Abstract
Background:
Pseudoexfoliation is a systemic disorder characterized by the deposition of extracellular matrix material. The microfibrillar material that gives rise to the condition is visible clinically in the anterior segment of the eye, and is also found in other tissues, including blood vessels, skin, gallbladder, kidneys, lungs, and heart.
Aims:
The present study aims to determine whether ocular pseudoexfoliation is associated with selected cardiovascular diseases.
Materials and Methods:
A cross-section comparison study was conducted with the help of the Veterans Health Administration databases, using the International Classification of Diseases, Ninth revision, Clinical Modification for pseudoexfoliation of lens capsule and pseudoexfoliation glaucoma. Selected cardiovascular diseases and risk factors for cardiovascular disease were identified using the appropriate medical codes. Patients with primary open-angle glaucoma, chronic sinusitis, and benign prostatic hyperplasia served as the comparison groups. A logistic regression model was used to control for age, gender, race, and major cardiovascular risk factors.
Results:
There were 6,046 case patients with pseudoexfoliation; approximately half were diagnosed with pseudoexfoliation glaucoma. Various stages of ischemic heart disease, cardiomyopathy, and aortic aneurysm were significantly associated with ocular pseudoexfoliation, after controlling for age, gender, race, and major cardiovascular risk factors. Associations, in general, were less demonstrable relative to the primary open-angle glaucoma comparison group.
Conclusion:
Associations of ocular pseudoexfoliation with cardiovascular diseases were generally fewer and less pronounced when compared to patients with primary open-angle glaucoma. These results add to the results of earlier studies, which suggest that open-angle glaucoma itself might be a risk factor for certain cardiovascular disorders.
Keywords: Cardiovascular disease, Glaucoma, Pseudoexfoliation glaucoma, Pseudoexfoliation lens capsule, Pseudoexfoliation syndrome
Introduction
Pseudoexfoliation is a systemic disorder characterized by the deposition of extracellular matrix material. The microfibrillar material that gives rise to the condition is readily visible clinically in the anterior segment of the eye, and is also found in other tissues, including blood vessels, skin, gallbladder, kidneys, lungs and heart[1–3] Several studies have reported an association between ocular pseudoexfoliation (PEX) and coronary artery disease,[4–6] asymptomatic myocardial diastolic dysfunction,[7] peripheral vascular disease,[8] abdominal aortic aneurysm,[9] and subclinical myocardial ischemia.[10] Despite accumulating evidence that pseudoexfoliation may be an independent risk factor for cardiovascular disease, other studies have failed to demonstrate any significant association with coronary artery disease,[11] or any increase in all-cause mortality, or cardiovascular mortality.[12,13] Given the clinical implications of a shared pathogenesis of an ocular disorder diagnosed at the slit lamp and systemic vascular disease, we explored the potential associations between PEX and cardiovascular diseases, using a large national cohort of beneficiaries in the Veterans Health Administration (VHA), with a cross-section study design.
Materials and Methods
The investigations were performed according to the guidelines of the Declaration of Helsinki. The protocol (Study # 1002-66-“Analysis of Retrospective Data Sets in Ophthalmology: Trends, Outcomes and Costs”) was approved by the Indiana University and the Clarian Health System Institutional Review Board in Indianapolis, Indiana, and the Veterans Administration Research and Development Committee for Compliance with Human Protection at the Richard L. Roudebush VAMC Indianapolis, Indiana.
The VHA national patient care database (Inpatient and Outpatient), which consolidates information from approximately 1,300 sites of care throughout the nation, was used to collect information on medical diagnoses from the fiscal year 2010. We used a cross-section study designed to determine whether an association existed between PEX (pseudoexfoliation of lens capsule and pseudoexfoliation glaucoma [PEXG]) and the major categories of cardiovascular disease, compared to large populations of patients with primary open-angle glaucoma (POAG), chronic sinusitis, and benign prostatic hyperplasia (BPH). For the purpose of this study, the acronym PEX will refer to both pseudoexfoliation of the lens capsule with and without glaucoma, and PEXG to those with glaucoma.
