Abstract
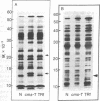
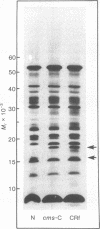
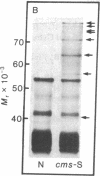
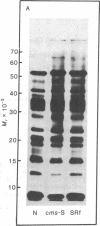
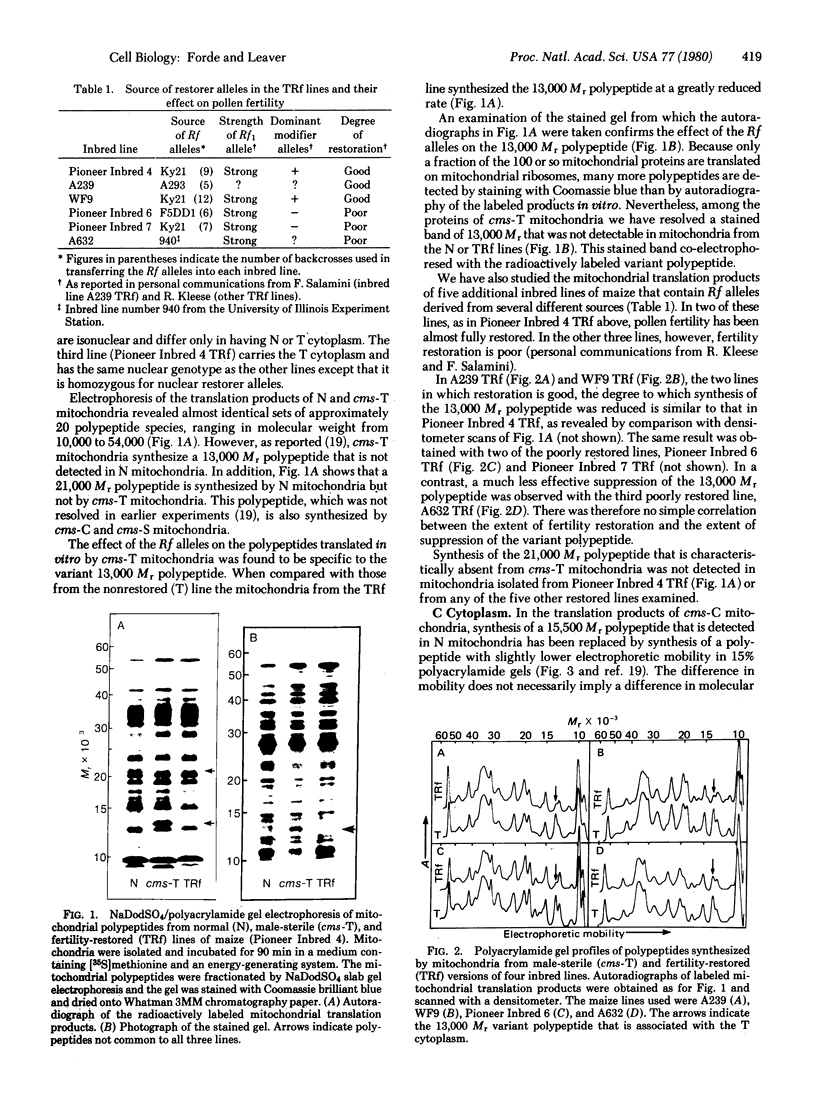
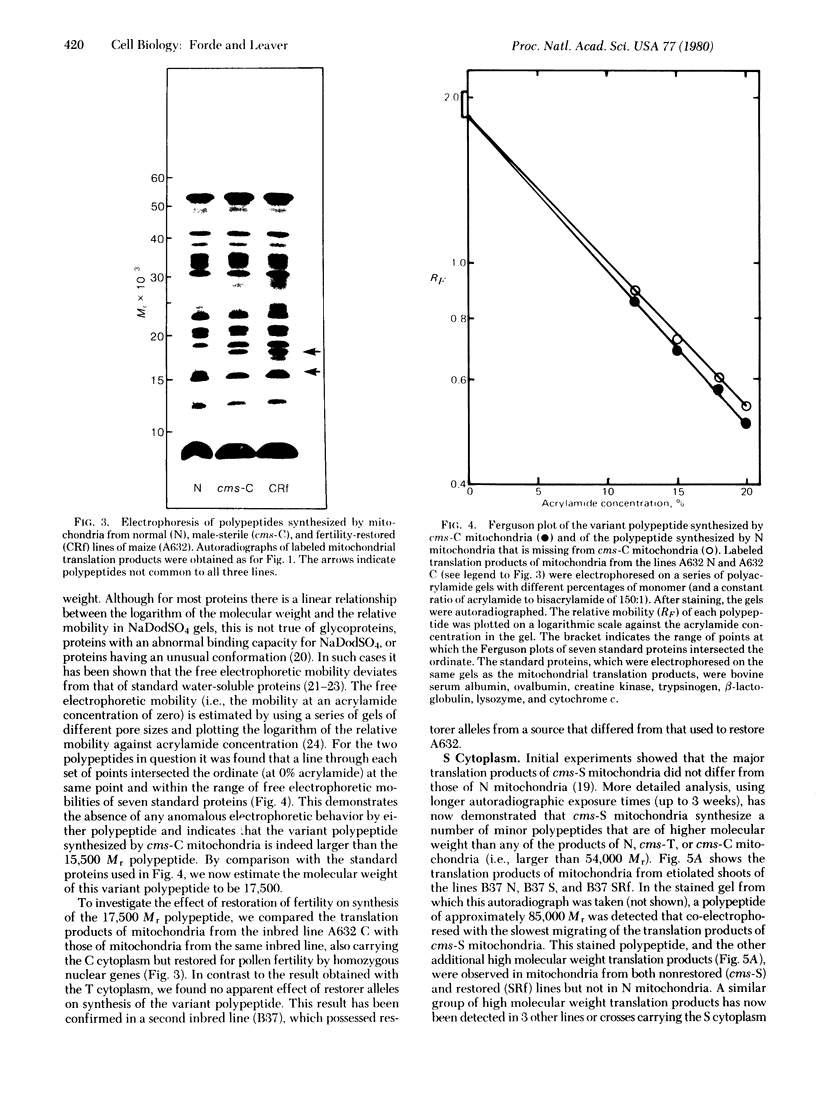
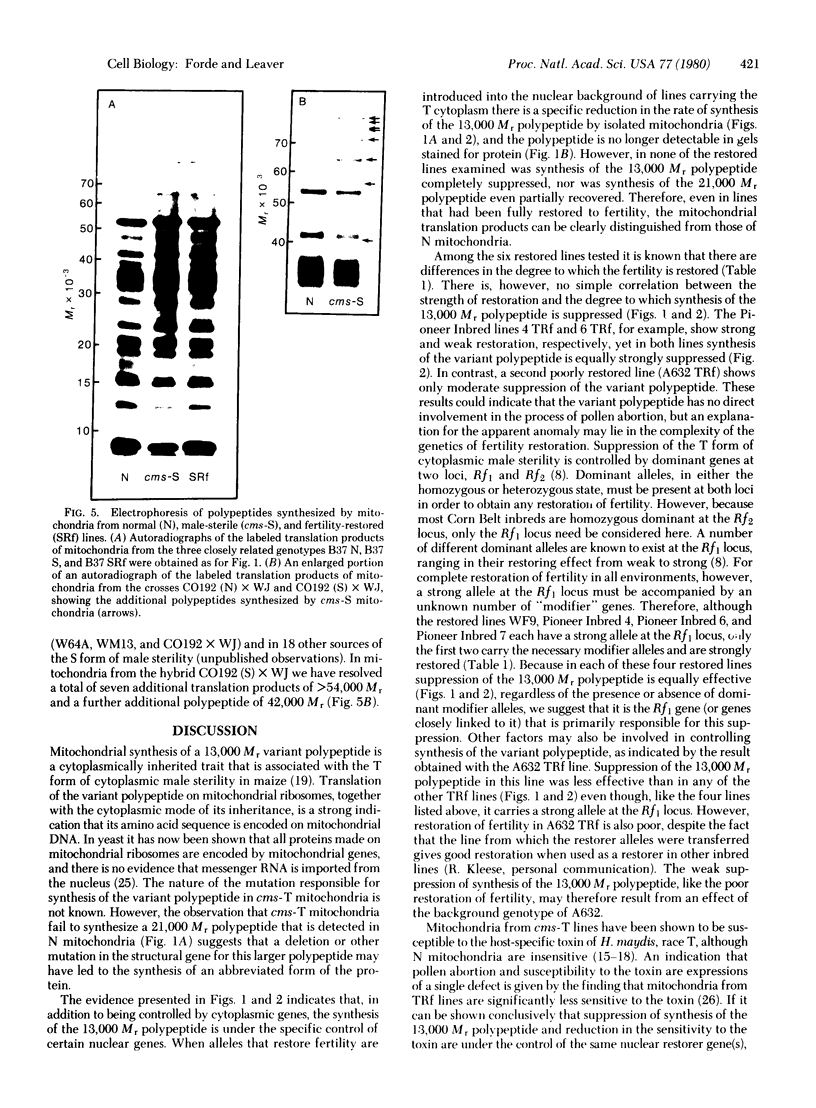
The polypeptides synthesized in vitro by mitochondria isolated from etiolated maize shoots of a number of different nuclear and cytoplasmic genotypes have been compared by using polyacrylamide gel electrophoresis. We have previously shown that mitochondria from maize plants carrying the T or C forms of cytoplasmically inherited male sterility (cms-T and cms-C mitochondria) can be distinguished from each other and from the mitochondria of normal (N) plants by the synthesis of a single additional or variant polypeptide species. Using lines that carry the T cytoplasm, and that differ principally in the presence or absence of nuclear “restorer” alleles that suppress the male-sterile phenotype, we find that these nuclear genes specifically suppress synthesis of the 13,000 Mr variant polypeptide. A 21,000 Mr polypeptide that is synthesized by N mitochondria is not detectable among the translation products of cms-T mitochondria from either restored or nonrestored lines. Results obtained with a number of lines possessing dominant restorer alleles from different sources indicate that it is the restorer gene at the Rf1 locus that is primarily responsible for regulating synthesis of the 13,000 Mr polypeptide. Mitochondria from lines with the S form of cytoplasmic male sterility (cms-S) were found to synthesize a group of minor polypeptides, ranging in molecular weight from 42,000 to 88,000, which were not detected in N, cms-T, or cms-C mitochondria. In the case of the S and C forms of male sterility no differences were found between the translation products of mitochondria from restored and nonrestored lines.
