insert was then calculated.[17]
Surface pH determination
For the determination of surface pH, the ocular insert was allowed to swell in the Petri dish at room temperature for 30 min in 0.1 ml of distilled water. The pH paper was kept on the surface and after1 min the colour that developed was compared with the standard colour scale.[17]
Drug content uniformity
The drug content of ocular inserts (5.5 cm diameter) was carried out by the assay method: three inserts were taken out from film and drug content determined. The uncoated ocular insert was dissolved in 10 ml of 0.1 N HCL while coated insert was dissolved in 10 ml of acetone, and the volume was made up to 100 ml with distilled water and the solution was filtered. The amount of drug in the filtrate was analyzed by measuring absorbance at 291 nm in a spectrophotometer (1601 Shimadzu, Kyoto, Japan). The experiment was done in triplicate.[17]
% Moisture absorption study
For the determination of % moisture absorption, the three ocular inserts were randomly selected and their initial weight was noted and then these were placed in the desiccators containing aluminium chloride within high (80%) humidity condition for 3 days. After 3 days, ocular inserts were reweighed and then % moisture was calculated using the following formula:
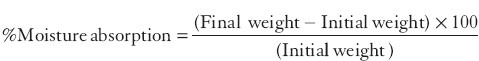
%Moisture loss study
Some of the ocular inserts were taken and their initial weight was noted and kept in desiccators containing anhydrous calcium chloride for 3 days and after 3 days inserts were taken out and reweighed. The percentage moisture loss was calculated using formula
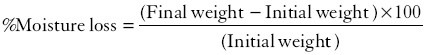
Fourier transform infrared spectroscopy
The FTIR spectra of pure drug and physical mixture (gatifloxacin, poly vinyl alcohol (PVA), and sodium alginate) were taken as KBr pellets in the range of 4000–650 cm-1 (Perkin Elmer Model 1600 FT-IR Spectrophotometer, USA). The infrared analysis of all inserts was carried out in the range of 4000–650 cm-1 by ATR-IR spectroscopy [Perkin Elmer Model 1600 FT-IR Spectrophotometer with ATR mode (Pike Miracle ATR Accessory, Perkin Elmer, USA)].
Drug permeation study
In vitro drug permeation studies were carried out by putting insert (5.5 mm diameter) on a Millipore membrane filter (0.80 μm), which was fixed between donor and receptor compartment of an all-glass modified Franz diffusion cell. The Millipore membrane filter was used to simulate corneal epithelial barrier as isolated cornea will not remain viable beyond 4 h. The required amount i.e. 10 ml of ringer solution was placed within the receptor compartment and then temperature of ringer solution was adjusted to 37 ± 0.5°C. The ringer solution was stirred at a low speed using magnetic stirrer. At specific time intervals, 1 ml aliquot of solution was withdrawn from the receptor compartment and replaced with fresh bicarbonate ringer solution. The aliquot was analyzed for the drug content using a UV-VIS spectrophotometer at 291 nm after appropriate dilution against reference using a bicarbonate ringer solution as a blank.
Release kinetics
The mechanism of drug release was investigated by fitting the drug release data into zero order, first order, Higuchi kinetics, and Korsmeyer–Peppas equations. The goodness of fit of drug release was evaluated by the regression coefficient (R2) value.
Eye irritation study
Ocular irritation studies were performed on six albino rabbits each weighing 1–2 kg of either sex. The sterile formulations were instilled twice a day for a period of 7 days and rabbits were observed periodically for redness, swelling, and watering of the eye.
In vivo drug release study
Selected ocular inserts (GTF 12 and GTF 13) sterilized using γ-irradiation was used for in vivo drug release studies. The protocol on the use of animals (albino rabbit) was approved by the Institutional Ethics Committee. Two group containing six healthy albino rabbits were used to study the drug release in vivo from formulations which showed the satisfactory in vitro drug release.[18] Each rabbit was kept in good hygienic condition in order to avoid vulnerability to any disease including ophthalmic type. Selected ocular insert was placed in the cul-de-sac of each rabbit while the other eye served as a control. At periodic intervals (2, 4, 6,8,10, and 12 h), the insert was taken out carefully from the cul-de-sac of each rabbit and analyzed for the remaining drug content. The drug remaining was subtracted from the initial drug content of the insert, which gave the amount of drug released in the rabbit eye.
