Abstract
Background/Aim:
The aim of the present investigation is to prepare and evaluate in situ gel-forming ophthalmic drug delivery system of moxifloxacin hydrochloride.
Materials and Methods:
Sodium alginate, a novel ophthalmic gel-forming mucoadhesive polymer, which gets converted to gel in the presence of divalent-cations (calcium ion) present in the lachrymal fluid, was used as the gelling agent. Hydroxy propyl methyl cellulose (HPMC) is a mucoadhesive polymer used as viscosity enhancer. Suitable concentrations of buffering agents were used to adjust the pH to 6.5. All the formulations were sterilized in an autoclave at 121°C for 15 minutes. The formulations were evaluated for clarity, pH measurement, gelling capacity, drug content estimation, rheological study, in vitro diffusion study, antibacterial activity, isotonicity, and eye irritation study.
Results:
The developed formulations exhibited sustained release of drug from formulation over a period of 10 hours thus increasing residence time of the drug. The optimized formulations were tested for eye irritation on albino rabbit (male). The formulations were found to be non-irritating with no ocular damage or abnormal clinical signs to the cornea, iris or conjunctiva observed.
Conclusion:
These in situ gelling systems containing gums may be a valuable alternative to the conventional systems.
Keywords: Gelling capacity, in situ gel, in vitro diffusion study, moxifloxacin hydrochloride, rheological evaluation
INTRODUCTION
Major problem in ocular therapeutics is the attainment of optimal drug concentration at the site of action, which is compromised mainly due to precorneal loss resulting in only a small fraction of the drug being ocularly absorbed. The effective dose administered may be altered by increasing the retention time of medication into the eye by using in situ gel-forming systems. Ophthalmic drug delivery is an extremely interesting and highly challenging endeavor.[1,2] The anatomy, physiology, and biochemistry of the eye render this organ exquisitely impervious to foreign substances. The challenge to the formulator is to circumvent the protective barriers of the eye without causing permanent tissue damage.[3] Ophthalmic ointments ensure superior drug bioavailability by increasing the contact time, minimizing the dilution by tears, and resisting nasolacrimal drainage. Major disadvantage of ointment, providing blurred vision, due to this it could be used either night time or for treatment on the outside and edges of the eyelids. Suspension as ophthalmic delivery systems relies on the assumption that particles may persist in conjunctival sac. Precorneal drug loss can be minimal, such as retarding drainage by using diffusion-controlled, nonerodible polymeric insert. The major disadvantage of inserts is the lack of patient acceptance owing to difficult administration. The development of newer, more sensitive diagnostic techniques and therapeutic agents render urgency to the development of more successful ocular delivery systems. The primitive ophthalmic solution, suspension, and ointment dosage forms are clearly no longer sufficient to combat these diseases, and current research and development efforts to design better therapeutic systems are the primary focus of this research work. The aim of the present investigation is to formulate an in situ gel using novel gum system. In situ gel solution increases the residence time and also sustain the release mechanism of the drug.
MATERIALS AND METHODS
Moxifloxacin hydrochloride was obtained from Torrent Pharmaceuticals, Ahemadabad, India. Sodium alginate and HPMC-E 50LV was obtained from the nice chemicals, Ahmedabad, India. HPMC-K 4M was obtained from Signet Chemicals, Japan. Benzalconium chloride was obtained from S.D Fine chemicals, Mumbai, India. All the polymers received were of pharmaceutical grade and were used as received. Other materials and solvents used were of analytical grade. Distilled water was prepared in laboratory using all glass distillation apparatus.
