Abstract
Background:
Herpes zoster is an intractable painful condition, more severe in elderly patients. The pain during the first 30 days of onset is known as Acute Herpetic Neuralgia. Multiple treatments using non-steroidal anti-inflammatory drugs (NSAIDs), opioids, and tricyclic anti-depressants are available, but their side effects limit their use in geriatric patients. Gabapentin is also used in chronic neuropathic pain; however, its role in acute herpetic neuralgia is less explored.
Aim:
This study was aimed to determine dose related efficacy and safety of gabapentin in reducing pain of acute herpetic neuralgia in geriatric patients.
Materials and Methods:
In this placebo-controlled, four-week trial including 56 subjects, 42 patients received gabapentin in the dosage of 300 mg (n=15), 600 mg (n=14), and 900 mg(n=13) per day in divided doses and 14 patients received placebo within 72 hours of onset of herpes zoster.
Results:
Subjects receiving gabapentin had a statistically significant reduction (P<0.0001) in visual analog scale (VAS) score as compared to placebo, emphasizing the efficacy of gabapentin in the treatment of acute pain associated with herpes zoster on each assessment (weeks 1, 2, 3, and 4). Gabapentin in doses of 600 mg/day and 900 mg/day was better than 300 mg/day in each visit. However, no difference was observed between gabapentin 600 mg/day and 900 mg/day group at any point of time (P>0.05).
Conclusion:
The results of this study show that gabapentin is effective in acute herpetic neuralgia in different doses with 600 mg/day being the more appropriate dose in terms of safety and efficacy.
Keywords: Acute herpetic neuralgia, gabapentin, geriatric patients, herpes zoster, post herpetic neuralgia, visual analogue scale
Introduction

Acute herpetic neuralgia (AHN) is characterized by pain which is more severe in elderly patients.[1] Conventionally, the treatments used for pain alleviation in acute herpetic neuralgia are non-steroidal anti-inflammatory drugs (NSAIDs), oxycodone, morphine, amitryptyline, and nortryptyline having variable efficacy and side effects.[2,3] Gabapentin is used successfully in the treatment of post herpetic neuralgia (PHN) and other painful conditions,[4,5] but its use in pain of acute herpes zoster is yet to be explored. This study was done to compare the efficacy and safety of different doses of gabapentin in geriatric patients with acute herpetic neuralgia.
Materials and Methods
The study was done from September 2007 through February 2011 including geriatric patients of more than 60 years of age with acute herpes zoster presenting within 72 hours of onset, having pain rating 60 or above at baseline on 100 mm Visual Analogue Scale (VAS). Exclusion criteria included the following: patients with acute herpes zoster presenting after 72 hours of onset; pain of herpes zoster rating less than 60 at baseline; prior treatment with gabapentin; history of hypersensitivity to the drug or its ingredients; significant hematological disease; immuno-compromised state; and significant systemic disease or hepatic and renal disease.
Eligible subjects who gave informed written consent underwent physical examination. Blood samples were taken for routine hematology and chemistry. Medical histories and demographics were obtained.
This study was a double-blind, controlled trial approved by ethical committee of the institute. The subjects were controlled for age and sex. They were assigned into four groups to receive gabapentin 300 mg/day (G 300), 600 mg/day (G 600), 900 mg/day (G 900) in divided doses and placebo. At each visit (weeks 0, 1, 2, 3, and 4), subjects were assessed for pain (on 100 mm Linear VAS with “0 mm” as no pain to “100 mm” as worst possible pain) by an independent assessor. Adverse events were recorded on visual rating scale from 0 to 3 as nil (-), mild (+), moderate (++), and severe (+++), respectively. Oral antiviral treatment (acyclovir 800 mg five times/day, for seven days) was given to all the patients of herpes zoster as per the guidelines of treatment of same.
Statistical analysis
For statistical analysis, we applied analysis of variance (ANOVA) test and “t” test by using Epi Info software for the comparison of the safety and efficacy with placebo and different doses of gabapentin.
Results
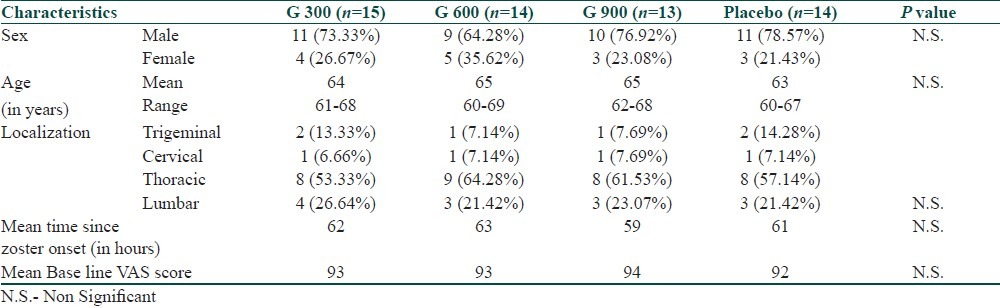
A total of 549 patients visited the dermatology outdoor for herpes zoster. Out of these, 189 subjects were above 60 years of age. Among them, 56 patients were eligible for the study according to inclusion and exclusion criteria [Table 1]. The disease characteristic of the patients showed that the most common area of localization was thoracic followed by lumbar region. The mean time since zoster onset was ranging from 59-63 hours and the mean VAS score was between 92-94 hours at first visit in all the patients.
