Abstract
The juxtaglomerular (JG) cell product renin is rate-limiting in the generation of the bioactive octapeptide angiotensin II. Rates of synthesis and secretion of the aspartyl protease renin by JG cells are controlled by multiple afferent and efferent pathways originating in the CNS, cardiovascular system and kidneys and making critical contributions to the maintainance of extracellular fluid volume and arterial blood pressure. Since both excesses and deficits of angiotensin II have deleterious effects it is not surprising that control of renin is secured by a complex system of feedforward and feedback relationships. Mice with genetic alterations have contributed to a better understanding of the networks controlling renin synthesis and secretion. Essential input for the setting of basal renin generation rates is provided by β-adrenergic receptors acting through cAMP, the primary intracellular activation mechanism for renin mRNA generation. Other major control mechanisms include COX-2 and nNOS affecting renin through PGE2, PGI2, and nitric oxide. Angiotensin II provides strong negative feedback inhibition of renin synthesis, largely an indirect effect mediated by baroreceptor and macula densa inputs. Adenosine appears to be a dominant factor in the inhibitory arms of the baroreceptor and macula densa mechanisms. Targeted gene mutations have also shed light on a number of novel aspects related to renin processing and the regulation of renin synthesis and secretion.
Introduction
The antihypertensive, antifibrotic, and antiproliferative effects of systemic administration of inhibitors of renin, angiotensin II receptors, or angiotensin II converting enzyme underscore the important contribution of the renin-angiotensin system (RAS) to the progression of cardiovascular and renal diseases. The formation of angiotensin II in the circulation is primarily regulated by changes in the activity of the aspartyl protease renin, which is largely a product of the juxtaglomerular granular (JG) cells at the glomerular vascular pole of the kidney. Thus, the rate of renin synthesis in JG cells and the rate of renin secretion are the main determinants of circulating angiotensin II. Under steady-state conditions synthesis and secretion of renin tend to change in parallel since transcriptional and posttranscriptional regulation of renin mRNA and secretion of renin from storage granules are partly under the control of the same intracellular mechanisms.
Commensurate with the central role of angiotensin II in volume homeostasis, synthesis and secretion of renin by JG cells is regulated by multiple inputs that in general reflect the status of extracellular fluid volume. The most important among the macroscopic control mechanisms of renin release in vivo are blood pressure (“the renal baroreceptor mechanism”), renal sympathetic tone through β-adrenergic receptors, and tubular NaCl concentration in the macula densa segment of the nephron (“the macula densa mechanism”). A number of pharmacological, ablative, and cellular strategies have been employed to establish the distinct nature and contribution of individual regulatory pathways in controlling renin secretion. However, the cellular mechanisms underlying control of renin secretion by physiological regulatory systems in the intact animal have been difficult to identify because of complex interactions between feedforward and feedback regulation. Mice with defined gene manipulations offer a new venue to further explore the mechanisms responsible for control of renin synthesis and secretion in the intact organism. The approach in the majority of studies has been to draw conclusions from phenotypic changes caused by defined alterations of the genotype. In this review we will focus mostly on those studies of renin secretion in which interference with recognized signaling pathways has occurred at the level of receptors, ligands, ligand synthesis, and membrane transduction mechanisms, all interventions that could potentially shed light on the acute regulation of renin release. A graphical preview of the selected topics is given in Fig. 1.
Fig 1.
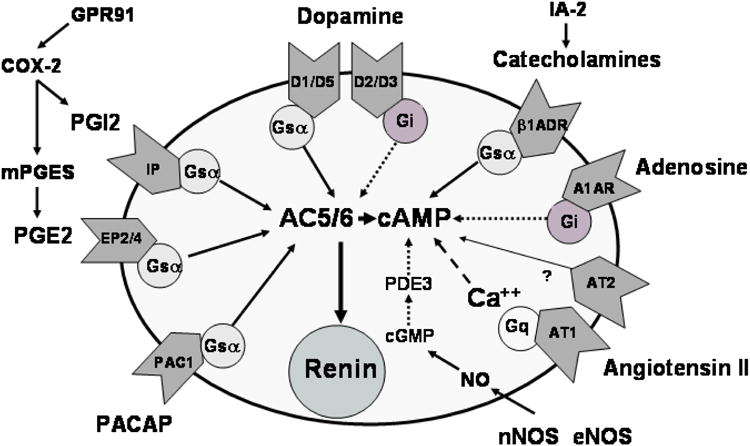
Schematic overview of the majority of genetic targets discussed in this review and their effects on renin release. Oval indicates a representative JG cell and effects on renin release are indicated by arrows with solid arrows delineating direct effects (or stimulatory effects with positive deflections) and broken arrows indicating inverse effects (or inhibitory effects with positive deflections). Pathways with an even number of broken arrows (zero or two) are stimulatory and pathways with an uneven number of broken arrows are inhibitory.
It has become apparent that the generation of angiotensin II in the circulation is only part of the functionality of the RAS. Evidence is mounting that angiotensin II can be generated in a tissue-specific fashion and exert local effects, for example in the brain, heart, or kidney, but a discussion of these local effects will not be attempted here 1, 2. Furthermore, in this short review we cannot address the important progress that has been made in defining the sequences in the renin DNA and their binding complexes that promote, enhance, or silence renin gene transcription, for example by the activation of nuclear hormone receptors such as the vitamin D3 receptor or PPAR 3-5.
Angiotensin II
Mice with deletions in the non-renin components of the renin-angiotensin system have confirmed that the expression and secretion of renin is under strong inhibitory control of angiotensin II. Mice with null mutations in the genes for angiotensinogen (AGT), angiotensin converting enzyme (ACE), and angiotensin II receptors show consistent and massive upregulation of renin expression and plasma renin concentrations 6-11. The overexpression of renin in these conditions is typically associated with upstream recruitment and ectopic location of renin-expressing cells 7, 11-13. Disruption of AT1a signaling contributes most to these abnormalities; intrarenal renin expression was found to be essentially normal in AT1b or AT2-deficient mice 13, 14. In fact, plasma angiotensin II levels were lower in AT1a/AT1b/AT2 triple knockout mice than in mice with AT1 receptor deficiency suggesting that AT2 receptors may have effects that are directionally opposite to those elicited by AT1 receptors 15.