International Classification of Diseases, Clinical Modification, Ninth revision, (ICD-9-CM) was used to identify patients with pseudoexfoliation of the lens capsule (366.11) and pseudoexfoliation glaucoma (365.52). Case-patients consisted of persons with either diagnosis. Patients with ICD-9-CM diagnosis of POAG (365.11), chronic sinusitis (473, 473.0-473.9), and BPH (600.00-600.9) made up the comparison groups. Patients in the comparison group could not have had ICD-9-CM diagnoses of PEX or PEXG, through a two-year, look-back period. Patients with BPH exceeded 600,000, so a 20% sample was randomly selected.
The following cardiovascular diseases were identified through the ICD-9-CM codes: acute myocardial infarction (410); acute, subacute, and chronic ischemic heart disease (411; 412); angina pectoris (413); ‘other forms’ of chronic ischemic heart disease (414); congestive heart failure (428); cardiomyopathy (425.0 [idiopathic], 425.1 [obstructive], and 425.9 [other]); and aortic aneurysm (441 [abdominal and thoracic]). Other forms of chronic ischemic heart diseases (coded as 414) typically identify conditions like coronary calcification or narrowing of coronary arteries, on angiography. The following risk factors for cardiovascular disease were also captured: systemic hypertension (ICD-9-CM: 401.0, 401.1, 401.9), hyperlipidemia (ICD-9-CM: 272), diabetes (ICD-9-CM: 250), and tobacco exposure (ICD-9-CM: 305.1). For patients with multiple heart disorders and cardiovascular risk factors, each condition was treated as an independent variable.
Statistical study
Demographic findings and frequencies of cardiovascular disease and cardiovascular risk factors were calculated for case and comparison groups. P-values for continuous variables were obtained by the Kruskal–Wallis test and P-values for categorical variables were obtained by the chi square test. The relative risks for individual cardiovascular diseases and risk factors for cardiovascular diseases with PEX and POAG were examined using the logistic regression analysis, and they were reported as an odds ratio, with a 99% confidence interval (CI), after adjustment for age, race, gender, hypertension, hyperlipidemia, tobacco exposure, and diabetes. The results were also reported for pseudoexfoliation of the lens capsule and PEXG, combined and separately. All analyses were conducted with Statistical Analysis Software (SAS®), Version 9.2, Cary, NC.
Results
After the inclusion and exclusion criteria were applied, there were 6,046 case-patients with PEX. Cases were almost equally divided between pseudoexfoliation of the lens capsule (n = 3,004) and PEXG (n = 3,042). The control groups consisted of 105,019 patients with POAG, 115,584 with chronic sinusitis, and 125,924 with BPH. Although the majority of patients in the study were men (case patients = 96.8%; control groups range: 86.6 to 97.4%), there were differences in racial composition and age distribution [Table 1]. Blacks made up 2.4% of the patients in the case group, yet represented from 4.6 to 15.6% of the control groups (P < 0.01). Patients with PEX were nearly five years older on an average, compared to persons with POAG (77.1 vs. 72.3 years) and with BPH (77.1 vs. 71.8 years); and were 20 years older than those with chronic sinusitis (77.1 vs. 57.1 years) [Table 1].
Table 1.
