Abstract
Background:
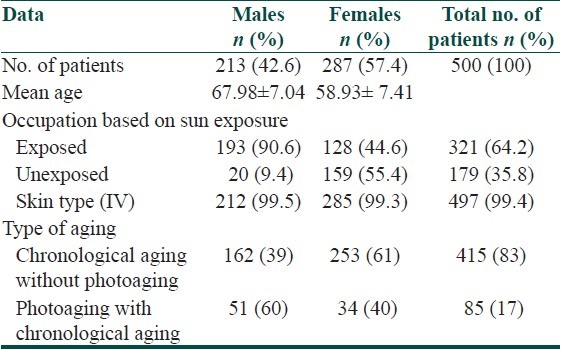
Skin is a window to aging changes, a biological reality. There is a dearth of studies regarding the various chronological (intrinsic) aging and photoaging (extrinsic) changes seen in Asians. This study was undertaken to detect the clinical pattern of aging skin changes and dermatoses seen in the elderly.
Materials and Methods:
This was a descriptive study conducted on 500 consecutive elderly individuals attending the Dermatology out-patient department. The severity of photoaging was graded using Glogau scale.
Results:
Most of the population had skin type IV and V. Majority (415, 83%) of our cases had chronological aging without photoaging and the remaining 85 (17%) individuals had photoaging along with chronological aging. The common skin changes due to chronological aging were thin skin, fine wrinkles, xerosis, and loss of elasticity. Photoaging changes such as dyspigmentation, freckles, thick skin, deep wrinkles, melasma, citrine skin, senile purpura, pseudostellate scar, acrokeratoelastoidosis marginalis, and lentigines were less frequent in our study. Smoking and prolonged sun exposure was the risk factors aggravating photoaging. The most common dermatosis was pruritus in 248 (49.6%) individuals, of which 149 (29.8%) had pruritus associated with xerosis. Contact dermatitis was more common in males. Fungal infections were frequently seen in females. Seborrhoeic keratosis (253, 50.6%) was the most common benign neoplasm more commonly seen in males. Cutaneous malignancies were less common in our study population.
Conclusion:
Photoaging changes were less common than chronological aging changes in skin type IV. Chronological changes were more frequent in females than males, while photoaging was more frequent in males.
Keywords: Aging skin, photoaging, chronoaging, dermatoses
Introduction

Aging is a “biological reality, which has its own dynamic, beyond human control.”[1] It is a complex process, in which there is progressive functional decline due to the accumulation of molecular damage over time.[2,3] Human skin like all other organs, undergoes chronological aging and aging as a consequence of environmental damage (photoaging). Aging skin has a marked susceptibility to dermatologic disorders due to the structural and physiologic changes that occur as a consequence of intrinsic and extrinsic aging.[4] Major advances in medical field and overall improvement in socioeconomic status have led to a significant increase in life expectancy. There is a longer-life time exposure to various toxic agents, irritants, environmental factors in the elderly individuals, which may have a profound effect on their health.
Skin changes in the elderly may occur due to natural aging process, or due to any dermatosis, the pattern, of which may be unique/ different in the aged population. Thus, the ambit of dermatological care needed for the elderly population is different from that for other age groups. There is also dearth in literature addressing the changing pattern of aging skin dermatoses in different population, which need to be studied in greater detail to better design the dermatological care that is to be offered to the elderly. The aim of the study was to study the frequency and the pattern of aging skin changes and dermatoses in the elderly and to differentiate chronological aging and photoaging skin changes.
Materials and Methods
This was a hospital- based descriptive study done in 500 consenting elderly individuals, conforming to the inclusion and exclusion criteria who attended the Dermatology out-patient department of JIPMER, Puducherry from February 2010 to March 2011, after obtaining Institute ethics committee clearance. All females aged 50 years and above and males aged 60 years and above were included in the study. Patients with genodermatoses, which may interfere with aging skin changes, photosensitivity disorders (excluding photoaging), premature aging, inherited disorders of DNA instability, albinos, and connective tissue disorders and patients suffering from chronic medical disorders likely to interfere with assessment of skin aging were excluded from the study.
A detailed history pertaining to the following parameters-demographic data, presenting illness, personal history/ factors aggravating aging skin changes, presence of medical/ surgical/ congenital diseases was elicited as per the proforma enclosed. A thorough dermatological examination was performed to look for the chronological aging and photoaging skin changes and dermatoses. Examination of hair, nails, palms, soles, genitalia and teeth was done. Relevant investigations, histopathology and dermoscopy were performed when required to confirm the diagnosis.
Severity grading of photoaging was done using Glogau scale as given in Table 1.[5]
Table 1.
Glogau scale for grading the severity of photoaging

The data collected was tabulated in Microsoft Excel Worksheet and computer-based analysis was performed using the SPSS 13.0 software (SPSS, Chicago, IL, USA). For comparison of means, unpaired t test was used. For comparison of proportions, Chi square test was used. In cases where any one of the cell value was less than five, Fisher's exact test was used.
Results
A total of 500 elderly individuals were included in the study with a female to male ratio of 1.34:1. The demographic data of the study population are tabulated in Table 2.
Table 2.
Demographic data
Hair examination
Most (375, 75%) of our study population had white hair, followed by grey hair in 114(22.8%) patients and black hair in eleven (2.2%) patients. Above the age of 60 years, most of the men had white hair when compared to women this difference was statistically significant as observed in Table 3.
Table 3.
Frequency of the distribution of hair color

Male pattern baldness was observed in 119(55.39%) males and diffuse thinning of hair was observed in 193(67.24%) females.
Nail
The commonest nail finding observed was loss of lustre present in 254 (50.8%) individuals. The distribution of other nail findings based on gender is given in Table 4.
Table 4.