It has been a widely accepted notion that the effect of angiotensin II on renin expression is the reflection of a direct interaction of the peptide with JG cells. Several findings from genetically modified mice call this concept into question. Cross-transplantation of kidneys between AT1a-deficient and wild type mice results in a significant increase in renin expression only when AT1a receptors are missing in the entire organism. Selective absence of AT1a receptors in the kidneys did not cause detectable elevations of renal renin mRNA, suggesting that upregulation of renin expression is not the direct consequence of angiotensin withdrawal from AT1 receptors in JG cells 16. That the stimulation of renin expression by withdrawal of angiotensin II effects is not primarily mediated by AT1 receptors on JG cells is supported by earlier findings in chimeric mice carrying both AT1a-/- and AT1a+/+ cells. In these mice increases in renin expression and JG cell hyperplasia were seen independent of whether individual JGAs did or did not express AT1 receptors 17. In both studies increased renin expression correlated with the reduced arterial blood pressure suggesting that at least part of the “feedback” effect of angiotensin II is baroreceptor-mediated. Increased arterial blood pressure rather than any direct effects of angiotensin II may also be responsible for the inhibition of renin expression in AGT-deficient mice on high salt intake 18-20.
G protein Gsα and cAMP
Substantial early experimental evidence has supported an important role for Gsα in activating adenylate cyclase (AC) and stimulating renin synthesis and secretion through its catalytic product cAMP 21. The absolutely critical contribution of the Gsα-cAMP-PKA signaling cascade in both basal and regulated renin secretion has been affirmed in mice with selective deletion of Gsα in JG cells 22. Cell-specific Gsα deletion was achieved by crossing mice that express Cre recombinase under control of the endogenous renin promoter with animals in which exon 1 of the GNAS gene is flanked by loxP sites 12, 23. In mice homozygous for the loxP-flanked GNAS gene and heterozygous for the modified renin locus, native Gsα was found to be significantly reduced in the whole kidney and almost completely abolished in single JG cells at the DNA, mRNA and protein level 22. This genetic deletion of Gsα in JG cells was associated with a marked reduction of renin expression and renin secretion under basal conditions. Renal cortical renin mRNA was reduced to 23% of control and renin content of isolated JG cells to about 10% of control indicating a greatly reduced rate of renin production. Furthermore, concentrations of renin in plasma and in the medium of isolated JG cells were reduced to 10% and 5% of control respectively indicating a critical role of Gsα signaling in renin secretion 22. Thus, Gsα-dependent signal processing is needed in a non-redundant fashion for the maintenance of high basal levels of renin expression and renin release. Furthermore, the stimulation of renin secretion caused acutely by furosemide, hydralazine, or isoproterenol was virtually abolished in the Gsα-deficient mice. To the extent that furosemide stimulates renin secretion through the macula densa and hydralazine through the baroreceptor, these data suggest that Gsα is required for the major physiological inputs that regulate renin secretion in vivo. Suppression of renin expression in the absence of Gsα signaling is already apparent prenatally; the appearance of renin in distal arcuate arteries, the earliest site for renin expression in the metanephric kidney, was missing in mice with JG cell specific deletion of Gsα 24.
A somewhat unexpected observation has been that the strong stimulation of renin synthesis and secretion caused by either converting enzyme or angiotensin receptors blockers was completely abrogated in mice with JG cell-specific deletion of Gsα 25. This finding sheds additional light on the phenomenon often described as the “short loop feedback” inhibition of renin expression. While there is substantial support for the concept that angiotensin II has a direct inhibitory effect on JG cells, mediated presumably by an increase in cytosolic calcium, there is no direct support for the assumption that the stimulatory pathway is simply the reverse, i.e. caused by a direct effect of angiotensin II on the JG cells to decrease cellular calcium 26. The requirement for Gsα for the stimulation of renin release by angiotensin II withdrawal indicates a role for activation of Gsα-dependent pathways. Co-administration of propranolol, indomethacin, and L-NAME mimicked the effect of Gsα deficiency 25, indicating that that angiotensin II withdrawal produces Gsα activation through stimulation of catecholamines, prostaglandins, and nitric oxide, and that these agents are the actual ligands that effect renin stimulation.
Cyclooxygenase and Prostaglandins
Studies of the functional connection between renin and the prostanoid system are founded on the finding that arachidonic acid increases and indomethacin reduces plasma renin levels 27. Numerous subsequent studies have identified various metabolites of arachidonic acid as potential regulators of renin secretion 28 with prostaglandins of the E and I series being the most consistent stimulators of renin release 29.
The effects of PGE2 are elicited by the activation of four types of G protein coupled receptors (EP1 to EP4). Studies in mice with deletion of single PGE2 receptors have identified the Gsα-coupled EP4 as the most important receptor for stimulation of renin. Basal plasma renin concentration was significantly lower in EP4-deficient than WT mice while there was no difference in EP1-, EP2-, or EP3-deficient mice 30. Renin release in response to a high PGE2 concentration was significantly reduced in isolated perfused kidneys of EP4-/- and EP2-/- mice while the renin-stimulating threshold concentration was increased in EP4-/- mice suggesting that PGE2 acts through EP4 and EP2 to increase renin release 30. Stimulation of renin release by chronic administration of furosemide was significantly reduced in EP4-/- mice together with a reduced stimulation of renin expression 31 although the acute administration of bumetanide in isolated kidneys did not show a difference in the renin release response between EP4-/- mice and all other strains 30. The effect of PGE2 on renin release is probably a direct effect since isolated JG cells have been shown to express EP4 and EP2 mRNA and to respond to PGE2 with increased renin secretion 29, 32. In view of the strong evidence involving PGE2 in renin secretion, it is surprising that mice deficient in microsomal PGE2 synthase did not show a significant reduction in basal or furosemide-stimulated renin mRNA, suggesting that alternative pathways may exist for the synthesis of PGE2 33.
Studies in prostacyclin receptor (IP)-deficient mice have confirmed that prostacyclin is another prostaglandin with renin-stimulatory properties. Besides having a reduced response to the stimulatory effect of furosemide, IP-deficient mice have a markedly reduced response of renin expression and release in response to renal arterial constriction or salt depletion whereas mice with deficiencies in the four PGE2 receptors showed normal responsiveness 31, 34. Somewhat unexpectedly, mice deficient in the FP receptor the prostanoid receptor that mediates the effects of PGF2α, also had reduced levels of plasma renin and angiotensin II concentrations 35. At the same time the number of renin-expressing cells appeared to be reduced, but exactly how PGF2α may stimulate renin release or increase the number of JG cells is unknown.
Mice with deletion in PGHS-2 (COX-2) have aided in solidifying the notion that the formation of PGE2 is a critical regulatory input to the formation and release of renin by JG cells. The presence of COX-2 in macula densa and TAL cells had suggested that this pathway may be of particular importance for macula densa control of renin 36. Evidence in support of this notion was provided by studies in COX-2 deficient mice showing that renin enzyme activity, plasma renin and renal renin mRNA and protein expression were markedly reduced 37, 38. 37, 39. In contrast, plasma renin concentration and renal renin expression were not different from control in COX-1 deficient mice 40. It is of note that the stimulatory effect of a chronic low salt diet on renin expression was virtually absent in COX-2-/- mice 38. The magnitude of the renin release response to acute stimulation by furosemide, hydralazine, or isoproterenol were markedly diminished in the absence of COX-2 37, as it is in most states of chronically suppressed renin.