Characteristics of patients with pseudoexfoliation, cases and comparison groups
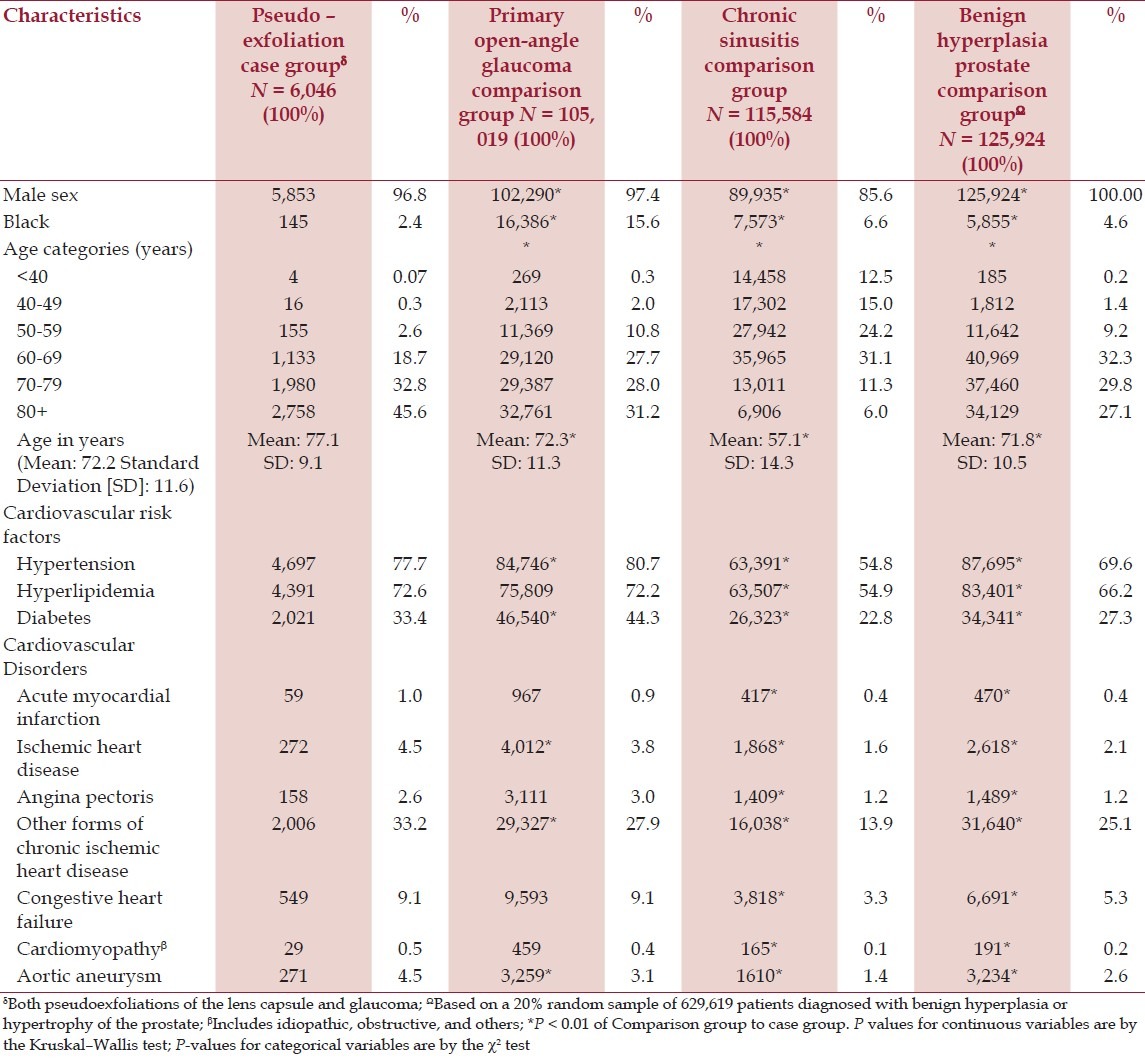
The distribution of cardiovascular disease risk factors (confounding variables) varied across groups. The prevalence of diabetes mellitus, for example, ranged from 27.3% in patients with BPH to 44.3% in patients with POAG (P < 0.01). Systemic hypertension was found in 54.8% of those with chronic sinusitis and 80.7% of patients with POAG [Table 1].
The distribution of cardiovascular diseases (unadjusted prevalence) also varied. A history of previous myocardial infarction, for instance, was three times higher in patients with PEX and POAG compared to patients with chronic sinusitis or BPH [Table 1]. Congestive heart failure was also more prevalent among those with POAG than in those with chronic sinusitis [Table 1].