Preparation of in situ gel
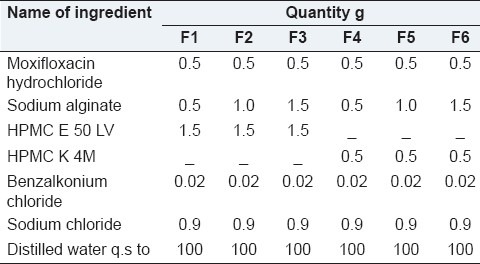
The polymeric solution was prepared by dispersing required quantity of sodium alginate as main polymer and HPMC- E 50 LV, HPMC- K4M as co-polymers in water using a magnetic stirrer until the polymers completely dissolve. Aqueous solution of moxifloxacin hydrochloride was added in to the polymeric solution with continuous stirring.[4] Buffering and osmolality agents were added to the resulting solution along with benzalkonium chloride. The pH of the solution was adjusted to 6.5 using 0.1 N NaoH/0.1 N HCl. The in situ gel formulations are depicted in Table 1.
Table 1.
Formulation design of in situ gelling system
Physical parameters
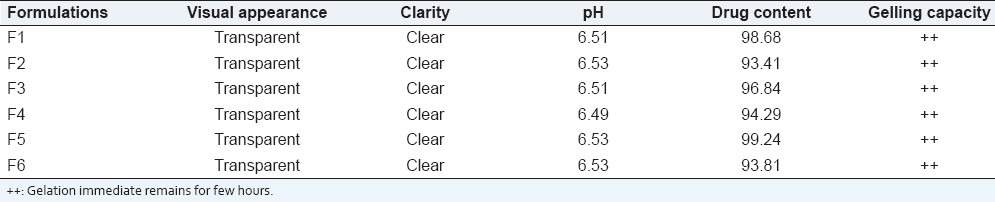
The formulated in situ gel solution was tested for clarity, pH, gelling capacity, and drug content estimation. The results are as shown in Table 2.
Table 2.
Evaluation parameters of formulations
Gelling capacity
The gelling capacity of the prepared formulation was determined by placing a drop of the formulation in a vial containing 2 ml of freshly prepared simulated tear fluid and visually observed. The time taken for its gelling was noted.[5,6]
Rheological studies
The viscosity measurements were carried out using Brookfield viscometer LVDV-E model. The in situ gel formulations were placed in the sampler tube. The samples were analyzed at 37°C ± 0.5°C by a circulating bath connected to the viscometer adaptor prior to each measurement.[7–10] The angular velocity of the spindle was increased 1 to 4 and the viscosity of the formulation was measured.
Drug content estimation
The drug content estimation was carried out by diluting 1 ml of prepared formulation in 100 ml of distilled water and analyzed using UV-visible spectrophotometer (Shimadzu UV-1700 PC, Shimadzu Corporation, Japan) at 290 nm.
In vitro drug release studies
The in vitro release of moxifloxacin hydrochloride from the prepared formulations was studied using a modified diffusion testing apparatus. The freshly prepared simulated tear fluid (pH 7.4) was used as a diffusion medium. Semi permeable membrane, previously soaked in the diffusion medium for overnight, was tied to one end of a specially designed glass cylinder (open at both ends) having inner diameter of 3.4 cm. Two milliliter of formulation was accurately pipette into the glass cylinder known as donor chamber. The cylinder was suspended in a beaker (Acceptor chamber) containing 100 ml of diffusion medium so that the membrane just touches the surface of the medium. Acceptor chamber was maintained at a temperature of 37 ± 2°C with a stirring rate of 50 rpm using magnetic stirrer. About 1 ml of sample was withdrawn at a time interval of 1 hour and replaced with an equal volume of fresh diffusion medium. The aliquots were diluted with the diffusion medium and analyzed at 290 nm using UV spectrophotometer. In a similar manner, 2 ml of pure drug solution (0.5% w/v in distilled water) and 2 ml marketed product (MILFLOX) were studied in a similar manner.