Gender wise distribution of nail changes
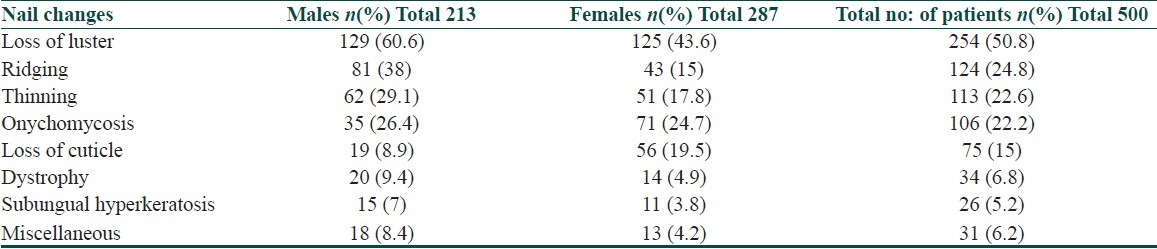
Onychomycosis was common in females (71, 24.7%) when compared to males (35, 26.4%) and this difference was found to be statistically significant (P=0.0015, Fisher's test). Lustreless nails, thinning of nails, ridging, and loss of cuticle were more common in males when compared to females, which was statistically significant.
The other nail findings observed less frequently were flattening in six (1.2) individuals, blackish discoloration in five (1%) individuals, thickening, shininess and splitting in three (0.6%) individuals each, longitudinal melanonychia, median canalicular dystrophy, and pitting in two (0.4) individuals each, beau's line, leukonychia, paronychia, pallor, onycholysis, and splinter hemorrhage in one (0.2) individual each.
Oral mucosa and teeth
The most common finding was dental caries observed in 186(73.4%) individuals followed by loss of teeth observed in 110(45.4%) individuals. The other abnormalities observed were staining 27(11.4%), lichen planus 6(2.7%) and candidiasis 5(1.7%). Loss of teeth was more common in males (69, 31.5%) when compared to females (41, 13.9%) and this difference was statistically significant (P<0.0001, Fisher's test).
Palms and soles
Acrokeratoelastoidosis marginalis [Figure 1] was seen in two (0.9%) females. Other findings observed were scaling (11, 2.2%), pigmentation (6, 1.2%), fissuring (3, 4.2%), corn foot (2, 0.4%), and intertrigo (1, 0.2%).
Figure 1.
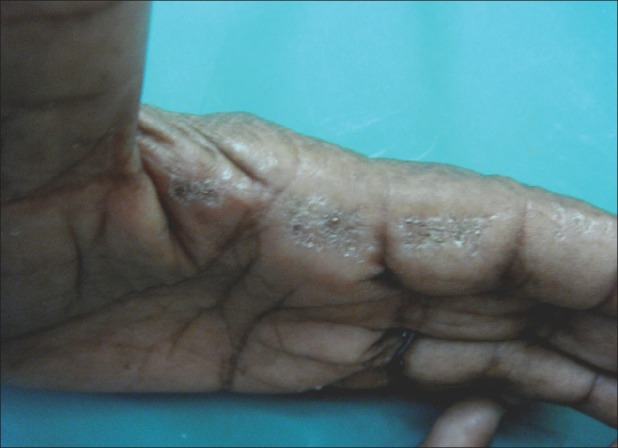
Acrokeratoelastoidosis marginalis characteristically seen at margin of fingers
Cutaneous Changes
Cutaneous changes over exposed skin
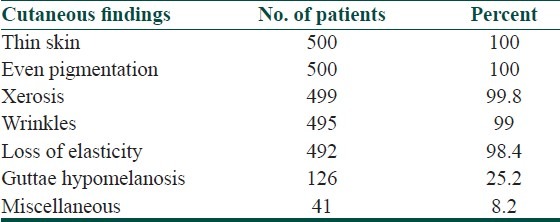
The cutaneous findings observed in more than 50 percent of cases were xerosis, loss of elasticity, fine wrinkles, thin skin [Figure 2], sunken eyes and acrochordon. The other less frequently observed cutaneous changes were sallowness 159(31.8%), dyspigmentation 65(13%), freckles 24(4.8%), comedones 23(4.6%), angular cheilitis (3.2%), thick skin (3.2%), deep wrinkles (2.4%), guttae hypomelanosis (2.2%, [Figure 3]), sebaceous hyperplasia (1.6%, [Figure 4]), senile purpura [Figure 5] and cherry angioma (1%) each, lichen sclerosus et atrophicus (0.8%), pseudostellate scar (0.4%), colloid milium, lentigines and milia (0.2%) each. Forty four (8.8%) individuals out of the 500 had mild aging skin changes.
Figure 2.
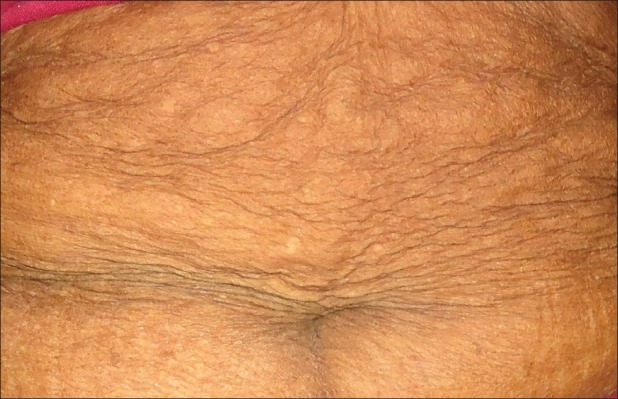
Thinning of skin with fine wrinkles seen over the abdomen
Figure 3.
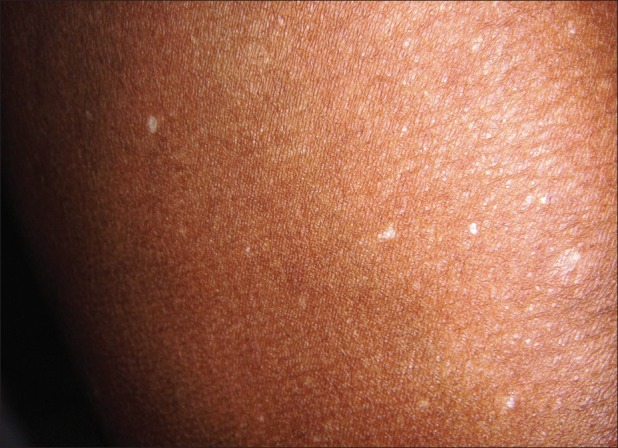
Idiopathic guttate hypomelanosis over the thighs
Figure 4.