After adjustment for age, race, gender, hypertension, hyperlipidemia, diabetes, alcohol use, and tobacco exposure there were statistically significant associations of PEX with all forms of chronic ischemic heart disease, cardiomyopathy, and aortic aneurysm, compared to patients with BPH [Table 2]. Except for the miscellaneous ICD-9-CM coding category referred to as ‘other forms,’ all Odds Ratios were significant at the 1% level. Associations detected with the chronic sinusitis comparison group included acute, subacute, and chronic ischemic heart disease, and cardiomyopathy [Table 3]. The category of ‘other forms’ of chronic ischemic heart disease was the only positive association of PEX in the POAG cohort, although cardiomyopathy was associated with just pseudoexfoliation of the lens capsule [Table 4]. This relatively ill-defined ICD-9-CM category, usually identified patients with signs of structural coronary artery disease (e.g. coronary artery calcification or vascular narrowing on angiography), who did not fit into the standard coronary artery syndrome diagnosis.
Table 2.
Risks of cardiovascular disease in ocular pseudoexfoliation with benign hyperplasia of prostate comparison groupδ
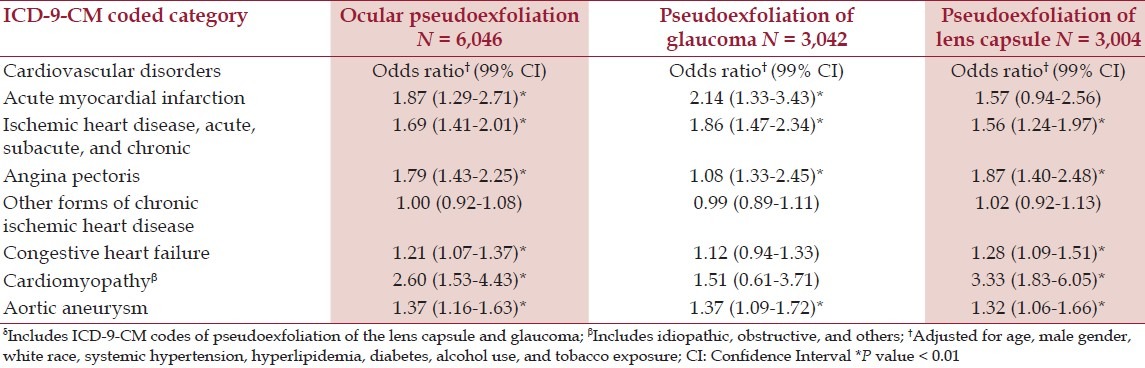
Table 3.
Risks of cardiovascular disease in ocular pseudoexfoliation with chronic sinusitis comparison groupδ
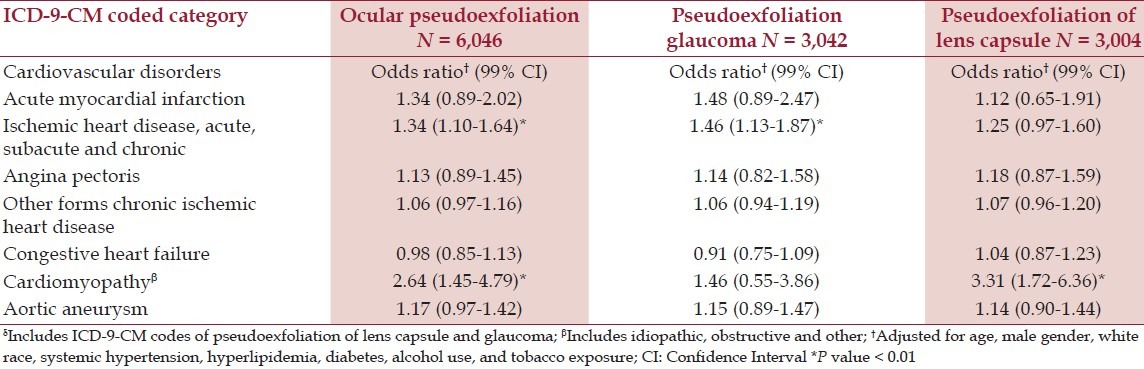
Table 4.