It is noteworthy that chronic inhibition of COX-2 enzyme activity by inhibitors such as rofecoxib does not always produce the same inhibition of renin expression that is seen with genetic COX-2 deletion 41, 42. A possible explanation for this dissociation may lie in the observation that pharmacological COX-2 inhibition is associated with a marked upregulation of renal COX-2 expression so that increased COX-2 enzyme levels may partly compensate for enzyme blockade 41. Other evidence for unidentified modifying factors in this pathway is the observation that the reduction in plasma renin concentration was more pronounced in mice with the COX-2 null mutation bred into congenic C57Bl/6, BALB/c, or 129 J backgrounds compared to that seen in a mixed genetic background 37, 39, 43.
Studies in COX-2 deficient mice have also provided insights about another important interaction in the relationships between prostaglandins and renin, the feedback inhibition of COX-2 expression by angiotensin II (Fig. 2). One critical experimental observation is that inhibition or genetic deletion of COX-2 attenuates the stimulatory effect of angiotensin II blockade on renin expression 37, 43. No difference in the stimulatory effect of captopril was observed between wild type and COX-1 deficient mice 40. Thus, a reduction in angiotensin II signaling, probably at the level of the MD and/or TAL cells, appears to lessen tonic feedback inhibition of COX-2 and thereby enhance renin expression. This is strongly supported by the observation that COX-2 expression is markedly upregulated in angiotensin-II-blocked or -deficient mice 37, 43-47. A fivefold upregulation of COX-2 and of urinary PGE2 excretion has also been observed in the low renin model of JG cell-specific deletion of Gsα 22. Interestingly, whereas AT1a receptors on macula densa cells appear to mediate inhibition of COX-2 expression, AT2 receptors may cause stimulation of COX-2 expression. This is based in part on the observation that the AT1 receptor blocker candesartan elevated COX-2 expression to a lesser extent in AT2 knockouts than in wild type mice 48. Furthermore, the stimulatory effect of candesartan in wild type mice was lessened by the AT2 receptor blocker PD123319. Thus, although under most physiological conditions COX-2 and renin expression change in parallel, during primary changes of renin expression angiotensin II feedback inhibition of COX-2 can dissociate the expression of COX-2 and renin.
Fig. 2.
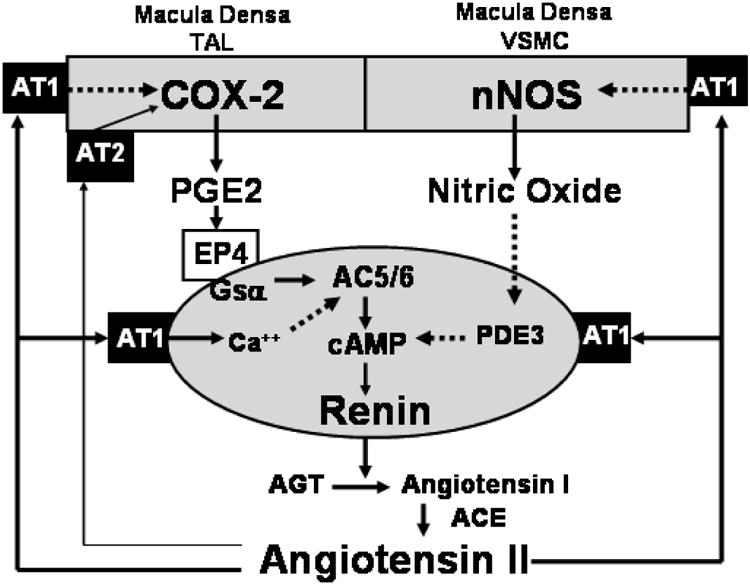
Angiotensin II feedback inhibition of renin synthesis and secretion is mediated by AT1 receptors on both JG cells and on macula densa cells. Activation of JG cell receptors directly inhibits renin whereas activation of macula densa cell receptors (perhaps also of TAL and VSMC receptors) inhibits renin indirectly through downregulation of COX-2 and nNOS. Feedback inhibition by angiotensin II is not necessarily symmetrical: reductions of angiotensin II levels rely more exclusively on the indirect pathways than increases of angiotensin II.
Solid arrows denote direct relationships or positive effects whereas broken arrows denote inverse relationships or negative effects.
TAL: thick ascending limb of Henle's loop; VSMC: vascular smooth muscle cells; PDE3: phosphodiesterase 3; AC5/6: adenylyl cyclase 5 or 6.
Nitric Oxide
The notion that NO may contribute to the regulation of renin secretion has seemed plausible because of the high levels of expression of NOS in cells of the macula densa and vascular endothelium, two cell types in close anatomical association with JG cells. Nevertheless, conclusions from studies with NO synthase blocking agents have been contradictory. Perhaps the most convincing evidence for an important role of NOS in renin secretion comes from studies in isolated perfused kidneys from either nNOS or eNOS deficient mice, in which renin secretion is markedly reduced (to 10% or 30% respectively) 49. Since perfusion pressure is servo-controlled in this setting, indirect mediation of the effect of NOS deficiency on renin by baroreceptor activation is unlikely. In intact nNOS-deficient mice plasma renin concentration is reduced, supporting tonic stimulation of renin secretion by NO 39, 49. Furthermore, the stimulation of renin synthesis and secretion caused by reduced Na intake was absent in mice without nNOS 50. In contrast to plasma renin, renal renin mRNA expression and renal renin content were found to be unchanged in nNOS knockout compared to wild type mice 51. The strength of the evidence from these studies is somewhat marred by the fact that the recombination strategy used in the available nNOS knockout mice permits the translation of an enzymatically active NOS1 isoform called NOS1β 52. This isoform has recently been detected in macula densa cells so that residual NOS activity in the vicinity of JG cells cannot be excluded in the “knockout” animals 53.
Evidence for a role of NO generated by eNOS in basal renin release is less convincing. Plasma renin concentration of eNOS-deficient mice was not different from wild types in two studies 49, 54 and paradoxically increased in another 55. An increase in plasma renin concentration accompanied by an increase in plasma aldosterone was also observed in triple knockout mice devoid of all 3 NOS isoforms 56. Since these mice show signs of cardiac and renal failure leading to a markedly reduced life span it is likely that a reduction of renal perfusion overcomes any inhibitory effects of NOS deficiency on renin release. The observations in the triple knockouts notwithstanding, renal renin expression and plasma renin concentration were found to be significantly reduced in nNOS/eNOS double knockout mice generated in our laboratory (unpublished). Furthermore, whereas NOS inhibition with L-NAME reduced plasma renin in WT, eNOS-/- and nNOS-/-, it had no effect on plasma renin in the double knockouts suggesting that iNOS does not play a major role under normal conditions and that both eNOS and nNOS facilitate renin release.