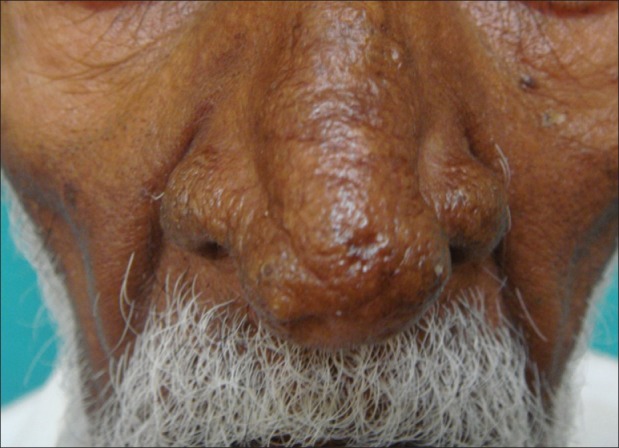
Sebaceous hyperplasia over the nose
Figure 5.
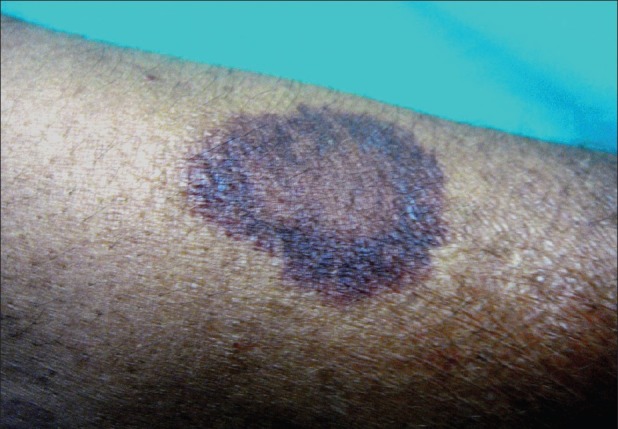
Senile purpura over the forearm
Of all the cutaneous findings present in exposed skin, the statistically significant differences were observed in fine wrinkles and angular cheilitis, which were more common in females, and deep wrinkles and sunken eyes, were more common in males.
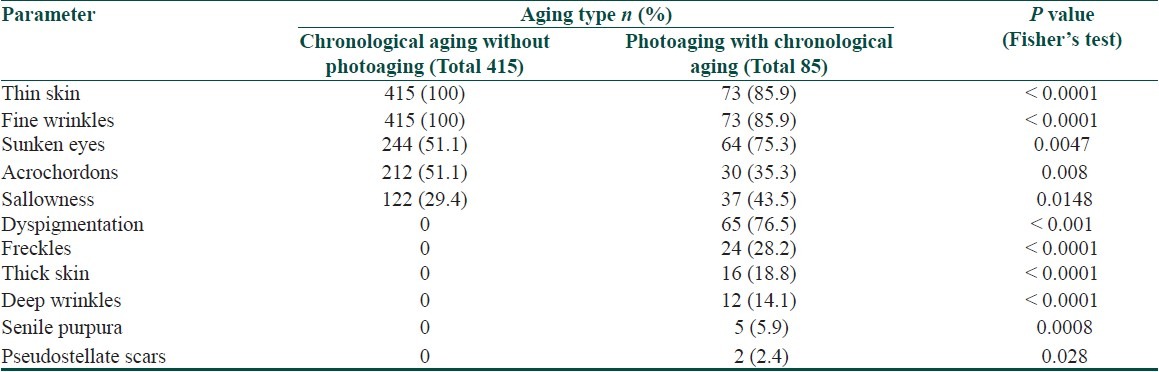
All elderly individuals with chronological aging alone had thin skin and fine wrinkles [Figure 2] and all individuals with thick skin and deep wrinkles [Figure 6] had photoaging with chronological aging as observed in Table 5. Acrochordons were common in individuals with chronological aging alone while sunken eyes and sallowness were common in individuals with photoaging along with chronological aging. Freckles, dyspigmentation, senile purpura and pseudostellate scars were seen only in individuals who had photoaging along with chronological aging. The statistically significant findings based on the type of aging are provided in Table 5.
Figure 6.
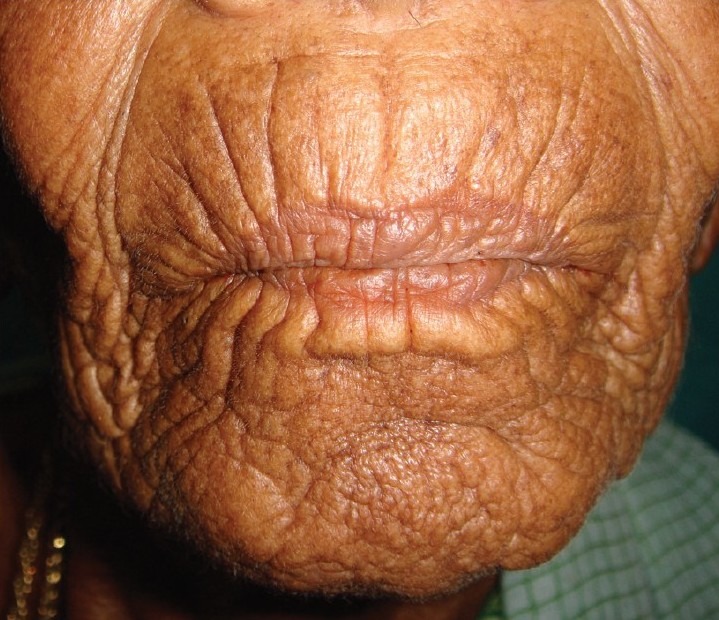
Deep wrinkles prominent around the mouth
Table 5.