Risks of cardiovascular disease in ocular pseudoexfoliation with primary open-angle glaucoma comparison groupδ
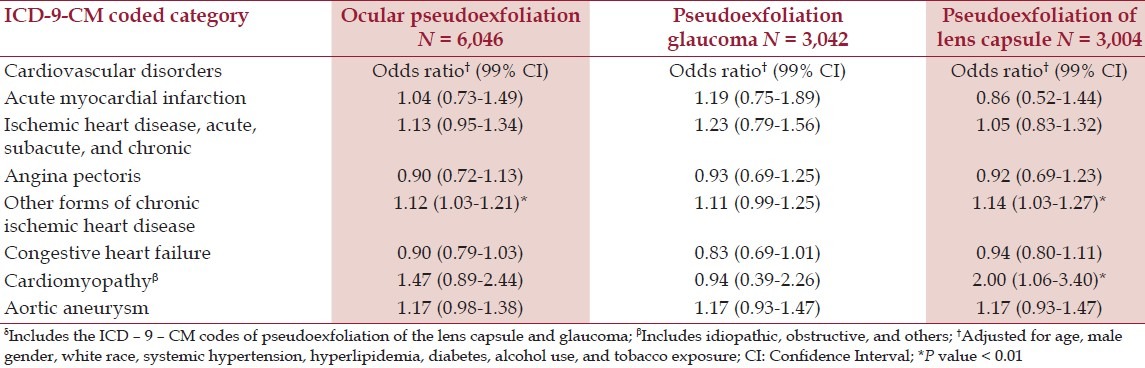
The single strongest correlation was with cardiomyopathy (OR 2.60 99% CI 1.53-4.43), reported with the BPH comparison group. The subset analysis (i.e. analyses with and without glaucoma) revealed that most associations were positive for both, except in five situations, where three were noted only for pseudoexfoliation of the lens capsule and two only for PEXG [Tables 2 and 4].
Discussion
This study, using administrative databases, showed statistically significant associations of PEX and PEXG with a variety of cardiovascular disorders, after controlling for age, gender, race, hypertension, hyperlipidemia, diabetes mellitus, and alcohol and tobacco use [Tables 2 and 4]. These disorders included past acute myocardial infarction; acute, subacute, and chronic ischemic heart disease; angina pectoris; other forms of chronic ischemic heart disease; congestive heart failure; cardiomyopathy; and aortic aneurysm. These associations varied, however, according to the control group. The most positive associations were found with the BPH comparison population and the least with POAG [Tables 2 and 4]. The only disorder that was consistently associated with PEX in all three comparison groups was cardiomyopathy, which, to our knowledge, has not been linked previously with PEX.
The fact that the relative risk of cardiovascular diseases and PEX was least when compared to a population of patients with POAG suggests that glaucoma itself may be an important confounding variable. This observation is supported by a number of previous studies that have shown a correlation between POAG and atherosclerosis, or other risks factors for vascular disease. In a review of this subject by Pache and Flammer, the authors found 16 studies showing a positive association of elevated blood pressure, smoking, or elevated serum lipids with POAG.[14] There were, however, approximately a third of as many studies reporting no association, or an association with arterial hypotension, rather than high blood pressure.[14]
There are other challenges to establishing causal associations between PEX and commonly found heart diseases, among an elderly population. For one, the proposed pathways of causation between cardiovascular disease and PEX go in both directions (reverse causality).[15] In other words, some investigators hypothesize that cardiovascular risk factors may affect the development of glaucoma, while others suggest that pseudoexfoliation material might affect the development of vascular disease. Another problem is the expanding number of newly recognized, potentially independent risk factors for cardiovascular disease, such as, inflammation (as measured by the C-reactive protein), vitamin D level, and hours of regular sleep.[16–19] Finally, the web of causation for most age-related cardiovascular disorders is incompletely understood, and for those conditions typically attributed to ischemia, the presumed pathogenesis can be quite different. Congestive heart failure and cardiomyopathy, for example, are both commonly attributed to ischemic heart disease, but can also be caused by toxins or an infectious disease.