Stability of ocular insert
Stability studies were carried out on ocular insert formulations according to ICH guidelines.[19] A sufficient number of ocular inserts (packaged in aluminum foils) were stored in a humidity chamber with a relative humidity of 75 ± 5% and temperatures of 40 ± 2°C and at room temperature. Samples were withdrawn at time 0, 3 weeks, 3 weeks, 6 weeks, 3 months, and 6 months. Ocular inserts were also evaluated for their physical characteristics (color) and also analyzed for drug concentration. The degradation rate constant was determined from the plot of logarithm of the remaining drug vs. time.
RESULTS AND DISCUSSION
In the present investigation, an attempt has been made to prepare ocular inserts of gatifloxacin using different polymers concentration, effect of cross-linking agent and different coating polymers. Polyvinyl alcohol and sodium alginate were used in the present study as good film forming property.[20,21] Glycerin was employed as plasticizer in the preparation to get films with good elasticity.[22] Calcium chloride, which is a known cross-linking agent, was used to harden the ocular insert for sustaining the drug release.[23]
Thickness uniformity
The thickness of ocular inserts was found to be in 0.40 ± 0.00 to 0.45 ± 0.06 millimeters [Table 2].
Table 2.
Physicochemical characteristics of various batches of gatifloxacin ocular inserts
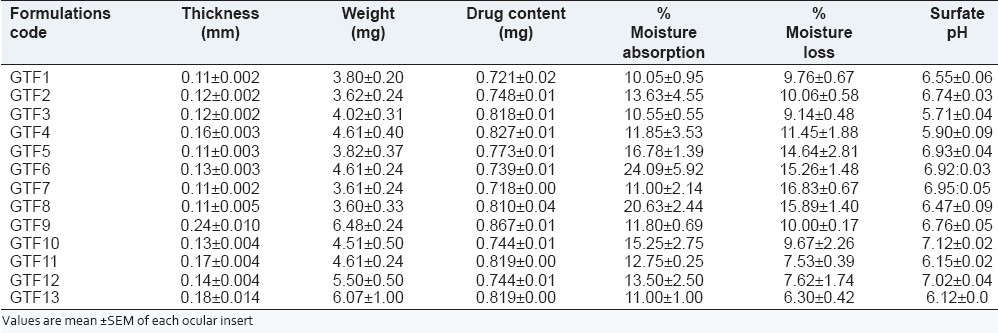
Formulation GTF 9 was showed maximum thickness due to contribution of higher concentration of plasticizer and polymer as per reported in the previous literature.[24]
Uniformity of weight
Uniformity of weight for all formulation was found varies from 3.60 ± 0.33 to 6.48 ± 0.24. The weight of formulation GTF9 was showed maximum due to contribution of higher concentration of polymers and plasticizer effect.
Surface pH
The surface pH of the prepared ocular inserts was found to be in between 5.71 ± 0.044 and 7.12 ± 0.025 [Table 2]. This indicates that the prepared inserts would not alter the pH of tear fluid in the eye.
Drug content uniformity
The drug content of various ocular inserts was found to be between 0.718 ± 0.002 and 0.867 ± 0.007 mg [Table 2]. The estimation of drug content was found to be almost same with their low standard deviation values. The minimum standard deviation value in all formulation indicated that the process was reproducible.
% Moisture absorption and loss study
The % moisture absorption value for all inserts was found in range of 10.05 ± 0.95 to 24.09 ± 5.92. The highest moisture absorption was marked for formulation GTF6 (24.09 ± 5.92%) as presented in Table 2. This might be due to the presence of higher concentration of hydrophilic polyvinyl alcohol in formulation. As the higher amount of moisture the films become soft and may be effect on the formulation integrity, as reported in the past literature.[25]
The percentage moisture loss was determined in triplicate. The moisture loss was occurred, when all the formulations were kept in very dry condition. The moisture loss for all formulations varied between 6.30 ± 0.42 and 16.83 ± 16.83 ± 0.67 [Table 2]. Formulation GTF13 showed maximum moisture loss i.e. 6.30 ± 0.42 under dry conditions.