Table 1.
Demographic and disease characteristic of the patients
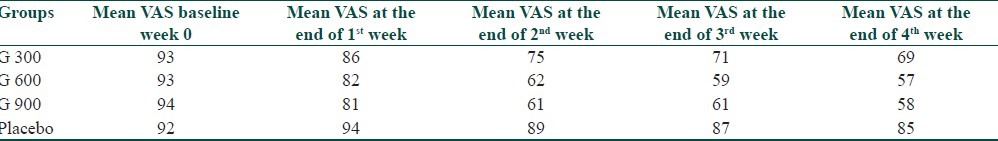
Pain assessment in patients showed highly significant change from baseline VAS score to 4th week of the therapy in gabapentin groups (P<0.001) as compared to placebo. There was a continuous decrease VAS in gabapentin group with its highest effect at the end of second week and then plateau effect was observed at the end of 3rd week onwards [Table 2].
Table 2.
Summary of average (mean) pain score on 100 mm Linear Visual Analogue Scale
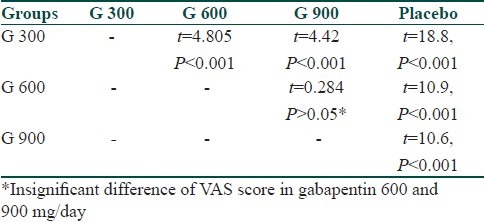
A significant difference in VAS score among different groups was observed after the end of 4th week of treatment (P<0.001) by ANOVA test [Table 3]. Along with ANOVA we also applied Student's “t” test among different groups. At the end of 4th week of treatment we found that there was significant difference in all groups except gabapentin 600mg and 900mg/day which showed insignificant difference in VAS score (t=0.284, P>0.05) [Table 4].
Table 3.
ANOVA comparison of VAS scoring in different groups at 4th week
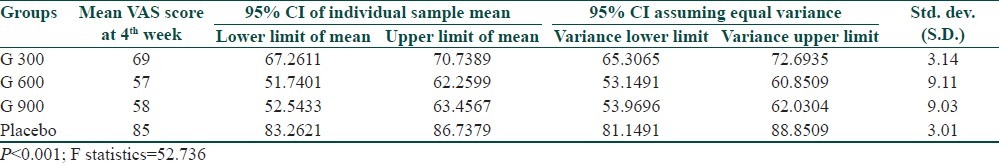
Table 4.
Comparison of VAS in different groups by t test at 4th week
Safety and adverse effects
The therapeutic efficacy of any drug is determined both by clinical efficacy and its side effects. Measures of frequency, nature, and severity of adverse events were derived from a total of 42 subjects receiving gabapentin. Overall, the most frequently reported adverse effects were somnolence (38.5%) and dizziness (23.79%) followed by gastrointestinal side effects (11.24%) among all the patients receiving gabapentin in different doses [Table 5]. However, all these effects were more prominent in 900 mg/day doses and these effects were reduced after 3rd week of therapy [Table 4].
Table 5.
Adverse effects in various doses of gabapentin and placebo

Discussion
AHN is defined by the International Herpes Management Forum (IHMF®) as the pain from onset of prodrome to 30 days; pain between 30 days from prodrome onset to 4 months is subacute herpetic neuralgia; and pain persisting beyond 4 months from onset of the prodrome of herpes zoster is post herpetic neuralgia.[2] The role of gabapentin in reducing the pain of post herpetic neuralgia is well established.[4,6] In most of the earlier studies, gabapentin was started after 30 days to 3 months from the onset of zoster. These studies showed significant role of gabapentin in the chronic pain of PHN. In our study, gabapentin was started as early as possible after the diagnosis of zoster.
The pathophysiology of pain in acute herpetic neuralgia differs from that of PHN. In acute zoster, the skin is inflamed, already partially denervated and the dorsal-root ganglion shows inflammation, hemorrhagic necrosis, and neuronal loss.[7] On the other hand, PHN is due to the continuing inflammation in peripheral nerves for weeks to months leading to demyelination, wallerian degeneration, and sclerosis.[8] After the injury, peripheral neurons discharge spontaneously, have lower activation thresholds, display exaggerated responses to stimuli, and there is an altered central nervous system signal processing. [9] Gabapentin has been reported to relieve pain in patients with intractable neuropathic pain and reflex sympathetic dystrophy allowing the reduction or termination of other analgesic medications and relieving symptoms associated with painful disease manifestations.[10] It also reduces spontaneous pain and tactile allodynia in patients with peripheral or central pain.[5] We utilized the property of gabapentin to act both centrally and peripherally by starting gabapentin within 72 hours of onset of zoster.
