Abstract
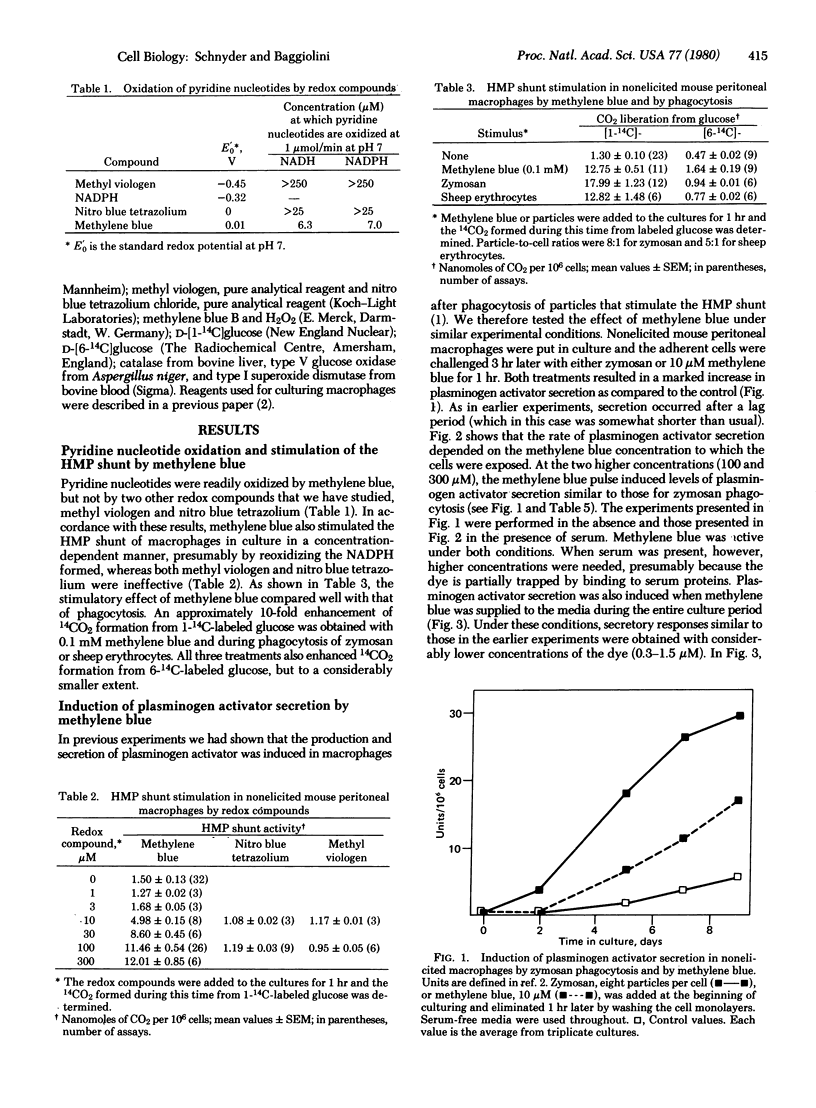
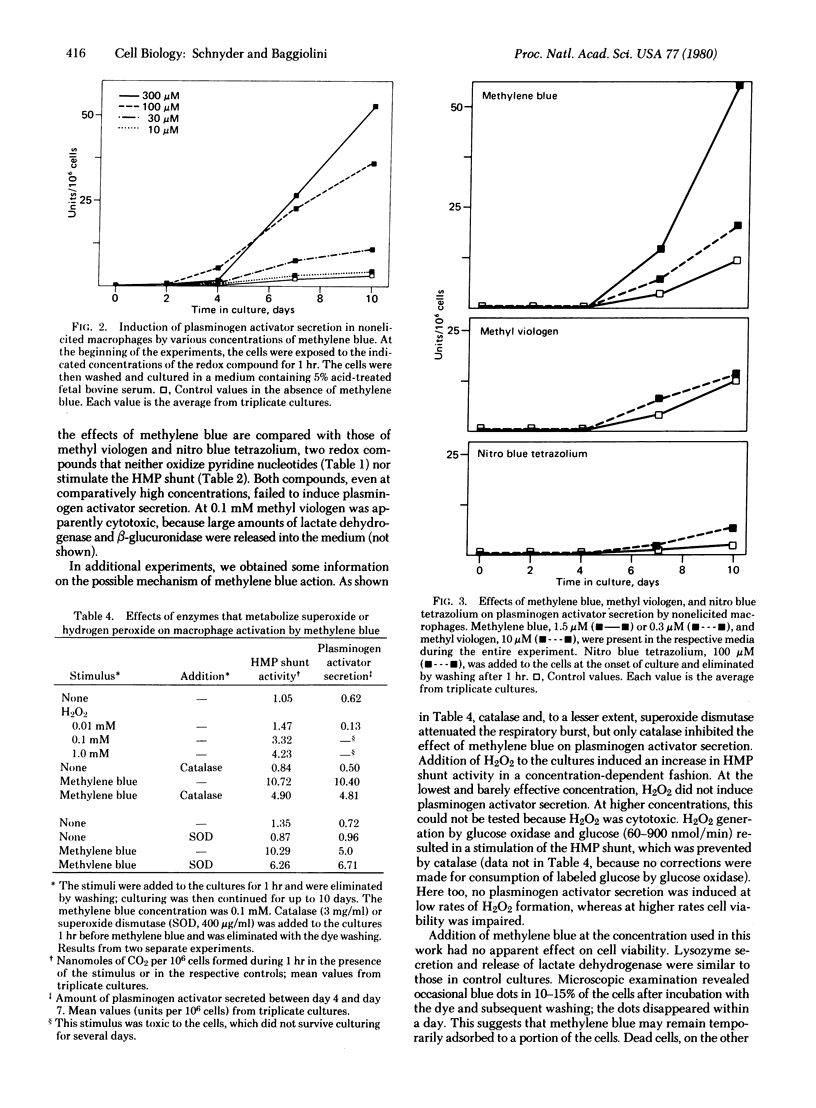
Resident peritoneal macrophages were obtained from untreated mice and were cultured in medium 199 with or without 5% acid-treated fetal bovine serum. Three hours after harvesting, redox compounds--i.e., methylene blue, methyl viologen, or nitro blue tetrazolium--were added to the cultures of adherent cells. After 1 hr, the cells were washed and culturing was continued in the absence of redox compounds. The effects of the redox compounds were tested by assaying for hexose monophosphate (HMP) shunt activity and for plasminogen activator secretion, and the results were compared with the effects induced by phagocytic stimuli. Methylene blue caused a concentration-dependent stimulation of the HMP shunt, whereas methyl viologen and nitro blue tetrazolium were ineffective. Shunt stimulation by methylene blue was followed, after a lag of 2-4 days, by plasminogen activator secretion. The rate of secretion was dependent on the methylene blue concentration used. Methyl viologen and nitro blue tetrazolium were again ineffective, whereas phagocytosis of zymosan or sheep erythrocytes, which stimulates the HMP shunt, induced plasminogen activator secretion at rates similar to those induced by methylene blue. These results add further evidence to our hypothesis that the HMP shunt-dependent metabolic burst is involved in macrophage activation. Because methylene blue mimics the action of zymosan it appears that shunt stimulation by itself initiates the activation process independently of phagocytosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Gordon S., Unkeless J. C., Cohn Z. A. Induction of macrophage plasminogen activator by endotoxin stimulation and phagocytosis: evidence for a two-stage process. J Exp Med. 1974 Oct 1;140(4):995–1010. doi: 10.1084/jem.140.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimetzek V., Sorg C. Lymphokine-induced secretion of plasminogen activator by murine macrophages. Eur J Immunol. 1977 Mar;7(3):185–187. doi: 10.1002/eji.1830070314. [DOI] [PubMed] [Google Scholar]