Fourier transform infrared (FTIR) spectroscopy
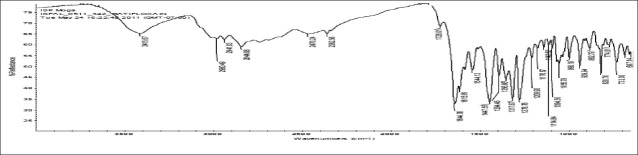
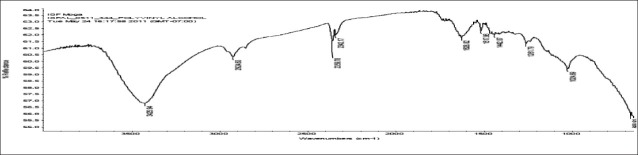
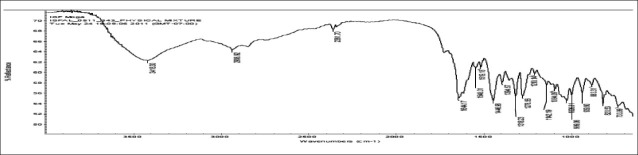
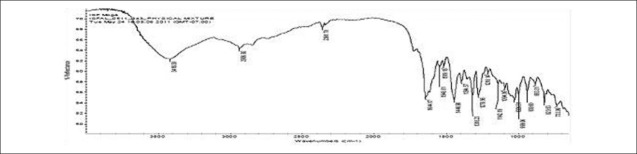
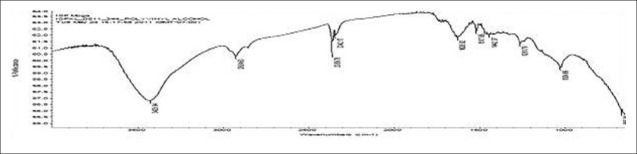
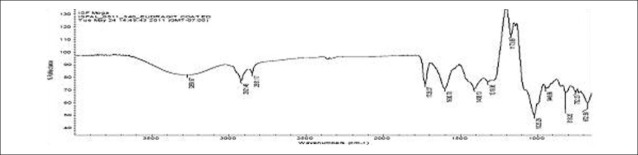
FTIR spectra of gatifloxacin sesquihydrate 1615 cm-1 [Figure 1] showed aromatic stretching 1644 cm-1, 1544 cm-1 and C-H bending for 820 cm-1 beside spectra also showed carboxylic acid C = O stretching 1644 cm-1 and 1726 cm-1, C-N stretching at 1356 cm-1. Spectra of physical mixture of drug, PVA and sodium alginate showed peaks at 3418 cm-1 due to stretching of hydroxyl groups, 2936 cm-1 due to C–H stretching and 1026 cm-1 C–O–C stretching [Figures 2 and 3] (which appears to be contributed by PVA and sodium alginate) along with pure drug peaks [Figure 4]. The ATR spectra blank insert of PVA-alginate showed shifting of hydroxyl peak from 3418 cm-1 to 3266 cm-1 due to intermolecular hydrogen bonding C-H stretching at 2922 cm-1 and C–O–C stretching at 1027 cm-1 [Figure 5]. The ATR spectra of optimized patch also showed the peak at 1728 cm-1. The ATR spectra of optimized ocular inserts (cross-linked and coated) showed aromatic C = C stretching at usual positions indicating incorporation of gatifloxacin sesquihydrate and peaks for ester at 1729 cm-1, as the acrylate polymers are esters [Figure 6]. It would be important to mention here an ATR spectrum provides information of the surface functional groups.
Figure 1.
FTIR spectra of gatifloxacin
Figure 2.
FTIR spectra of PVA
Figure 3.
FTIR spectra of sodium alginate
Figure 4.
FTIR spectra of physical mixture of drug and polymers
Figure 5.
ATR-IR spectra of blank ocular insert
Figure 6.