Antimicrobial Activity
Antimicrobial activity was determined by agar diffusion test employing cup plate technique. The drug was allowed to diffuse through a solid agar medium. The standard minimum inhibitory concentration (MIC 2 μg/ml) of control and developed formulations containing moxifloxacin were prepared. A total of 60 ml of nutrient agar media was prepared and sterilized at 15 lb/sq-inch pressure for 18 minutes in an autoclave; 0.5 ml of microorganism suspension was poured into the above medium which is maintained at temperature of 52°C to 58°C. This will be done in an aseptic condition. Immediately 20 ml of the microbial agar suspension was poured into each petriplate. After solidification of the media, sterile solutions of moxifloxacin hydrochloride (standard solutions) and the developed formulations diluted suitably with sterile distilled water (test solutions) were poured in to the cup of sterile nutrient agar Petri plates. This was previously seeded with test organisms (Escherichia coli and Staphylococcus aureus). After allowing diffusion of the solutions for 2 hours, the agar plates were incubated at 37°C for 24 hours. The Zone of inhibition (ZOI) was measured around each cup and compared with that of control. The entire operation was carried out in a laminar airflow unit. Each formulation solution was tested in triplicate. Both positive and negative controls were maintained throughout the study.[11]
Ocular irritancy
Ocular irritation study was performed on optimized formulation in four albino rabbits (male), each weighing about 2 to 3 kg, and 0.1 ml of the optimized sterile moxifloxacin hydrochloride formulation was instilled in to cul-de-sac twice a day for a period of 14 days. The rabbits were monitored periodically for redness, swelling, watering of the eye.[12]
Accelerated stability studies
Optimized sterile formulation was subjected to stability testing. Sterile optimized ophthalmic formulation was filled in glass vials, closed with gray butyl rubber closures and sealed with an aluminium caps. The vials contain optimized formulation were kept in stability chamber, maintained at 40 ± 2°C and 75 ± 5 % RH for one month. Samples were withdrawn weekly and estimated for drug content, pH, visual appearance, gelling capacity and in vitro drug release.[13]
RESULTS AND DISCUSSION
In the present investigation, efforts were made to prepare the sustained release moxifloxacin hydrochloride in situ gel forming ophthalmic solution using polymers such as sodium alginate and HPMC. Sodium alginate a novel ophthalmic gel-forming mucoadhesive polymer, which gets converted to gel in the presence of divalent-cations (calcium ion) present in the lachrymal fluid (pH 7.4), was used as the gelling agent.
The prepared in situ gel formulations were evaluated for clarity, pH measurement, gelling capacity, drug content estimation, rheological study, in vitro diffusion study. The pH of in situ gel solution was found to be around 6.5 for all the formulations. The formulation should have an optimum viscosity that will allow for easy instillation into the eye, which would undergo a rapid sol to gel transition (triggered by ion exchange) as shown in Table 2.
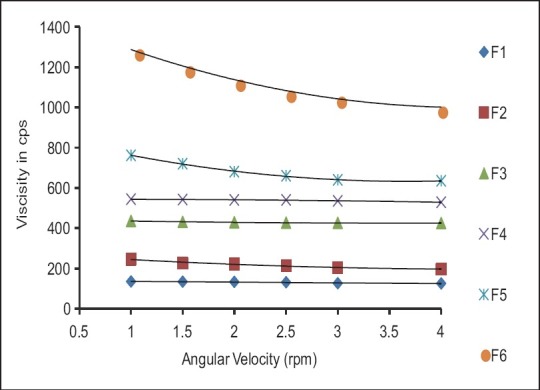
Rheological evaluation of all the formulation exhibited Newtonian flow before gelling and exhibited pseudoplastic flow after gelling in the eye. There was increase in the viscosity after gelling. Additionally, the gel formed in situ should maintain its integrity without dissolving or eroding for a prolonged period. Results are as shown in Table 3 and Figures 1 and 2.
Table 3.