Expression of nNOS in macula densa cells was observed to be significantly increased in mice with gene deletions of the AT1a receptor, of both AT1a and AT1b receptors, and of angiotensinogen, suggesting that angiotensin II exerts an inhibitory influence on nNOS expression 47, 57, 58. Upregulation of nNOS in AT1a/AT1b knockout mice was noted to extend to the preglomerular vasculature 59. The implication of these observations is that angiotensin II inhibits nNOS expression in macula densa and vascular smooth muscle cells just as it inhibits COX-2 expression in the macula densa (Fig. 1).
Catecholamines
In metanephric kidneys of mice with deletion of β1 and β2 adrenergic receptors the early expression of renin in large renal vessels is largely absent, but renin-positive cells later appear in the typical JG arteriolar location 60. This observation implies that the early expression of renin in large renal vessels appears to be under sympathetic control, and that the subsequent establishment of the adult-type JG location does not require an earlier vascular expression.
Adult β1/β2 adrenergic receptor-deficient mice have greatly reduced levels of renin in plasma and renal tissue, indicating that renal sympathetic input and circulating catecholamines via β-receptors represent a dominant stimulatory input for basal renin expression throughout life 61. Changes of renin release by furosemide, inhibition of angiotensin II receptors or formation, or dietary salt intake occurred in the absence of β1/β2 adrenergic receptors, but the magnitude of the secretory response to both acute or chronic perturbations was markedly smaller in all conditions 61. A generalizable conclusion from these and similar observations in other studies is that the size of the releasable renin pool as determined by renin synthesis in the entire JG cell population is the main determinant of the amount of renin that is released in response to an acute regulatory need (Fig. 3).
Fig. 3.
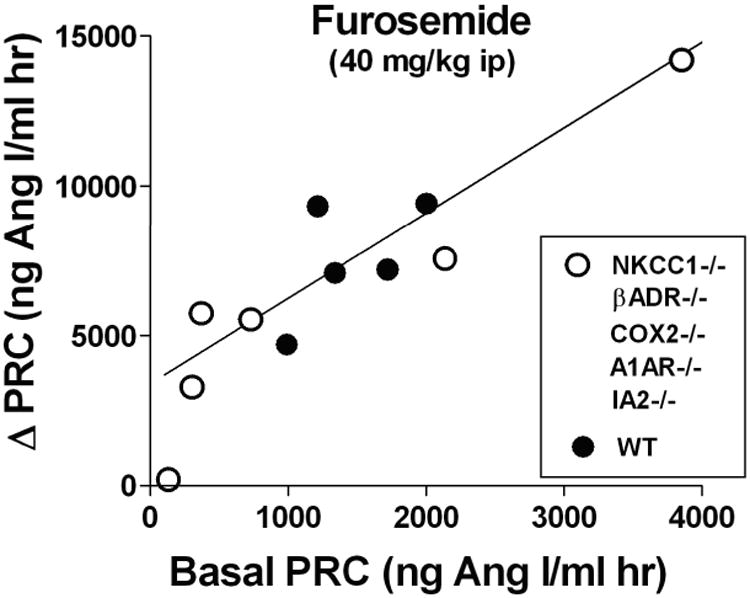
Relationship between basal plasma renin concentration (PRC) and the increase (Δ) of PRC caused by intraperitoneal injection of 40 mg/kg furosemide.
Each symbol is the mean value of 5-10 measurements of PRC in wild type (black dots) and various mutant mouse models (open circles; mutants are used to extend the limited range of basal PRC in wild type to the higher values of NKCC1-/- and the lower values of the other mutant strains). Data are taken from references 37, 61, 71, 77, 94.
Recent studies have identified two of the adenylyl cyclases that are activated by the interaction of adrenoceptors with Gsα. The response of renin release to an injection of isoproterenol was significantly reduced in mice deficient in either AC5 or AC6, suggesting that these Ca-inhibited adenylyl cyclases are causal in the generation of cAMP that mediates the increase in renin secretion with β-adrenergic stimulation 62. In contrast to the β1/β2 adrenergic receptor knockout mice, basal plasma renin and renal renin synthesis were increased in AC6-/- and normal in AC5-/-, indicating redundancy and the capacity to compensate for the loss of an individual AC.
The pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates renin secretion in the isolated perfused rat kidney as well as in JG cells in primary culture 63. This effect is mediated by the PAC1 receptor for PACAP since PAC1-deficient mice have a significantly lower plasma renin concentration and since renin release in isolated kidneys from PAC1-deficient mice did not respond to PACAP.
A previously unknown role of IA-2 proteins inregulation of renin secretion, probably in the β-adrenergic pathway, has recently been discovered. IA-2 proteins are best known for their role as the major autoantigen in type I diabetes mellitus 64, 65. The structure of IA-2 and IA-2β indicates that they are members of the tyrosine phosphatase family although they lack enzymatic activity 66. IA-2 proteins are integral proteins of dense core vesicle membranes in cells with secretory activity as well as in autonomic nerve varicosities and synaptic vesicles 66, 67. The location of IA-2 proteins suggests a role in secretory processes, and this notion has been confirmed in regard to the release of insulin and luteinizing hormone LH 68-70. Plasma renin concentration and renal renin expression were reduced by about 50% in mice with IA-2/IA-2β double deletion, with smaller changes in mice with the respective single deletions 71. The regulation of renin secretion by furosemide or salt intake was intact, although the response magnitude was reduced commensurate with the lower renin expression levels. The effect of IA-2 and IA-2β is probably a consequence of a reduced tonic β-adrenergic input since the expression of IA-2 proteins co-localized with tyrosine hydroxylase, but not with that of renin, and since β-adrenergic blockade with propranolol did not further reduce renin secretion in the IA-deficient animals 71. The use of IA-2 gene manipulation thus identified an unexpected effect that indicates a contribution of IA-2 and IA-2β to tonic adrenergic transmitter release.
Adenosine
The nucleoside adenosine exerts its effects throughout the body by activation of specific G protein coupled receptors. Over a concentration range between 10−10 and 10−6 M, adenosine causes dose-dependent inhibition of renin secretion in isolated JG cells and kidney slices, indicating a direct inhibitory interaction of adenosine with JG cells 72, 73. Studies with selective receptor agonists have established that the inhibitory effect is mediated by the A1 adenosine receptor subtype (A1AR) 72, 73. Mice with deletion of A1AR have elevated plasma renin concentrations, supporting a tonic inhibitory influence of adenosine on renin release 74-76. Interestingly, repeat measurements of plasma renin concentration over a two week period have shown a significantly greater variability in individual A1AR-deficient compared to WT mice. The greater renin instability may reflect the fact that the tubuloglomerular feedback (TGF) mechanism is absent in A1AR-/- mice, and that GFR is therefore predicted to be less stabile, resulting in greater variability of NaCl concentration at the macula densa. Thus, the purported role of TGF to stabilize NaCl excretion may be achieved not only by controlling GFR, but also by minimizing variations in renin secretion.