ATR-IR spectra of optimized ocular insert
In vitro diffusion study
The in vitro permeation studies of gatifloxacin ocular inserts were carried out through the Millipore membrane filter clamped between donor and receptor compartment of modified Franz diffusion cell. The results are shown in Figure 7, which indicate that the in vitro drug release from ocular inserts (GTF1 to GTF9) was sustained for 8 h. On cross-linking with CaCl2 (GTF9 and GTF10), the release was extended up to 10 h. Ocular insert coated with Eudragit RS-100 (GTF11) could sustain the drug release up to 11 h and % cumulative drug release was 83.53%. Formulation GTF13 (coating with Eudragit RL-100), however, could maximum % drug release i.e. 89.53 and sustain up to 11 h. Eudragit RS and Eudragit RL are copolymers of acrylic acid and methacrylic acid esters containing 5% and 10% trimethylammoniummethacrylate chloride, respectively. The in vitro drug release was increased from formulation GTF12 (coated Eudragit RL-100) due to the presence higher water permeability functional quaternary ammonium groups of Eudragit RL-100 (10%).[26] Thus, Eudragit RL-100 (GTF12) appears to be the best polymer with respect to % cumulative drug release and sustaining of the drug release.
Figure 7.
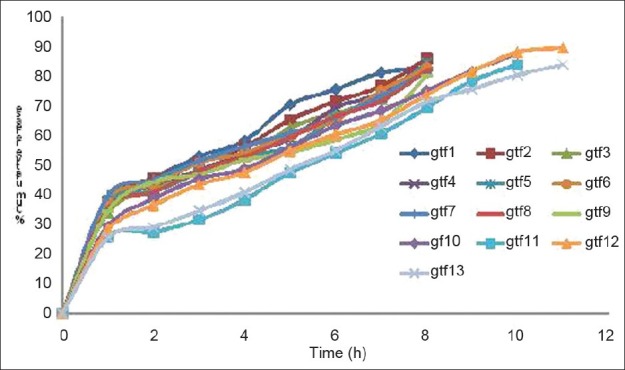
In vitro release profile of gatifloxacin through different ocular inserts
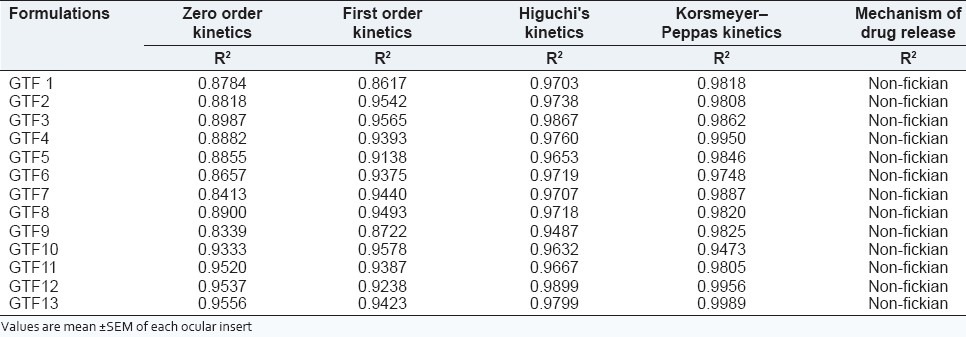
All the formulation were best fitted into Korsmeyer-Peppas equation and values for ‘n’ (diffusion exponent) were >0.89 indicating super case II type of release of drug from ocular inserts. This indicates that drug release is by the non-Fickian mechanism [Table 3].
Table 3.
Kinetic profile of release study data from ocular inserts
In vivo drug release study
Figure 8 presents the cumulative drug release (%) from formulation GTF 12 and GTF 13 at different time intervals. The GTF 12 showed maximum cumulative drug release i.e. 92.36% and sustained up to 12 h. However, GTF 13 formulation showed 81.87% cumulative drug release and sustained up to 12 h. The in vivo study suggests that the GTF 12 showed maximum cumulative drug release and better sustained effect as compared with GTF 13, due to the presence of higher quaternary ammonium compound group (10%) in Eudragit RL-100 as compared than in Eudragit RS-100 (5%), the GTF 12 have tendency to stick more with ocular mucosa. Moreover, the presence of the quaternary ammonium compound group renders positive charge to the polymers by which it can interact with anionic mucin and thereby increases its residence on corneal surface. For all formulation, rabbits which are subjected to for in vivo studies didn’t show any irritation, inflammation, and abnormal discharge which confirmed the safety of the polymers used in the formulation.
Figure 8.