Rheological studies of formulations
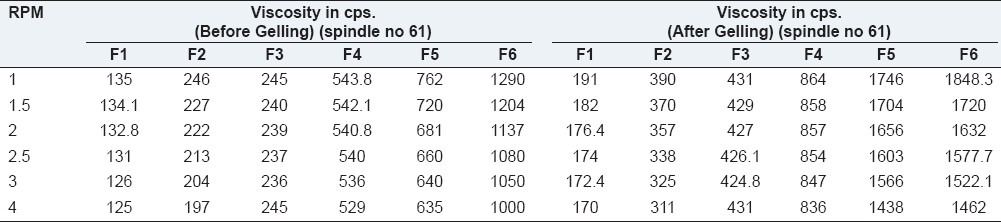
Figure 1.
Formulations F1 to F6 (Before gelling)
Figure 2.
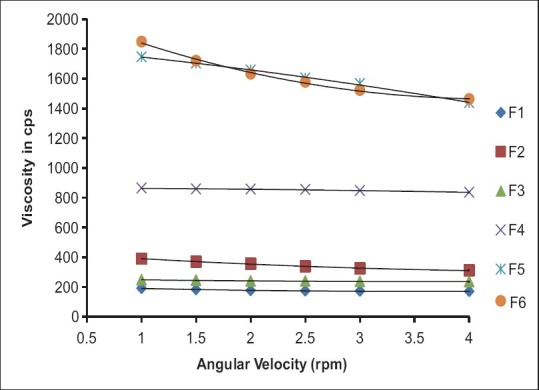
Formulations F1 to F6 (After gelling)
From the in vitro results it was observed that percentage release of the drug from the developed formulations F1 (93.86%), F2 (92.78%), F3 (89.97%), F4 (82.80%), F5 (80.49%), and F6 (78.71%) as shown in Figure 3. Formulation F6 showed more sustained release compared to other formulations. This could be the reason of higher concentration of Sodium alginate and HPMC K4M among the developed formulations. By observing the drug release profile it can be conclude that release is not stagnant even end of 10 hours. Formulation F6 showed highest zone of inhibition values against S. aureus (28.66 mm) and E. coli (30.99 mm), respectively, compared to other developed formulations. Hence, F6 formulation was taken for further study.
Figure 3.
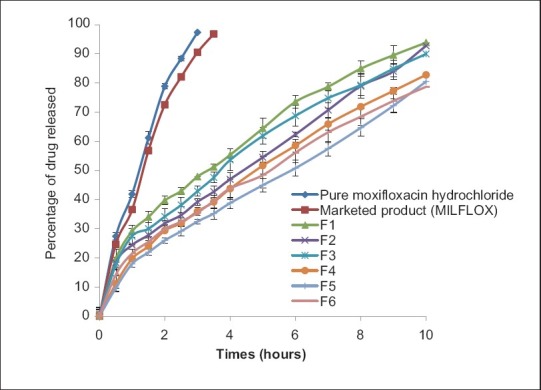
Comparative in vitro diffusion profile of pure drug, marketed product and F1 to F6 formulations
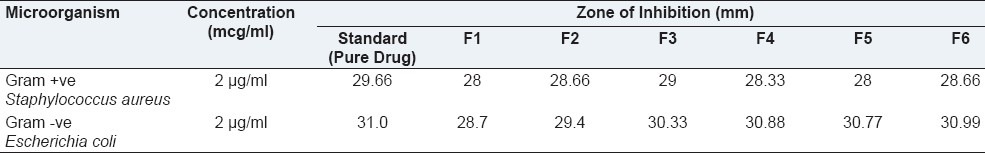
Antimicrobial efficacy study was performed on F6 formulation using Gram +ve S. aureus and Gram -ve E. coli organism. The zone of inhibition of F6 ophthalmic formulation found to be 28.66 and 30.99 mm, respectively, for Gram +ve S. aureus and Gram -ve E. coli organism. The results of antimicrobial activity are as shown in the Table 4. The study indicated moxifloxacin hydrochloride retained its antimicrobial activity when formulated as gel forming ophthalmic system against both selected S. aureus and E. coli.
Table 4.