The role of adenosine in pressure-mediated changes in renin secretion has been examined in A1AR deficient mice both in vivo and with the isolated perfused kidney. Stimulation of renin secretion by low renal perfusion pressure has been found to remain intact 76, 77, but the inhibitory effect of an increase in perfusion pressure on renin secretion was entirely absent in isolated perfused kidneys 76. A similar asymmetric effect of A1AR-deficiency on renin release pertains in regard to the macula densa mechanism. Examination of the stimulatory arm by furosemide-induced blockade of NaCl transport showed maintenance of increased renin release in A1AR-deficient mice 77, 78. On the other hand, intravenous injection of NaCl causing increased NaCl concentration and transport at the macula densa reduced plasma renin concentration in WT mice, but it did not alter it in A1AR knockout mice 77. Thus, activation of A1AR by adenosine contributes to the inhibition of renin secretion by increased perfusion pressure or increased NaCl at the macula densa while the stimulatory arms of these regulatory pathways are largely A1AR-independent.
Connexins
The usefulness of genetically modified mice is exemplified by the identification of the important role of gap junctional coupling in the function of the juxtaglomerular apparatus and the regulation of renin secretion. Although it was known that the renal vasculature and the JG cells express a number of connexins (Cx37, Cx40, Cx43, Cx45) and that gap junctional coupling is likely to account for electrotonic spreading of vasomotor responses along the vasculature in general 79, 80, the use of mice with targeted deletions permitted conclusions about the role of specific connexin proteins in renin secretion. Cx40 is the dominant connexin linking JG cells to each other, to neighboring mesangial cells, and to vascular endothelial cells. Its inactivation at the gene level is associated with dramatic abnormalities in the JG cell phenotype 81, 82. In the adult animal, renal vascular renin expression is normally restricted to cells in the media of the distal-most part of the afferent arteriole; in Cx40-deficient mice an expansion and redistribution of renin-expressing cells into the extraglomerular mesangium, glomerular tuft, and interstitial space is observed 83. Together with a change towards a mesenchymal cell appearance, this suggests cell dedifferentiation since gap junction coupling is a characteristic of fully differentiated cells. Total renal renin content and plasma renin under basal conditions were significantly elevated indicating that gap junctional coupling of JG cells by Cx40 exerts an inhibitory influence, perhaps through a tonic depolarizing effect and an upward shift of cytosolic calcium. The critical role of Cx40-coupling among JG cells is emphasized by the finding that the phenotype of global Cx40 deficiency is fully mimicked by JG cell-specific deletion of Cx40 84 whereas selective deletion of Cx40 from endothelial cells did not alter renin secretion, the distribution of JG cells, or blood pressure 84, 85. Interestingly, elevations of plasma renin and renin mislocation were observed in transgenic mice expressing a mutant Cx40 previously found in a patient with atrial fibrillation and hypertension 86, 87. The cause for the recruitment of “new” renin-producing cells and the implicit expansion into neighboring domains is still unknown, but this abnormality emphasizes the restrictive effect of Cx40 coupling among JG cells.
Baroreceptor-mediated inhibition or stimulation of renin secretion was found to be largely obliterated in Cx40-deficient mice suggesting that the synchronized response of JG cells to the pressure signal is achieved by Cx-40 dependent cell-to-cell communication 82, 87. Macula densa regulated renin release as assessed by responsiveness to furosemide has also been found to be attenuated in Cx40-deficient mice 83. Since signaling through the MD pathway is thought to involve the release of prostaglandins and possibly of other diffusible mediators it is conceivable that an increased distance of renin-producing cells from the MD imposes geometrical restrictions to transmitter efficiency. It is important to note that the secretory machinery itself seems to be intact since the stimulatory response to isoproterenol and the inhibitory effect of L-NAME were maintained in Cx40-deficient mice 82, 88. The increase in renin production is the likely cause of the elevated blood pressure of Cx40-deficient mice 81, 82, 87 although decreased levels of eNOS expression leading to reduced NO production may contribute to the hypertension 89.
Maintenance of JG cell connectivity in the absence of Cx40 by replacing its coding sequence with that of Cx45, another connexin isoform normally found in JG cells, resulted in partial rescue of the Cx40-deficient phenotype 90. Plasma renin activity was similar to wild type, and it responded to angiotensin II with a decrease and to enalapril with an increase, changes not seen in the Cx40 null animals. Since the conductivity of Cx45 is significantly lower than that of Cx40, these results are somewhat surprising, and they raise the question of whether some property of the channel other than conductance could be responsible for the effect on renin expression. The observation that Cx45 can substitute for Cx40 in its role to suppress renin expression is also surprising in view of the observation that deletion of Cx37 has no effect on JG cell renin formation, release, or localization pattern even though Cx37 is normally expressed by JG cells 91. The effect of Cx45 knockin described above does not imply that Cx45 is normally involved in the control of renin synthesis and release. JG cell-specific deletion of Cx45 had no effect on the RAS and did not alter blood pressure or the regulation of renin release 92. The reason for this appears to be the absence of demonstrable expression of Cx45 in JG cells in contrast to arteriolar smooth muscle cells where Cx45 was readily found 92. On the other hand, a role of Cx45 had been suggested in studies in which Cx45 was conditionally deleted using Cre recombinase under control of the nestin promoter 93. Nestin-driven Cre activity was shown to cause reporter expression in afferent and efferent arterioles, mesangial cells, as well as JG cells. The phenotype of these mice largely mimicked that of Cx40-deficient animals in that blood pressure was elevated and renin expression and secretion was increased. In view of the extensive spectrum of cells expressing nestin and therefore of Cx45 deletion in other than JG cells the effect on the RAS in these animals may have been exerted indirectly. Overall, participation of gap junctional connectivity in renin responsiveness is in retrospect perhaps not surprising; synchronization between ensembles of JG cells may be necessary for physiological inputs to cause changes in renin secretion sufficiently large to change plasma renin.
Emerging Areas of Interest
Inhibitors of Na,K,2Cl-cotransport activity such as furosemide or bumetanide are potent stimulators of renin secretion, an effect believed to be mainly due to inhibition of the kidney-specific NKCC2 isoform and subsequent activation of the macula densa pathway. However, a threefold increase of basal plasma renin and renal renin mRNA has also been observed in mice deficient in the ubiquitous NKCC1 isoform 94, 95. Furthermore cells from these mice did not show renin exocytosis in response to furosemide. Thus, NKCC1-mediated NaCl uptake in JG cells appears to exert direct inhibition of basal renin production and release presumably by determining resting membrane potential. Increased expression of renin and elevated plasma renin concentrations have also been observed in NHE2-deficient mice, an effect that seems to be secondary to stimulation of the macula densa COX-2 pathway 96. How NHE2-mediated transport interferes with COX-2 activity still remains to be determined, but changes in cell volume are speculated to play a critical role 96.