Cutaneous findings on exposed skin with statistically significant difference based on type of aging
Cutaneous changes over the unexposed skin
The cutaneous findings observed over the unexposed skin are shown in Table 6. The other less frequently observed cutaneous changes over the unexposed skin were cherry angioma in 31(6.2%) individuals, acrochordons in six (1.2%) individuals, lichen sclerosus et atrophicus in four (0.8%) individuals.
Table 6.
Frequency of distribution of cutaneous changes observed over unexposed skin
Idiopathic guttae hypomelanosis over unexposed skin was more common in males (72, 33.8%) when compared to females (54, 18.8%) and this difference was statistically significant.
Acrochordons were more commonly seen over the exposed skin (242, 86.4% patients) than in unexposed skin (6, 2.4% patients) and this difference was statistically significant (P<0.0001, Fisher's test).
Smoking
Out of the 500 individuals, 74(14.8%) males had history of smoking. Out of the 74 patients with history of smoking, 19(25.67%) patients had photoaging along with chronological aging and 55(74.32%) patients had chronological aging alone and this difference was statistically significant (P=0.0429, Fisher's test).
Associated Systemic Disorders
Diabetes mellitus (75, 28.9%) and hypertension (66, 25.5%) were the commonly associated medical disorders.
Dermatoses of the Elderly
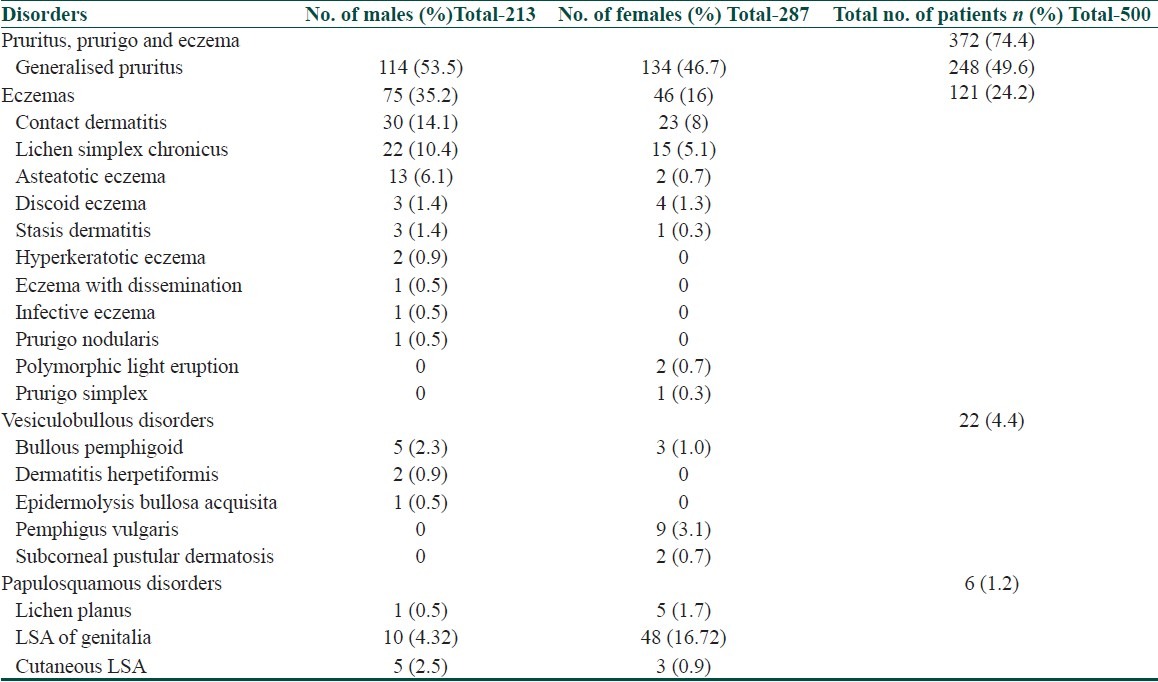
Generalised pruritus (248, 49.6%) was the most commonly observed dermatosis in our study population, of which in 149(29.8%) individuals it was associated with xerosis and the rest 99(19.8%) individuals had other associated pruritic diseases. The distributions of the other dermatoses are given in Table 7. All of these disorders were confirmed by biopsy except for the eczematous disorders. Lichen simplex chronicus (P=0.0378), asteatotic eczema (P=0.0007) and contact dermatitis (0.0390) were more common in males than in females.
Table 7.
Gender wise distribution of dermatoses
Infections and Infestations in the Elderly
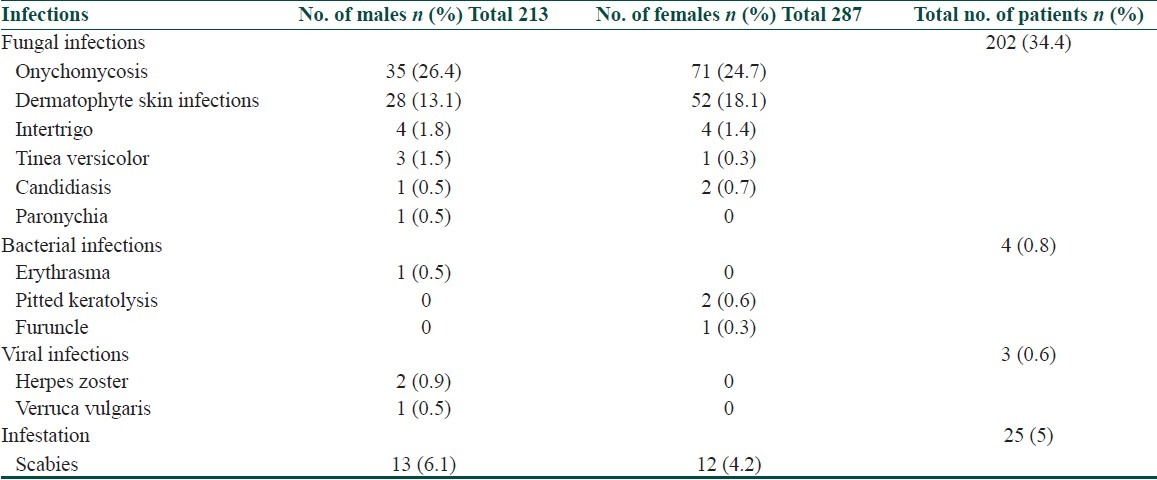
Infections and infestations in elderly were seen in 234 (46.8%) individuals. The commonest infection observed in our study population was fungal infection in 202 (34.4%) patients and onychomycosis was commoner in females 71(24.7%) when compared to males 35(26.4%) and this difference was found to be statistically significant (p=0.027, Fisher's test). The distribution of other infections and infestation based on gender are depicted in Table 8.