This study shows that gabapentin in all doses was significantly more efficacious than placebo (P<0.001). Comparison between different doses showed significantly less efficacy of 300 mg/day as compared to 600 and 900 mg per day (P<0.001) and dosage of 600 mg/day was equally efficacious to 900 mg/day (P>0.05). This effect can be explained on the basis of nonlinear-bioavailability relationship of gabapentin, i.e., at higher doses smaller percentage of drug is bioavailable.[11]
While reviewing the literature, we could find only two studies published in western text assessing the role of gabapentin in acute herpetic neuralgia. Berry and Peterson observed 66% reduction of pain as compared to placebo by using single dose 900 mg of gabapentin.[12] Our study also showed around 26-38% of reduction of pain in different groups as measured by VAS at the end of 4th week. It should be noted that in the former study, the effect was studied for six hours period only, while in our study, the effect was observed for four weeks period showing the long term efficacy of the drug. Another study by Dworkin et al., on gabapentin and CR-oxycodone (controlled release) showed significant reduction in pain with oxycodone in the first two weeks, but for gabapentin, the pain relief was observed only in the first week but not later.[13] In our study, the pain reduction started by the end of first week of therapy was highest at the end of second week and then plateau was observed in the 3rd to 4th week. The continuous reduction of pain by gabapentin in our study may be explained on the basis of starting of gabapentin within 72 hours which may have had a role in reducing the pain provocation potential at receptor level and its anti-nociceptive properties.
The safety profile showed significantly more somnolence, dizziness, and gastro-intestinal side effects in dose of 900 mg/day as compared with 300 and 600 mg/day [Table 5]. This highlights that the dose of 600 mg/day is therapeutically more effective and a safe dose. The most common side effect was somnolence, but it was not a source of stress in our patients, rather nocturnal somnolence was perceived as mark of pain relief.
Geriatric patients usually are on multiple drugs for different medical and surgical conditions. A drug which has less drug interaction potential is therefore needed. Gabapentin, in all doses undergoes minimal metabolism, has minimal protein binding and is excreted unchanged by kidneys which accounts for its less drug interactions.[12] As geriatric patients are at a higher risk to develop post herpetic neuralgia, gabapentin can be continued as single or combined therapy in cases of PHN.
The results of this study show that early initiation of gabapentin can be safe and effective alternative to analgesics and tricyclics in geriatric patients to reduce and prevent the pain in herpes zoster. Further trials can be done in future enrolling more number of patients with different combinations and different age groups to quantify the effect.

Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Rogers RS, 3rd, Tindall JP. Geriatric herpes zoster. J Am Geriatr Soc. 1971;19:495–504. doi: 10.1111/j.1532-5415.1971.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 2.Wood M. How can the burden of zoster-associated pain be reduced? Recommendations from the IHMF Workshop, Washington, D.C. Worthing United Kingdom: PPS Europe; 1993. May 15, Understanding zoster-associated pain; pp. 3–11. [Google Scholar]
- 3.Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 4.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: A randomized controlled trial. JAMA. 1998;280:1837–42. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 5.Rice AS, Maton S. Gabapentin in postherpetic neuralgia: A randomised, double blind, placebo controlled study. Pain. 2001;94:215–24. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 6.Muller SA, Winkelmann RK. Cutaneous nerve changes in zoster. J Invest Dermatol. 1969;52:71–7. doi: 10.1038/jid.1969.10. [DOI] [PubMed] [Google Scholar]
- 7.Watson CP, Deck JH, Morshead C, Van der Kooy D, Evans RJ. Postherpetic neuralgia: Further post-mortem studies of cases with and without pain. Pain. 1991;44:105–17. doi: 10.1016/0304-3959(91)90124-G. [DOI] [PubMed] [Google Scholar]
- 8.Wall PD. Neuropathic pain and injured nerve: central mechanisms. Br Med Bull. 1991;47:631–43. doi: 10.1093/oxfordjournals.bmb.a072497. [DOI] [PubMed] [Google Scholar]
- 9.Rosner H, Rubin L, Kestenbaum A. Gabapentin adjunctive therapy in neuropathic pain states. Clin J Pain. 1996;12:56–8. doi: 10.1097/00002508-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Attal N, Parker F, Brasseur L, Chauvin M, Bouhassira D. Program and abstracts of the 16th Annual Scientific Meeting of the American Pain Society. New Orleans, La: 1997. Oct 23-26, Efficacy of gabapentin on neuropathic pain: A pilot study. Abstract 653. [Google Scholar]
- 11.Goa KL, Sorkin EM. Gabapentin: A review of its pharmacological properties and clinical potential in epilepsy. Drugs. 1993;46:409–27. doi: 10.2165/00003495-199346030-00007. [DOI] [PubMed] [Google Scholar]
- 12.Berry JD, Petersen KL. A single dose of gabapentin reduces acute pain and allodynia in patients with herpes zoster. Neurology. 2005;65:444–7. doi: 10.1212/01.wnl.0000168259.94991.8a. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin RH, Barbano RL, Tyring SK, Betts RF, McDermott MP, Pennella-Vaughan J, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain. 2009;142:209–17. doi: 10.1016/j.pain.2008.12.022. [DOI] [PubMed] [Google Scholar]