Keywords: cytoplasmic male sterility, restoration of fertility, nuclear-mitochondrial interaction
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand H., Collins R. A. A regulatory system controlling the production of cytochrome aa3 in Neurospora crassa. Mol Gen Genet. 1978 Oct 25;166(1):1–13. doi: 10.1007/BF00379723. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Colson A. M., Goffeau A., Briquet M., Weigel P., Mattoon J. R. Nucleo-cytoplasmic interaction between oligomycin-resistant mutations in Saccharomyces cerevisiae. Mol Gen Genet. 1974;135(4):309–326. doi: 10.1007/BF00271146. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Forde B. G., Oliver R. J., Leaver C. J. Variation in mitochondrial translation products associated with male-sterile cytoplasms in maize. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3841–3845. doi: 10.1073/pnas.75.8.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Groot G. S., van Harten-Loosbroek N., Kreike J. Electrophoretic behaviour of yeast mitochondrial translation products. Biochim Biophys Acta. 1978 Feb 16;517(2):457–463. doi: 10.1016/0005-2787(78)90212-5. [DOI] [PubMed] [Google Scholar]
- Guerineau M. Expression of a yeast episome: RNA-DNA hybridization studies. FEBS Lett. 1977 Aug 15;80(2):426–428. doi: 10.1016/0014-5793(77)80491-2. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Kustermann-Kuhn B., Royer H. D. Synthesis of high molecular weight polypeptides in Escherichia coli minicells directed by cloned Saccharomyces cerevisiae 2-micron DNA. Gene. 1976;1(1):33–47. doi: 10.1016/0378-1119(76)90005-6. [DOI] [PubMed] [Google Scholar]
- JONES D. F. The interrelation of plasmagenes and chromogenes in pollen production in maize. Genetics. 1950 Sep;35(5-1):507–512. [PMC free article] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Pring D. R. Restriction endonuclease analysis of mitochondrial DNA from normal and Texas cytoplasmic male-sterile maize. Science. 1976 Jul 9;193(4248):158–160. doi: 10.1126/science.193.4248.158. [DOI] [PubMed] [Google Scholar]
- MITCHELL M. B., MITCHELL H. K. A nuclear gene suppressor of a cytoplasmically inherited character in Neurospora crassa. J Gen Microbiol. 1956 Feb;14(1):84–89. doi: 10.1099/00221287-14-1-84. [DOI] [PubMed] [Google Scholar]
- Miller R. J., Koeppe D. E. Southern corn leaf blight: susceptible and resistant mitochondria. Science. 1971 Jul 2;173(3991):67–69. doi: 10.1126/science.173.3991.67. [DOI] [PubMed] [Google Scholar]
- Ono B. I., Fink G., Schatz G. Mitochondrial assembly in respiration-deficient mutants of Saccharomyces cerevisiae. IV. Effects of nuclear amber suppressors on the accumulation of a mitochondrially made subunit of cytochrome c oxidase. J Biol Chem. 1975 Jan 25;250(2):775–782. [PubMed] [Google Scholar]
- Poyton R. O., Kavanagh J. Regulation of mitochondrial protein synthesis by cytoplasmic proteins. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3947–3951. doi: 10.1073/pnas.73.11.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. III. Physical characterization of isolated subunits and chemical evidence for two different classes of polypeptides. J Biol Chem. 1975 Jan 25;250(2):752–761. [PubMed] [Google Scholar]
- Pring D. R., Levings C. S. Heterogeneity of Maize Cytoplasmic Genomes among Male-Sterile Cytoplasms. Genetics. 1978 May;89(1):121–136. doi: 10.1093/genetics/89.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S., Hu W. W., Timothy D. H. Unique DNA associated with mitochondria in the "S"-type cytoplasm of male-sterile maize. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2904–2908. doi: 10.1073/pnas.74.7.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M. M. CYTOPLASMIC INHERITANCE OF MALE STERILITY IN ZEA MAYS. Science. 1931 Mar 27;73(1891):340–341. doi: 10.1126/science.73.1891.340. [DOI] [PubMed] [Google Scholar]
- Sainsard A. Mitochondrial suppressor of a nuclear gene in Paramecium. Nature. 1975 Sep 25;257(5524):312–314. doi: 10.1038/257312a0. [DOI] [PubMed] [Google Scholar]
- Trembath M. K., Monk B. C., Kellerman G. M., Linnane A. W. Biogenesis of mitochondria 40. Phenotypic suppression of a mitochondrial mutation by a nuclear gene in Saccharomyces cerevisiae. Mol Gen Genet. 1975 Oct 22;140(4):333–337. [PubMed] [Google Scholar]