- Lazdins J. K., Kühner A. L., David J. R., Karnovsky M. L. Alteration of some functional and metabolic characteristics of resident mouse peritoneal macrophages by lymphocyte mediators. J Exp Med. 1978 Sep 1;148(3):746–758. doi: 10.1084/jem.148.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M. Attachment of modified erythrocytes to phagocytic cells in absence of serum. Proc Soc Exp Biol Med. 1967 Feb;124(2):396–399. doi: 10.3181/00379727-124-31749. [DOI] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Rossi F. Reversible metabolic stimulation of polymorphonuclear leukocytes and macrophages by concanavalin A. Nat New Biol. 1973 May 23;243(125):111–112. [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Role of phagocytosis in the activation of macrophages. J Exp Med. 1978 Dec 1;148(6):1449–1457. doi: 10.1084/jem.148.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J Exp Med. 1978 Aug 1;148(2):435–450. doi: 10.1084/jem.148.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F., Zilversmit D. B. Release from alveolar macrophages of an inhibitor of phagocytosis. Am J Physiol. 1970 Apr;218(4):1118–1127. doi: 10.1152/ajplegacy.1970.218.4.1118. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: induction by concanavalin A and phorbol myristate acetate. Cell. 1977 Jul;11(3):695–705. doi: 10.1016/0092-8674(77)90086-1. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Reich E. Macrophage plasminogen activator: induction by products of activated lymphoid cells. J Exp Med. 1977 Feb 1;145(2):429–437. doi: 10.1084/jem.145.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Dukor P., Zurier R. B. Effect of cyclic AMP on release of lysosomal enzymes from phagocytes. Nat New Biol. 1971 Jun 2;231(22):131–135. doi: 10.1038/newbio231131a0. [DOI] [PubMed] [Google Scholar]