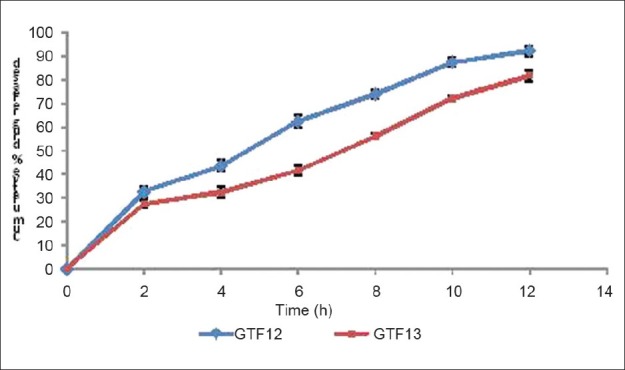
In vivo drug release profile from optimized gatifloxacin ocular insert
Rabbit eye irritation
The prepared optimized ocular inserts of gatifloxacin i.e. GTF12 showed satisfactory ocular tolerance. No any sign of ocular damage or irritation were visible. Only slight redness in rabbit eyes was observed.
Stability of ocular insert
Finally, accelerated stability studies at elevated temperature and humidity revealed no significant changes in the color in the insert formulation. The degradation rate constant and shelf life values for GTF 12 and GTF 13 were found to be 1.10, 1.14 day-1 and 936, 792 days, respectively, suggesting there is no need to add overages to ensure 2 year shelf life. The stability study concluded that the formulation GTF 12 and GTF 13 showed lesser degradation rate and maximum shelf life as per the ICH guideline.
CONCLUSION
From the investigation, it can be concluded that the formulation based on PVA and sodium alginate, cross-linked by CaCl2, and coated with Eudrgit RL-100 (GTF12), well tolerated by the rabbit eye. The physicochemical properties of inserts were found in satisfactory range. The formulation GTF12 showed maximum in vitro drug release profile, better sustained action, and exhibited Korsmeyer-Peppas drug release kinetics (non-Fickian). Eudragit Rl-100, as a sustained drug release polymer showed promising sustained action in vivo condition in the rabbit model. Stability studies showed no significant changes in the inserts which suggest that the inserts were stable. However, more exhaustive preclinical and clinical studies need to be performed to show further information about these approaches.
ACKNOWLEDGEMENTS
Authors are grateful to Dr. Madhu Chitkara, Vice chancellor, Chitkara University, Rajpura, Punjab for financial and infrastructure support for the project.
Footnotes
Source of Support: Nil.
Conflict of Interest: The authors state no conflict of interest and have received no payment in preparation of this manuscript.
REFERENCES
- 1.Hardman JG, Limbird LE. Antimicrobial agents. In: Petri WA, editor. Goodman & Gillman's: The pharmacological basis of therapeutics. New York, NY: McGraw-Hill; 2001. pp. 1180–5. [Google Scholar]
- 2.Barbu E, Sarvaiya I, Green KL, Nevell TG, Tsibouklis J. Vinylpyrrolidone-co-(meth) acrylic acid inserts for ocular drug delivery: Synthesis and evaluation. J Biomed Mater Res A. 2005;74:598–606. doi: 10.1002/jbm.a.30329. [DOI] [PubMed] [Google Scholar]
- 3.Gangopadhyay N, Daniell M, Weih L, Taylor HR. Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers. Br J Ophthalmol. 2000;84:378–84. doi: 10.1136/bjo.84.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith A, Pennefather PM, Kaye SB, Hart CA. Fluoroquinolones: Place in ocular therapy. Drugs. 2001;61:747–61. doi: 10.2165/00003495-200161060-00004. [DOI] [PubMed] [Google Scholar]
- 5.Cutarelli PE, Lass JH, Lazarus HM, Putman SC, Jacobs MR. Topical fluoroquinolones: Antimicrobial activity and in vitro corneal epithelial toxicity. Curr Eye Res. 1991;10:557–63. doi: 10.3109/02713689109001764. [DOI] [PubMed] [Google Scholar]
- 6.Mallari PL, McCarty DJ, Daniell M, Taylor H. Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am J Ophthalmol. 2001;131:131–3. doi: 10.1016/s0002-9394(00)00642-5. [DOI] [PubMed] [Google Scholar]
- 7.Attia MA, Kassem MA, Safwat S. In vivo performance of [3H] dexomethasone ophthalmic film delivery systems in the rabbit eye. Int J Pharm. 1988;47:21–30. [Google Scholar]
- 8.Bharath S, Hiremath SR. Ocular delivery system of perfloxacin mesylate. Pharmazie. 1999;54:55–8. [PubMed] [Google Scholar]