This national cross-section comparison study, involving over 6,000 patients with PEX and over 100,000 patients in each comparison group found significant associations of PEX with many forms of heart disease, particularly in the BPH group, after adjustment for age, gender, race, and major cardiovascular risk factors [Tables 2 and 4]. The use of POAG as a comparison group served to offset any association that might exist between open-angle glaucoma and cardiovascular disease. This control group also helped to assure that persons with PEX were not inadvertently represented in the comparison group, as data were collected retrospectively. PEX should have been recognized and recorded in the electronic medical record with any reasonable evaluation of open-angle glaucoma, although we acknowledge that this might not always be the case. POAG as a control might have some drawbacks. Treatment of POAG with β-blockers, for instance, could theoretically reduce the prevalence of clinically detectable heart disease.
Our results showed that substantial differences in major risk factors for cardiovascular disease existed between groups. Variations of a similar nature have been found in other epidemiological studies, and may explain why reports on the relationship of PEX and cardiovascular disease have shown some inconsistencies in the literature.[4–6,11–13,20]
There are several weaknesses of this study, other than that the population was predominantly male and that de-identified data was collected retrospectively. The diagnosis of PEX could not be confirmed, and the diagnoses of cardiovascular diseases were not standardized. Although, at the slit lamp, few conditions could be mistaken for PEX, it is possible to overlook faint deposits and the diagnosis can be missed following cataract extraction. Controlling for cardiovascular risks factors is challenging using administrative datasets, but prior studies have also often lacked data related to tobacco use, alcohol consumption, socioeconomic status, and key hemostatic factors.[4–6,11–13,20]
Conclusion
Using a cross-section comparison study design, we found correlations between PEX and cardiovascular diseases, similar to those reported in the literature, and with cardiomyopathy, which had not been previously described. Attenuation of these associations when compared to a control population with POAG, however, suggests that the relationship may be confounded by factors mediated through glaucoma itself.
Footnotes
Source of Support: This research was supported by the Department of Veterans Affairs Health Service Research and Development Grant, HFP 04-148.
Conflict of Interest: None declared.
References
- 1.Streeten BW, Li ZY, Wallace RN, Eagle RC, Jr, Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol. 1992;110:1757–62. doi: 10.1001/archopht.1992.01080240097039. [DOI] [PubMed] [Google Scholar]
- 2.Streeten BW, Dark AJ, Wallace RN, Li Z-Y, Hoepner JA. Pseudoexfoliative fibrillopathy in the skin of patients with ocular pseudoexfoliation. Am J Ophthalmol. 1990;110:490–9. doi: 10.1016/s0002-9394(14)77871-7. [DOI] [PubMed] [Google Scholar]
- 3.Schlőtzer-Schrehardt U, Koca MR, Nauman GOH, Folkoholz H. Pseudoexfoliation syndrome. Ocular manifestation of a systemic disorder? Arch Ophthalmol. 1992;110:1752–6. doi: 10.1001/archopht.1992.01080240092038. [DOI] [PubMed] [Google Scholar]
- 4.Citirik M, Acaroglu G, Batman C, Yildiran L, Zilelioglu O. A possible link between the pseudoexfoliation syndrome and coronary heart disease. Eye. 2007;21:11–5. doi: 10.1038/sj.eye.6702177. [DOI] [PubMed] [Google Scholar]