Table 8.
Gender wise distribution of infections and infestation
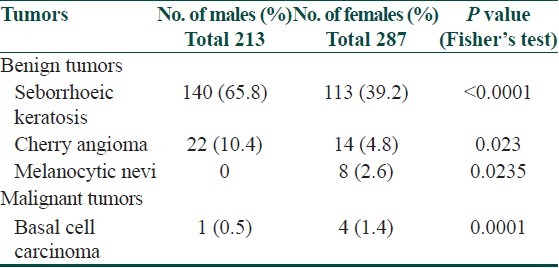
Benign and Malignant Tumors of the Skin in Elderly
The most common cutaneous benign tumor was seborrhoeic keratoses observed in 253 (50.6%) individuals. Seborrhoeic keratoses and cherry angiomas were more common in males when compared to females, and melanocytic nevi and basal cell carcinoma were more common in females than in males, which were statistically significant as observed in Table 9.
Table 9.
Gender-wise distribution of benign and malignant tumors
Miscellaneous Disorders
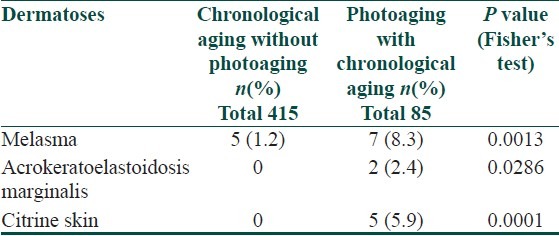
Melasma was more common in individuals with photoaging and acrokeratoelastoidosis marginalis and citrine skin were present only in individuals with photoaging as observed in Table 10.
Table 10.
Distribution of miscellaneous dermatoses based on type of aging
Discussion
The burden of dermatologic disease in the elderly is known to be substantial and is almost certainly underestimated, based on the available data.[6] India is the second largest populated country in the world, with 72 million elderly persons above 60 years of age as of 2001 and this number is likely to increase to 179 million in 2031, and further to 301 million in 2051.[7] This rapid demographic shift has created many challenges in management of diseases in the geriatric population. Dermatologists need to be aware of these aging changes as well as equip themselves to treat these conditions.
With aging there is a decline in the regular functions of skin resulting in some inevitable changes, such as roughness, wrinkling, laxity of the skin, and atypical presentations of dermatologic diseases are observed in elderly patients.[8]
We must distinguish process of aging into intrinsic aging caused by passage of time and photoaging caused by solar radiation. Chronological aging is a natural process characterized by thinning of skin, even pigmentation of skin, laxity, fine wrinkles, xerosis, slackness, benign neoplasms, seborrhoeic keratoses and cherry angiomas.[9] Intrinsic aging is influenced by ethnicity, anatomical variations and hormonal changes.[10]
Photoaging, which is due to extrinsic factors like exposure to UV light, lifestyle influence, smoking and nicotine,[10] is characterized by thick, dry and rough skin, deep wrinkles with actinic keratoses, irregular freckling, lentigines, guttae hypomelanosis, persistent hyperpigmentation, stellate pseudoscars, fine nodularity, fine elasticity, telangiectasia, venous lakes, purpura, comedones, sebaceous hyperplasia, citrine skin (Milian), cutis rhomboidalis nuchae (Jadassohn), nodular elastoidosis with cyst and comedones (Favre-Racouchot), diffuse elastoma (dubreuilh), elastic nodule of ear, acrokeratoelastoidosis marginalis, striated beaded lines, actinic granuloma and actinic comedonal plaque.[9]
With age, the epidermal dermal junction flattens, number of interdigitations decreases giving the skin a thin and sallowed appearance. Dry and flaky skin is the result of decrease in epidermal filaggrin and decrease of epidermal turnover rate by 50%. The number of melanocytes decreases by 20% giving the pale appearance to the skin and hair and decreased production of vitamin D by 70%. Involution of vertical capillary in dermal papillae causes pallor, decrease of skin temperature and changes in thermoregulation.[11]
The reduction in dermal bulk and accumulation of lipofuscin, a marker of cellular damage compounded by diminished neuromuscular control contributes to wrinkling.[12] Reduced synthesis and faster degradation of collagen contributes to impaired wound healing. Elastic fibers decreases in number and size so there is pronounced less elasticity in aged skin. Alteration of mucopolysaccharides affects skin turgor. The overall volume of subcutaneous fat tissue diminishes. Scalp hair becomes finer with age and the density of hair follicles decreases with age leading to baldness and is influenced by the hormonal changes in aging.[2] The prevalence of total tooth loss is greater among older age groups.[13] In the elderly, the nails become brittle, pale and dull.[14]
In the exposed areas of the senile skin, there is increase in elastic fibres and hypertrophic response (elastosis) leading to development of pebbly surface in contrast to the decrease in elastic fibres in aging skin elsewhere.[12] Dyspigmentation of skin is seen due to the localized proliferation of melanocytes in the sun exposed skin.[2]
There are published studies regarding skin disorders among the elderly population from different countries such as Taiwan, USA, Norway, the Philippines, Turkey and India.[15–17] Most of these studies are about detecting the prevalence or characteristic pattern of skin diseases, or identifying past or present skin complaints and the dermatologic findings in the elderly. But, there are very few studies regarding the various chronological (intrinsic) aging and photoaging (extrinsic) changes seen in Asians with skin type IV and V apart from the dermatoses affecting the elderly.