Significant increases in renal and plasma renin have been found in dopamine D3-receptor deficient mice, consistent with the expectation that the Gi-coupled D2-like receptors for dopamine (D2, D3, D4) inhibit renin release 97. Although the direct effect of activation of the Gs-linked D1-like receptors is stimulation of renin release, the D1 agonist fenoldopam caused the opposite, a significant inhibition of renin release 98. The mechanism of this indirect action of D1 activation appears to be inhibition of proximal tubular fluid reabsorption and subsequent suppression of cortical expression of COX-2 by an elevated NaCl at the macula densa. D1 agonists have in fact been shown to suppress COX-2, and the inhibition exerted by fenoldopam was converted to stimulation in COX-2-deficient mice 98, 99. Mice with a cell-specific deletion of the dopamine synthesizing enzyme aromatic amino acid decarboxylase in the proximal tubule have an array of disturbances that can be interpreted as representing the unchecked effects of angiotensin II excess 100.
GPR91, until now a G protein-coupled orphan receptor, has recently been identified as a receptor for succinate. It has been found to be localized in the apical membrane of cells of the cortical TAL including the MD and in endothelial cells of the afferent arteriole 101. Activation of GPR91 by succinate stimulates COX-2 activity, PGE2 release and presumably renin expression, an effect that is particularly pronounced in diabetic wild type mice and markedly reduced in GPR91-deficient animals 102.
Components of the olfactory system including the olfactory receptor Olfr90, the olfactory trimeric G protein Golf, and the olfactory adenylate cyclase isoform 3 (AC3) have been found to be expressed in MD cells 103. Plasma renin concentration in AC3-deficient mice was reduced by about 50% compared to wild types despite increased MD COX-2 expression and augmented nNOS activity suggesting that the change in renin expression was primary. The ligands that may activate olfactory receptors on MD cells in vivo remain to be determined.
Gene deletion studies have clarified some functional aspects of the issue that some mouse strains possess a single renin gene (Ren-1c) whereas other strains in addition carry a duplicated renin gene (Ren-1d and Ren-2). Deletion of the Ren-1d gene in two renin gene mice has shown that Ren-2 generates a fully functional renin providing sufficiently high levels of active plasma renin to essentially keep arterial blood pressure normal 104, 105. Because Ren-2 expression is known to be androgen-sensitive this effect is somewhat less effective in female animals. Nevertheless, an important characteristic of Ren-1d deficiency is that plasma levels of prorenin are markedly elevated and that the granulation of JG cells appears to be reduced 104. Thus, Ren-2 does not seem to carry the signals needed to be sorted to the regulated secretory pathway, but is preferentially secreted as prorenin through the constitutive pathway.
Cathepsin B is able to activate prorenin in vitro, and it has been considered a likely candidate as physiological prorenin-processing enzyme because of its localization in JG cell granules 106. However, mice with deletions of the cathepsin B gene have normal plasma levels of active renin and do not display any of the phenotypic hallmarks of renin deficiency 107. Thus, the issue of the prorenin activator in vivo still awaits resolution.
Isolated JG cells respond to hypotonicity-induced cell swelling with an increase in renin secretion. Recent studies using membrane capacitance changes as index of exocytosis have shown that the increase in capacitance caused by hypotonicity in JG cells from wild type mice was absent in cells from COX-2-deficient or AQP1-deficient mice 108. These observations indicate that hypotonicity causes water influx through AQP1 water channels, activation of COX-2, release of PGE2 and stimulation of renin exocytosis through the cAMP/PKA pathway. The novel findings of presence of both COX-2 and AQP1 in JG cells and of their functional interactions have interesting implications that will stimulate further investigations.
Conclusion
Genetic modification in mice has become the ultimate tool for the in vivo evaluation of the chronic effects of complete and “certified” inactivation of defined regulatory factors. Given the central role of the RAS in body fluid and blood pressure regulation it is not surprising that mutational approaches have been extensively used to gain further insights into this system. Improved understanding has been gained of the feedback inhibition of renin expression by angiotensin II, the central role of Gsβ signaling in renin control, the inclusion of COX-2 and nNOS products in regulatory pathways, the inhibitory role of adenosine in baroreceptor- and macula densa control, and the fundamental contribution of β-receptors to renin expression. New directions that have greatly benefited from the availability of novel mouse models include exciting information on the roles of connexins, GPR91, AC3, specific transport proteins, and numerous other factors. Combined with vastly improved phenotypic methods, the insights gained have been dramatic, confirming some hypotheses and dashing others. Insights with the “first generation” methodology of global over- or under-expression of genes have been important, but one may expect that widened application of improved tools for temporal and spatial control as well as insertions of known mutations will further expand the application of this approach. The expected extension of gene manipulations from mice to rats will be highly welcome in that it will facilitate and expand the phenotyping possibilities 109.
Acknowledgments
Research from the laboratory was supported by intramural funds of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD.