- 9.Hume LR, Lee HK, Benedetti L, Sanzgiri YD, Topp EM, Stella VJ. Ocular sustained delivery of prednisolone using hyaluronic acid benzyl ester films. Int J Pharm. 1994;111:295–8. [Google Scholar]
- 10.Wada T, Naka H, Tokushige H, Sakaki H, Ogawa T, Jensen H, Whitcup SM. Treatment of rabbit corneal infections with ophthalmic gatifloxacin: A concentration dependence study. Adv Ther. 2004;21:1–12. doi: 10.1007/BF02850260. [DOI] [PubMed] [Google Scholar]
- 11.Rathore MS, Majumdar DK. Effect of formulation factors on in vitro transcorneal permeation of gatifloxacin from aqueous drops. AAPS Pharm Sci Tech. 2006:7, 57. doi: 10.1208/pt070357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesavan K, Nath G, Pandit J. Preparation and in vitro antibacterial evaluation of gatifloxacin mucoadhesive gellan syetm. Daru. 2010;18:237–46. [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006;315:12–7. doi: 10.1016/j.ijpharm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Kalam MA, Sultana Y, Samad A, Ali A, Aqil M, Sharma M, et al. Gelrite- based in vitro gelation ophthalmic drug delivery system of gatifloxacin. J Dispers Sci Technol. 2008;29:89–96. [Google Scholar]
- 15.Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimization and in vitro characterisation. Eur J Pharm Biopharm. 2008;68:513–25. doi: 10.1016/j.ejpb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Abhilash AS, Jayaprakash S, Nagarajan M, Dhachinamoorthi D. Design and evaluation of timolol ocular inserts. Indian J Pharm Sci. 2005;67:311–4. [Google Scholar]
- 17.Balasubramaniam J, Srinatha A, Pandit JK, Nath G. In vitro microbiological evaluation of polyvinyl alcohol-based ocular inserts of ciprofloxacin hydrochloride. Indian J Pharm Sci. 2006;68:626–30. [Google Scholar]
- 18.Shankar V, Chandrasekaran AK, Durga S, Geetha G, Ravichandran VA. Design and evaluation of diclofenac sodium ophthalmic inserts. Acta Pharm Sci. 2006;48:5–10. [Google Scholar]
- 19.ICH, Q1A (R2): Stability Testing of New Drug Substances and Products, International Conference on Harmonization, Geneva. 2003 [Google Scholar]
- 20.Jain D, Carvalho E, Banthia AK, Banerjee R. Development of polyvinyl alcohol-gelatin membranes for antibiotic delivery in the eye. Drug Dev Ind Pharm. 2011;37:167–77. doi: 10.3109/03639045.2010.502533. [DOI] [PubMed] [Google Scholar]
- 21.Aburahma MH, Mahmoud AA. Biodegradable ocular inserts for sustained delivery of brimonidine tartarate: Preparation and in vitro/in vivo evaluation. AAPS Pharm Sci Tech. 2011;12:1335–47. doi: 10.1208/s12249-011-9701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundada AS, Shrikhande BK. Formulation and evaluation of ciprofloxacin hydrochloride soluble ocular drug insert. Curr Eye Res. 2008;33:469–75. doi: 10.1080/02713680802023104. [DOI] [PubMed] [Google Scholar]
- 23.Samanta A, Ghosal SK. Prolonged delivery of ciprofloxacin hydrochloride from hydrophilic ocular inserts. Acta Pol Pharm. 2004;61:343–9. [PubMed] [Google Scholar]
- 24.Gorle AP, Gattani SG. Development and evaluation of ocular drug delivery system. Pharm Dev Technol. 2010;15:46–52. doi: 10.3109/10837450902967947. [DOI] [PubMed] [Google Scholar]
- 25.Shinde AJ, Garala KC, More HN. Development and characterization of transdermal therapeutics system of tramadol hydrochloride. Asian J Pharm. 2008;2:265–9. [Google Scholar]
- 26.Khan S, Ali A, Singhavi D, Yeole P. Controlled ocular delivery of acyclovir through rate controlling ocular insert of Eudragit: A technical note. AAPS PharmSciTech. 2008;9:169–73. doi: 10.1208/s12249-008-9032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]