Chronological aging and skin
The age groups of elderly individuals included in different studies done in this regard are variable. The majority of the studies[8,11,15–19] had included an age group from 60- 65 years as elderly population. Sahoo et al[20] and Patange and Fernandez[21] had included an age from 55 years and that by Beauregard and Gilchrest[6] included an age group from 50 years as elderly. The present study included all the geriatric population with males above 60 years and females having age of 50 years and above. Female patients were chosen at a younger age, as in them the hormonal changes after menopause influences the aging skin changes,[22] which starts around their 50s.[12]
The common skin changes due to chronological aging in our study were thin skin (500, 100%), fine wrinkles (500, 100%), xerosis (498, 99.6%), and loss of elasticity (495, 99%) seen in almost all elderly individuals. Sunken eyes (308, 61.6%) and acrochordons (245, 49%) were seen in half of the individuals, more commonly over the neck.
Guttae hypomelanosis was seen in 130 (26%) individuals over exposed and unexposed skin, of which 119 individuals had over the unexposed skin alone and four individuals had over exposed skin alone. This finding was similar to that observed by Patange and Fernandez[21] (49, 24.5%) but higher than that studied by Sahoo et al[20] (13, 6.5%). Higher frequency of guttae hypomelanosis was reported in a study by Grover and Narasimhalu[16] (153, 76.5%). We observed senile comedones in 23 (4.6%) individuals, which were comparable to that observed by Grover and Narasimhalu[16] (13, 6.5%).
We had a higher prevalence of wrinkling and xerosis when compared to the other studies by Grover and Narasimhalu[16] [191 (95.5%), 171 (85.5%)] and Chopra et al[15] [110 (51.7%), 108 (50.8%)]. Probably the reason being, most of our population were agricultural labourers belonging to a lower socio economic group residing in the villages, and had not used cosmetics or sunscreens for preventing or camouflaging the aging skin changes.
Color change in hair is a distinctive feature of aging, most commonly represented by greying of the hair. White hair was seen more commonly among the males (207, 97.2%) in comparison to females (130, 90.9%) even after adjusting for age in our study. Hair loss in elderly women in our study was mostly diffuse thinning of hair (193, 67.24%) whereas males had androgenetic alopecia (119, 55.39%). Patange and Fernandez[21] observed male pattern baldness in 20 males and a diffuse pattern of hair loss in 94 females in their study of 200 patients.
The commonest nail finding in elderly in our study was loss of lustre (254, 50.8%), which was higher than that observed by Patange and Fernandez[21] (20.5%) and was lower than that reported by Grover and Narasimhalu[16] (128, 64%).
Photoaging and skin
The cutaneous changes due to chronic sun exposure were more common in males than in females in our study. Probably this was related to males being occupied mostly in outdoor occupation. Photoaging changes seen in our study were less common compared to other studies as majority of our population had skin type (IV), with a higher melanin content, which is photoprotective.[12]
The prevalence of cutaneous changes suggestive of photoaging such as dyspigmentation (65,13%), freckles (24,4.8%), thick skin (16,3.2%), deep wrinkles (12,2.4%), melasma (12,2.4%), citrine skin (5,1%), senile purpura (5,1%), pseudostellate scar (2,0.4%), acrokeratoelastoidosis marginalis (2,0.4%), and lentigines (1,0.2%) were less frequently seen in our study than that reported in the literature in various studies.
Beauregard and Gilchrest[6] observed elastosis in 95.6% of patients, lentigines in 70.6% of patients, senile purpura in 11.9% of patients and stellate pseudoscar in 5.9% of patients. The most common disorder due to chronic sun exposure in a study by Polat et al[17] was solar lentigo (48.8%).
Photoaging changes in our study were related to racial factors, occupation with prolonged sun exposure, male gender with outdoor occupation and smoking. Smoking is associated with increased risk of accelerated photoaging skin changes like deep wrinkles.[12] Smoking and prolonged sun exposure may play an important role as risk factors increasing the risk of photoaging by an odds ratio of 1.884 (P=0.0429) and 2.789 (P=0.0003) respectively. After menopause there is accelerated chronological aging in females leading to earlier cutaneous aging changes due to the decrease in estrogen.[22]
Dermatoses in the elderly
Among the various dermatoses seen in the elderly in our study, generalised pruritus (248, 49.6%), eczemas (121, 24.2%), vesiculobullous diseases (22, 4.4%) and adverse drug reactions (7, 1.4%) were the most common diagnosis made in our study.
Generalised pruritus is the most frequent complaint in dermatologic surveys in the elderly. It is the most commonly attributable to xerosis of skin followed by various causes like inflammatory eczema, lichen simplex chronicus, skin infection, psoriasis, urticaria, drug induced, anogenital itching, insect bites, and neuropsychiatric diseases, of which xerosis is the commonest.[12]
In our study generalised pruritus was seen in 248 (49.6%) individuals, of whom 149 (29.8%) individuals had pruritus, which was associated with xerosis and in the rest (99,19.8%) it was associated with other cutaneous disorders. This was also the frequently observed finding in many studies such as by Patange and Fernandez[21] (78.5%), Sahoo et al[20] (54%), in an English survey[6] (29.5%), Beauregard and Gilchrest[5] (29%), Liao et al[18] (14.2%) and by Yalcin et al[8] (473, 11.5%). The prevalence of pruritus recorded in various studies may vary depending on the specific population studied and definitive diagnosis.[11]
Of the other pruritic dermatoses recorded in our study, contact dermatitis was seen more frequently (10.6%) among the various eczemas (121, 24.2%) and was common in males than females. Elderly population is expected to have less incidence of contact dermatitis because of decreased occupational exposure and declining immune reactivity that occurs with age. Increased frequency of eczemas in the elderly population in our study may be because majority of them (319, 63.8%) were agricultural labourers with continued exposure to various allergens.