- 5.Andrikopoulos GK, Mela EK, Georgakopoulos CD, Papadopoulos GE, Damelou AN, Alexopoulos DK, et al. Pseudoexfoliation syndrome in Greek patients with cataract and its association to glaucoma and coronary artery disease. Eye. 2007;23:442–7. doi: 10.1038/sj.eye.6702992. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P, Wang JJ, Smith W. Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol. 1997;124:685–7. doi: 10.1016/s0002-9394(14)70908-0. [DOI] [PubMed] [Google Scholar]
- 7.Bojić L, Ermacora R, Polić S, Ivanisević M, Mandić Z, Rogosić V, et al. Pseudoexfoliation syndrome and asymptomatic myocardial dysfunction. Graefes Arch Clin Exp Ophthalmol. 2005;243:446–9. doi: 10.1007/s00417-004-1074-9. [DOI] [PubMed] [Google Scholar]
- 8.Praveen MR, Shah SK, Vasavada AR, Diwan RP, Shah SM, Zumkhawala BR, et al. Pseudoexfoliation as a risk factor for peripheral vascular disease: A case-control study. Eye. 2001;25:174–9. doi: 10.1038/eye.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher S, Schlötzer-Schrehardt U, Martus P, Lang W, Naumann GO. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet. 2001;357:359–60. doi: 10.1016/s0140-6736(00)03645-x. [DOI] [PubMed] [Google Scholar]
- 10.Demir N, Ulus T, Yucel OE, Kumral ET, Singar E, Tanboga HI. Assessment of myocardial ischaemia using tissue Doppler imaging in pseudoexfoliation syndrome. Eye (Lond) 2011;25:1177–80. doi: 10.1038/eye.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emiroglu MY, Coskun E, Karapinar H, Capkın M, Kaya Z, Kaya H, et al. Is pseudoexfoliation associated with coronary artery disease? N Am J Med Sci. 2010;2:487–90. doi: 10.4297/najms.2010.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrum KR, Hattenhauer MG, Dodge D. Cardiovascular and cerebrovascular mortality associated with ocular pseudoexfoliation. Am J Ophthalmol. 2000;129:83–6. doi: 10.1016/s0002-9394(99)00255-x. [DOI] [PubMed] [Google Scholar]
- 13.Ringvold A, Bilka S, Sandvik L. Pseudoexfoliation and mortality. Acta Ophthalmol Scand. 1997;75:255–6. doi: 10.1111/j.1600-0420.1997.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 14.Pache M, Flammer J. A sick eye in a sick body? Systemic findings in patients with primary open-angle glaucoma. Surv Ophthalmol. 2006;51:179–212. doi: 10.1016/j.survophthal.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Schlőtzer-Schrehardt U, Streeten BA. Pseudoexfoliation syndrome. In: Klintworth GK, Garner A, editors. Pathobiology of Ocular Disease. New York: Informa Healthcare; 2008. pp. 505–36. Ch 25. [Google Scholar]
- 16.Malhorta A, Loscalzo J. Sleep and cardiovascular disease: an overview. Prog Cardiovasc Dis. 2009;51:279–84. doi: 10.1016/j.pcad.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5:93–102. doi: 10.2174/157016107780368280. [DOI] [PubMed] [Google Scholar]
- 18.Motiwala SR, Wang TJ. Vitamin D and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:345–53. doi: 10.1097/MNH.0b013e3283474985. [DOI] [PubMed] [Google Scholar]
- 19.Madjid M, Willerson JT. Inflammatory markers in coronary heart disease. Br Med Bull. 2011;100:23–8. doi: 10.1093/bmb/ldr043. [DOI] [PubMed] [Google Scholar]
- 20.Tarkkanen A, Renuanen A, Kivelä T. Frequency of systemic vascular diseases in patients with primary open-angle glaucoma and exfoliation glaucoma. Acta Ophthalmol. 2008;86:598–602. doi: 10.1111/j.1600-0420.2007.01122.x. [DOI] [PubMed] [Google Scholar]