Zone of inhibition values of formulation F1 to F6 and pure drug at the concentration of 2 μg/ml against Staphylococcus aureus and Escherichia coli
Ocular irritation study was performed using healthy albino rabbits after getting prior permission from the institutional animal ethics committee. The eyes of each rabbits were examined at particular time interval after instillation of the optimized formulation (F6). There was no redness, continuous blinking, swelling or watering of eyes. No ocular damage or abnormal clinical signs to the cornea, iris or conjunctiva were visible. The result of ocular irritation studies indicates that formulations containing all ingredients are non-irritant to rabbit eye.
Accelerated stability studies were carried out at 40 ± 2°C at 75 ± 5 % RH for 1 month using stability chamber. The samples were analyzed periodically on every week, and found that there are no changes in visual appearance, clarity, pH, and gelation. Assay values after 1 month of storage are found almost same (deviating not more than one percent). Release profiles were similar to that of zero days.
CONCLUSION
Moxifloxacin hydrochloride, a broad spectrum antibacterial agent used in the treatment of ocular infections, was successfully formulated as in situ gel-forming eye drops using Sodium alginate as a gelling agent in combination with HPMC as a viscosity enhancing agent. Thus, the developed formulation is a viable alternative to conventional eye drops by virtue of its ability to enhance bioavailability through its longer precorneal residence time and ability to sustain drug release. Also important is the ease of administration afforded and decreased frequency of administration resulting in better patient acceptance.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ashim KM. Ophthalmic drug delivery system. Vol. 58. New York: Marcel Dekker Inc; 1993. pp. 105–10. [Google Scholar]
- 2.Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery an overview. Int J Pharm. 2004;269:1–14. doi: 10.1016/j.ijpharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Singh SK, Bandyopadhyay P. Pharmacia Corporation. Ophthalmic formulation with novel gum composition. 2006 US 7128928. [Google Scholar]
- 4.Thorsteinn L, Tomi J. Cyclodextrins in ocular drug delivery. Adv Drug Del Rev. 1999;36:59–78. [Google Scholar]
- 5.Gokulgandhi MR, Parikh JR, Megha Barot M, Modi DM. A pH triggered in situ gel forming ophthalmic drug delivery system for tropicamide. Drug Delivery Technology. 2007;5:44–9. [Google Scholar]
- 6.Zhidong L, Jiawei L, Shufang N, Hui L, Pingtian D, Weisan P. Study of an alginate/HPMC based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006;315:12–7. doi: 10.1016/j.ijpharm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Indu PK, Manjit S, Meenakshi K. Formulation and evaluation of ophthalmic preparations of acetazolamide. Int J Pharm. 2000;199:119–27. doi: 10.1016/s0378-5173(00)00359-8. [DOI] [PubMed] [Google Scholar]
- 8.Pandit D, Bharathi A, Srinatha R, Singh S. Long acting ophthalmic formulation of indomethacin: Evaluation of alginate gel systems. Indian J Pharm Sci. 2007;69:37–40. [Google Scholar]
- 9.Johan C, Katarina E, Roger P, Katarina J. Rheological evaluation of gelrite in situ gel for opthalmic use. Eur J Pharm Sci. 1998;6:113–6. doi: 10.1016/s0928-0987(97)00074-2. [DOI] [PubMed] [Google Scholar]
- 10.Katarina E, Johan C, Roger P. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur J Pharm Sci. 1998;6:105–12. doi: 10.1016/s0928-0987(97)00075-4. [DOI] [PubMed] [Google Scholar]
- 11.Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Control Release. 2001;69:379–88. doi: 10.1016/s0168-3659(01)00279-6. [DOI] [PubMed] [Google Scholar]
- 12.Draize J, Woodward G, Calvery O. Methods for the study of irritation and toxicity of substance applied topically to the skin and mucous membrane. J Pharm Col Exp Ther. 1994;82:377–90. [Google Scholar]
- 13.Mathews BR. Regulatory aspects of stability testing in Europe. Drug Dev Ind Pharm. 1999;25:831–56. doi: 10.1081/ddc-100102245. [DOI] [PubMed] [Google Scholar]