In our study, we observed pemphigus vulgaris in nine (3.1%) individuals and bullous pemphigoid in eight (1.6%) individuals respectively. Bullous pemphigoid is common after the age of 60 years due to the age associated increase in circulating antibodies and change in basement membrane.[12]
Adverse drug eruptions of all kinds increase in elderly in part because the elderly consume more medications.[12] Drug eruptions were reported in a recent study with incidence in elderly of about 50.1 per 1000 person years.[23] Yalcin et al[8] reported 1.4% of drug reaction cases, which was similar to that reported in our study (7,1.4%).
Infections and infestations
In our study, they comprised of 46.8% (234 out of 500). Higher frequency of fungal infections in the elderly is due to the decrease in immunity, high incidence of diabetes and improper skin care. The number of Langerhans cells decrease by 20 to 50% and this alteration also contributes to immunologic decline in senescence.[11]
The most common cutaneous infection in our study was fungal infection (202, 34.4%), of which we observed a higher frequency of onychomycosis (106, 22.2%) followed by the bacterial (4. 0.8%) and viral infections (3, 0.6%). Onychomycosis was recorded more commonly in females than males, may be attributable to the house hold work. We observed infestation with scabies in 25(5%) elderly individuals.
The most common dermatological diseases in a study by Cvitanovic et al[11] in population over 65 years old were actinic keratosis in 184 patients (22.38%), seborrhoeic keratosis in 156 (18.98%), discoid eczema in 77 patients (9.37%), allergic contact dermatitis in 60 (7.30%) patients, dermatophytosis in 56 (6.81%) patients, psoriasis in 51 (6.20%), verruca vulgaris in 39 (4.74%), skin tags in 27 (3.28%), naevi in 9 (1.09%) and acne in 1 (0.12%) patient.
In Taiwan[18] the most common cutaneous disorders in the elderly were dermatitis in 58.7%, fungal infections in 38%, pruritus in 14.2%, benign tumors in 12.8% and viral infections in 12.3%. Cutaneous malignant tumors were present in 2.1%. Sahoo et al[20] in a study done in India found that the commonest dermatoses were: pruritus in 54%, mycoses in 20%, xerosis in 12.5%, seborrhoeic keratosis in 10%, fibroma in 10% and psoriasis in 9%.
Very similar results are from Turkey[8] where five most frequent skin diseases in the elderly were eczema (20.4%), mycoses (15.8%–26%), pruritus (11.5%), psoriasis 8.1%, bacterial (7.3%) and viral (6.7%) infections. In Singapore,[11] the most common diagnosis, was endogenous eczema (8.4%), xerosis (5.9%) and contact dermatitis (6.7%). Seborrhoeic keratosis was the most common skin tumor 4.5% of all patients. Skin infestation with scabies was very common and was seen in 2.3%.[11] In Young's study[17] , the percentage of fungal infections was 4.5% and viral infections was 3.3%. These percentages were 3.4% and 4.0%, respectively, in Adam's study.[17]
Benign and malignant tumors of the skin
Seborrhoeic keratosis seems to be the most common cutaneous benign tumor in the elderly. Incidence of seborrhoeic keratosis in various studies ranges from 24.2% to 74.5%.[15,16,21] A higher prevalence was also seen in studies by Simth et al[19] (88%), and Beauregard and Gilchrest[6] (61.2%). In our study, we detected seborrhoeic keratoses in 253 (50.6%) individuals. Male gender and sun exposure were found to predispose towards development of seborrhoeic keratosis.
Acrochordons are pedunculated benign lesions composed of loose fibrous tissue occurring mainly over the neck and flexures.[24] We noted a higher frequency of acrochordons (245, 49%) when compared to Grover and Narasimhalu[16] (24.5%) and a lower frequency of cherry angioma (36, 7.2%) than that studied by Grover and Narasimhalu[16] (49.5%-75%) and Beauregard and Gilchrest[6] (53.7%).
Sebaceous hyperplasia represents hyperplastic sebaceous glands. In old age, although, there is decrease in sebum production, there is an increase in size of the sebaceous gland[2] . We observed sebaceous hyperplasia in eight (1.6%) individuals and its frequency was lower than that observed by Beauregard and Gilchrest[6] (17.5%).
Actinic keratosis in elderly has been frequently reported in studies done in Western countries than that compared to studies done in Asian population from Taiwan, Japan and Singapore.[17] Young's study showed that actinic keratosis is the second most common skin disorder, and in Adam and Reilly's study, it was the most frequent skin disorder in the elderly.[17] In Beauregard et al's[6] study, the percentage of actinic keratosis was 17.7% and that by Cvitanovic et al[11] was 22.38%. In our study, actinic keratosis was not observed probably because of the skin type IV/V, which is photoprotective due to a higher melanin content.[25]
The prevalence of malignant skin tumors in various studies[6,18] range between 4.4-29.8%. It includes mostly basal cell carcinoma, melanomas and squamous cell carcinoma. Our study had very low frequency of malignant tumors (5,1%) when compared to other studies, which may be explained on the basis of genetic variability or photoprotective role of melanin in skin type IV/V.
Associated systemic diseases
Diabetes mellitus (75, 28.9%) and hypertension (66, 25.5%) were most common associated medical disorders in our study, which was higher than that observed by Sahoo et al[20] in their study done in elderly from Orissa (diabetes mellitus in 21 (10.5%) patients and hypertension in 5 cases out of 200 cases).
The differences seen in various studies underscore the genetic makeup, racial influences, geographic location, skin color and duration of sun exposure, which may underlie these aging skin changes. Different patterns of dermatoses in various studies help in better understanding of the prevalent skin diseases in a particular region. With increasing geriatric population, better understanding of the physiological changes occurring with age and various dermatoses occurring with increased frequency in the elderly is required for provision of better dermatological health care.