References
- 1.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 2.Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 3.Pan L, Gross KW. Transcriptional regulation of renin: an update. Hypertension. 2005;45:3–8. doi: 10.1161/01.HYP.0000149717.55920.45. [DOI] [PubMed] [Google Scholar]
- 4.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todorov VT, Desch M, Schmitt-Nilson N, et al. Peroxisome proliferator-activated receptor-gamma is involved in the control of renin gene expression. Hypertension. 2007;50:939–944. doi: 10.1161/HYPERTENSIONAHA.107.092817. [DOI] [PubMed] [Google Scholar]
- 6.Tanimoto K, Sugiyama F, Goto Y, et al. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269:31334–31337. [PubMed] [Google Scholar]
- 7.Niimura F, Labosky PA, Kakuchi J, et al. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest. 1995;96:2947–2954. doi: 10.1172/JCI118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian B, Meng QC, Chen YF, et al. Blood pressures and cardiovascular homeostasis in mice having reduced or absent angiotensin-converting enzyme gene function. Hypertension. 1997;30:128–133. doi: 10.1161/01.hyp.30.1.128. [DOI] [PubMed] [Google Scholar]
- 9.Hilgers KF, Reddi V, Krege JH, et al. Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension. 1997;29:216–221. doi: 10.1161/01.hyp.29.1.216. [DOI] [PubMed] [Google Scholar]
- 10.Sugaya T, Nishimatsu S, Tanimoto K, et al. Angiotensin II type 1a receptor-deficient mice with hypotension and hyperreninemia. J Biol Chem. 1995;270:18719–18722. doi: 10.1074/jbc.270.32.18719. [DOI] [PubMed] [Google Scholar]
- 11.Oliverio MI, Madsen K, Best CF, et al. Renal growth and development in mice lacking AT1A receptors for angiotensin II. Am J Physiol. 1998;274:F43–50. doi: 10.1152/ajprenal.1998.274.1.F43. [DOI] [PubMed] [Google Scholar]
- 12.Sequeira Lopez ML, Pentz ES, Nomasa T, et al. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 13.Machura K, Steppan D, Neubauer B, et al. Developmental renin expression in mice with a defective renin-angiotensin system. Am J Physiol Renal Physiol. 2009;297:F1371–1380. doi: 10.1152/ajprenal.00378.2009. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Li W, Yoshida H, et al. Targeting deletion of angiotensin type 1B receptor gene in the mouse. Am J Physiol. 1997;272:F299–304. doi: 10.1152/ajprenal.1997.272.3.F299. [DOI] [PubMed] [Google Scholar]
- 15.Gembardt F, Heringer-Walther S, van Esch JH, et al. Cardiovascular phenotype of mice lacking all three subtypes of angiotensin II receptors. Faseb J. 2008;22:3068–3077. doi: 10.1096/fj.08-108316. [DOI] [PubMed] [Google Scholar]
- 16.Crowley SD, Gurley SB, Oliverio MI, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsusaka T, Nishimura H, Utsunomiya H, et al. Chimeric mice carrying ‘regional’ targeted deletion of the angiotensin type 1A receptor gene. Evidence against the role for local angiotensin in the in vivo feedback regulation of renin synthesis in juxtaglomerular cells. J Clin Invest. 1996;98:1867–1877. doi: 10.1172/JCI118988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kihara M, Umemura S, Yabana M, et al. Dietary salt loading decreases the expressions of neuronal-type nitric oxide synthase and renin in the juxtaglomerular apparatus of angiotensinogen gene-knockout mice. J Am Soc Nephrol. 1998;9:355–362. doi: 10.1681/ASN.V93355. [DOI] [PubMed] [Google Scholar]
- 19.Tamura K, Umemura S, Sumida Y, et al. Effect of genetic deficiency of angiotensinogen on the renin-angiotensin system. Hypertension. 1998;32:223–227. doi: 10.1161/01.hyp.32.2.223. [DOI] [PubMed] [Google Scholar]
- 20.Umemura S, Kihara M, Sumida Y, et al. Endocrinological abnormalities in angiotensinogen-gene knockout mice: studies of hormonal responses to dietary salt loading. J Hypertens. 1998;16:285–289. doi: 10.1097/00004872-199816030-00005. [DOI] [PubMed] [Google Scholar]
- 21.Hackenthal E, Paul M, Ganten D, et al. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Kim SM, Oppermann M, et al. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292:F27–37. doi: 10.1152/ajprenal.00193.2006. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Gavrilova O, Liu J, et al. Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci U S A. 2005;102:7386–7391. doi: 10.1073/pnas.0408268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neubauer B, Machura K, Chen M, et al. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol Renal Physiol. 2009;296:F1006–1012. doi: 10.1152/ajprenal.90448.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Kim SM, Eisner C, et al. Stimulation of renin secretion by angiotensin II blockade is Gsalpha-dependent. J Am Soc Nephrol. 2010;21:986–992. doi: 10.1681/ASN.2009030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichihara A, Suzuki H, Murakami M, et al. Interactions between angiotensin II and norepinephrine on renin release by juxtaglomerular cells. Eur J Endocrinol. 1995;133:569–577. doi: 10.1530/eje.0.1330569. [DOI] [PubMed] [Google Scholar]
- 27.Larsson C, Weber P, Anggard E. Arachidonic acid increases and indomethacin decreases plasma renin activity in the rabbit. Eur J Pharmacol. 1974;28:391–394. doi: 10.1016/0014-2999(74)90296-9. [DOI] [PubMed] [Google Scholar]
- 28.Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81–227. [PubMed] [Google Scholar]
- 29.Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol Renal Physiol. 1996;271:F659–F669. doi: 10.1152/ajprenal.1996.271.3.F659. [DOI] [PubMed] [Google Scholar]
- 30.Schweda F, Klar J, Narumiya S, et al. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol. 2004;287:F427–433. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 31.Nusing RM, Treude A, Weissenberger C, et al. Dominant role of prostaglandin E2 EP4 receptor in furosemide-induced salt-losing tubulopathy: a model for hyperprostaglandin E syndrome/antenatal Bartter syndrome. J Am Soc Nephrol. 2005;16:2354–2362. doi: 10.1681/ASN.2004070556. [DOI] [PubMed] [Google Scholar]
- 32.Friis UG, Stubbe J, Uhrenholt TR, et al. Prostaglandin E2 EP2 and EP4 receptor activation mediates cAMP-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol Renal Physiol. 2005;289:F989–997. doi: 10.1152/ajprenal.00201.2005. [DOI] [PubMed] [Google Scholar]
- 33.Francois H, Facemire C, Kumar A, et al. Role of microsomal prostaglandin E synthase 1 in the kidney. J Am Soc Nephrol. 2007;18:1466–1475. doi: 10.1681/ASN.2006040343. [DOI] [PubMed] [Google Scholar]
- 34.Fujino T, Nakagawa N, Yuhki K, et al. Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I2 receptor IP. J Clin Invest. 2004;114:805–812. doi: 10.1172/JCI21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Y, Lucitt MB, Stubbe J, et al. Prostaglandin F2alpha elevates blood pressure and promotes atherosclerosis. Proc Natl Acad Sci U S A. 2009;106:7985–7990. doi: 10.1073/pnas.0811834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris RC, McKanna JA, Akai Y, et al. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SM, Chen L, Mizel D, et al. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol. 2007;292:F415–F422. doi: 10.1152/ajprenal.00317.2006. [DOI] [PubMed] [Google Scholar]