Conclusion
Our study has shown that in individuals with skin type IV (dark–skinned), photoaging changes were seen less commonly than chronological aging changes. Though photoaging changes were less common in dark skinned individuals, prolonged sun exposure was a risk factor for photoaging. The cutaneous changes over the exposed and unexposed skin were commoner in females than in males reflecting the influence of hormonal changes (decreased estrogen) after menopause, leading to earlier cutaneous aging changes in females. However, photoaging along with chronological aging was more common in males attributable to their occupational exposure to sun. Dermatoses are seen in the elderly due to both intrinsic and extrinsic factors. Contact dermatitis was common in males due to their occupational exposure and onychomycosis was common in females due to the household, in which majority of the females were involved. Malignant tumors were less commonly seen in our study population due to the skin type (IV) of majority of the individuals. However, benign tumors are common in the elderly population, which need to be identified earlier and treated. As the geriatric population is increasing worldwide, and the demand for their care is also increasing, it is essential to identify the dermatoses in them and to provide them with proper care.

Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.World Health Organization. [Last accessed on 27.04.2011];Definition of an older or elderly person. Available from: http://www.who.int/healthinfo/survey/ageingdefinitionolder/en/index.html . [Google Scholar]
- 2.Millington GWM, Graham-Brown RAC. Skin and skin disease throughout life. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Vol. 8. Oxford: Wiley-Blackwell publication; 2010. pp. 21–9. [Google Scholar]
- 3.Magalhaes JPD. What is aging.? Definitions and concepts in gerontology. [Last accessed on 27.04.2011]. Available from: http://www.senescence.info/
- 4.Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Review. Am J Clin Dermatol. 2009;10:73–86. doi: 10.2165/00128071-200910020-00001. [DOI] [PubMed] [Google Scholar]
- 5.Brannon H. Glogau Classification of Photoaging-Classifying the Severity of Wrinkles. [Last accessed on 23/8/2011]. Available from: http://dermatology.about.com/od/wrinkles/a/glogau.htm .
- 6.Beauregard S, Gilchrest BA. A survey of skin problems and skin care regimens in the elderly. Arch Dermatol. 1987;123:1638–43. [PubMed] [Google Scholar]
- 7.Rajan SI, Sarma PS, Mishra US. Demography of Indian aging, 2001-2051. J Aging Soc Policy. 2003;15:11–30. doi: 10.1300/J031v15n02_02. [DOI] [PubMed] [Google Scholar]
- 8.Yalcin B, Tamer E, Toy GG, Oztas P, Hayran M, Alli N. The prevalence of skin diseases in the elderly: analysis of 4099 geriatric patients. Int J Dermatol. 2006;45:672–6. doi: 10.1111/j.1365-4632.2005.02607.x. [DOI] [PubMed] [Google Scholar]
- 9.Fenske NA, Lober CW. Aging and its effects on the skin. In: Moschella SL, Hurley HJ, editors. Dermatology. 3rd ed. Philadelphia: WB Saunders; 1992. pp. 107–22. [Google Scholar]
- 10.Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin aging: a review. Int J Cosmet Sci. 2008;30:87–95. doi: 10.1111/j.1468-2494.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 11.Cvitanovi H, Eva EK, Kuljanac I, Jancic E. Skin disease in a geriatric patients Group.Coll. Antropol. 2010;34(Suppl):247–251. [PubMed] [Google Scholar]
- 12.Yaar M, Gilchrest BA. Aging of skin. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick' s Dermatology in General Medicine. 7th ed. New York: McGraw- Hill Companies; 2008. pp. 963–73. [Google Scholar]
- 13.Hunt RJ, Hand JS, Kohout FJ, Beck JD. Incidence of tooth loss among elderly lowans. Am J Public Health. 1988;78:1330–32. doi: 10.2105/ajph.78.10.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Berker DAR, Baran R. Rook's Textbook of Dermatology. 8th ed. Vol. 65. Oxford: Wiley- Blackwell publication; 2010. Disorders of nails; p. 7. [Google Scholar]
- 15.Chopra A, Kullar J, Chopra D, Dhaliwal SR. Cutaneous physiological and pathological changes in elderly. Indian J Dermatol Venereol Leprol. 2000;66:274. [PubMed] [Google Scholar]
- 16.Grover S, Narasimhalu C. A clinical study of skin changes in geriatric population. Indian J Dermatol Venereol Leprol. 2009;75:305–6. doi: 10.4103/0378-6323.51266. [DOI] [PubMed] [Google Scholar]
- 17.Polat M, Yalcın B, Calıskan D, Alli N. Complete dermatological examination in the elderly: An exploratory study from an outpatient clinic in Turkey. Gerontology. 2009;55:58–63. doi: 10.1159/000129683. [DOI] [PubMed] [Google Scholar]
- 18.Liao YH, Chen KH, Tseng MP, Sun CC. Pattern of skin diseases in a geriatric patient group in Taiwan: A 7-year survey from the outpatient clinic of a University Medical Center. Dermatology. 2001;203:308–13. doi: 10.1159/000051778. [DOI] [PubMed] [Google Scholar]
- 19.Smith, Derek R, Leggat, Peter A. Prevalence of skin disease among the elderly in different clinical environments. Australasian Journal on Aging. 2005;24:71–6. [Google Scholar]
- 20.Sahoo A, Singh PC, Pattnaik P, Panigrahi R. Geriatric dermatoses in southern Orissa. Indian J Dermatol. 2000;45:66–8. [Google Scholar]
- 21.Patange SV, Fernandez RJ. A study of geriatric dermatoses. Indian J Dermatol Venereol Leprol. 1995;61:206–8. [PubMed] [Google Scholar]
- 22.Verdier-Sevrain S, Bonte F, Gilchrest B. Biology of estrogens in skin: implications for skin aging. Exp Dermatol. 2006;15:83–94. doi: 10.1111/j.1600-0625.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 23.Carneiro SC, Azevedo-e-Silva MC, Ramos-e-Silva M. Drug eruptions in the elderly. Clin Dermatol. 2011;29:43–8. doi: 10.1016/j.clindermatol.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Quinn AG, Perkins W. Non melanoma skin cancer and other epidermal skin tumors. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Oxford: Wiley- Blackwell publication; 2010. pp. 40–1. [Google Scholar]
- 25.Verma SB. Dermatology for the elderly: An Indian perspective. Clin Dermatol. 2011;29:91–6. doi: 10.1016/j.clindermatol.2010.07.012. [DOI] [PubMed] [Google Scholar]