- 38.Yang T, Endo Y, Huang YG, et al. Renin expression in COX-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol. 2000;279:F819–825. doi: 10.1152/ajprenal.2000.279.5.F819. [DOI] [PubMed] [Google Scholar]
- 39.Paliege A, Mizel D, Medina C, et al. Inhibition of nNOS expression in the macula densa by COX-2-derived prostaglandin E(2) Am J Physiol Renal Physiol. 2004;287:F152–159. doi: 10.1152/ajprenal.00287.2003. [DOI] [PubMed] [Google Scholar]
- 40.Cheng HF, Wang SW, Zhang MZ, et al. Prostaglandins that increase renin production in response to ACE inhibition are not derived from cyclooxygenase-1. Am J Physiol Regul Integr Comp Physiol. 2002;283:R638–646. doi: 10.1152/ajpregu.00150.2002. [DOI] [PubMed] [Google Scholar]
- 41.Hocherl K, Kammerl MC, Schumacher K, et al. Role of prostanoids in regulation of the renin-angiotensin-aldosterone system by salt intake. Am J Physiol Renal Physiol. 2002;283:F294–301. doi: 10.1152/ajprenal.00347.2001. [DOI] [PubMed] [Google Scholar]
- 42.Kammerl MC, Nusing RM, Seyberth HW, et al. Inhibition of cyclooxygenase-2 attenuates urinary prostanoid excretion without affecting renal renin expression. Pflugers Arch. 2001;442:842–847. doi: 10.1007/s004240100616. [DOI] [PubMed] [Google Scholar]
- 43.Cheng HF, Wang JL, Zhang MZ, et al. Genetic deletion of COX-2 prevents increased renin expression in response to ACE inhibition. Am J Physiol Renal Physiol. 2001;280:F449–456. doi: 10.1152/ajprenal.2001.280.3.F449. [DOI] [PubMed] [Google Scholar]
- 44.Cheng HF, Wang JL, Zhang MZ, et al. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest. 1999;103:953–961. doi: 10.1172/JCI5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto T, Kihara M, Sato K, et al. Expression of cyclooxygenase-2 in the juxtaglomerular apparatus of angiotensinogen gene-knockout mice. Nephron Physiol. 2006;102:p1–8. doi: 10.1159/000088312. [DOI] [PubMed] [Google Scholar]
- 46.Wolf K, Castrop H, Hartner A, et al. Inhibition of the renin-angiotensin system upregulates cyclooxygenase-2 expression in the macula densa. Hypertension. 1999;34:503–507. doi: 10.1161/01.hyp.34.3.503. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang X, Le TH, Roncal C, et al. Th1 inflammatory response with altered expression of profibrotic and vasoactive mediators in AT1A and AT1B double-knockout mice. Am J Physiol Renal Physiol. 2005;289:F902–910. doi: 10.1152/ajprenal.00141.2005. [DOI] [PubMed] [Google Scholar]
- 48.Zhang MZ, Yao B, Cheng HF, et al. Renal cortical cyclooxygenase 2 expression is differentially regulated by angiotensin II AT(1) and AT(2) receptors. Proc Natl Acad Sci U S A. 2006;103:16045–16050. doi: 10.1073/pnas.0602176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castrop H, Schweda F, Mizel D, et al. Permissive role of nitric oxide in macula densa control of renin secretion. Am J Physiol Renal Physiol. 2004;286:F848–857. doi: 10.1152/ajprenal.00272.2003. [DOI] [PubMed] [Google Scholar]
- 50.Sallstrom J, Carlstrom M, Jensen BL, et al. Neuronal nitric oxide synthase-deficient mice have impaired renin release but normal blood pressure. Am J Hypertens. 2008;21:111–116. doi: 10.1038/ajh.2007.16. [DOI] [PubMed] [Google Scholar]
- 51.Wagner C, Godecke A, Ford M, et al. Regulation of renin gene expression in kidneys of eNOS- and nNOS-deficient mice. Pflugers Arch. 2000;439:567–572. doi: 10.1007/s004249900214. [DOI] [PubMed] [Google Scholar]
- 52.Huang PL, Dawson TM, Bredt DS, et al. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 53.Lu D, Fu Y, Lopez-Ruiz A, et al. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol. 2011;298:F1465–1471. doi: 10.1152/ajprenal.00650.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beierwaltes WH, Potter DL, Shesely EG. Renal baroreceptor-stimulated renin in the eNOS knockout mouse. Am J Physiol Renal Physiol. 2002;282:F59–64. doi: 10.1152/ajprenal.0144.2001. [DOI] [PubMed] [Google Scholar]
- 55.Shesely EG, Maeda N, Kim HS, et al. Elevated blood pressure in mice lacking endothelial nitric oxide synthase. Proc Nat Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakata S, Tsutsui M, Shimokawa H, et al. Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation. 2008;117:2211–2223. doi: 10.1161/CIRCULATIONAHA.107.742692. [DOI] [PubMed] [Google Scholar]
- 57.Kihara M, Umemura S, Kadota T, et al. The neuronal isoform of constitutive nitric oxide synthase is up- regulated in the macula densa of angiotensinogen gene-knockout mice. Lab Invest. 1997;76:285–294. [PubMed] [Google Scholar]
- 58.Kihara M, Umemura S, Sugaya T, et al. Expression of neuronal type nitric oxide synthase and renin in the juxtaglomerular apparatus of angiotensin type-1a receptor gene-knockout mice. Kidney Int. 1998;53:1585–1593. doi: 10.1046/j.1523-1755.1998.00904.x. [DOI] [PubMed] [Google Scholar]
- 59.Park S, Harrison-Bernard LM. Augmented renal vascular nNOS and renin protein expression in angiotensin type 1 receptor null mice. J Histochem Cytochem. 2008;56:401–414. doi: 10.1369/jhc.2007.950220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neubauer B, Machura K, Schnermann JB, et al. Renin expression in large renal vessels during fetal development depends on functional {beta}1/{beta}2-adrenergic receptors. Am J Physiol Renal Physiol. 2011;301:F71–F77. doi: 10.1152/ajprenal.00443.2010. [DOI] [PubMed] [Google Scholar]
- 61.Kim SM, Chen L, Faulhaber-Walter R, et al. Regulation of renin secretion and expression in mice deficient in beta1- and beta2-adrenergic receptors. Hypertension. 2007;50:103–109. doi: 10.1161/HYPERTENSIONAHA.107.087577. [DOI] [PubMed] [Google Scholar]
- 62.Aldehni F, Tang T, Madsen K, et al. Stimulation of renin secretion by catecholamines is dependent on adenylyl cyclases 5 and 6. Hypertension. 57:460–468. doi: 10.1161/HYPERTENSIONAHA.110.167130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hautmann M, Friis UG, Desch M, et al. Pituitary adenylate cyclase-activating polypeptide stimulates renin secretion via activation of PAC1 receptors. J Am Soc Nephrol. 2007;18:1150–1156. doi: 10.1681/ASN.2006060633. [DOI] [PubMed] [Google Scholar]
- 64.Lan MS, Wasserfall C, Maclaren NK, et al. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1996;93:6367–6370. doi: 10.1073/pnas.93.13.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabin DU, Pleasic SM, Shapiro JA, et al. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol. 1994;152:3183–3188. [PubMed] [Google Scholar]
- 66.Lan MS, Lu J, Goto Y, et al. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994;13:505–514. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- 67.Solimena M, Dirkx R, Jr, Hermel JM, et al. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. Embo J. 1996;15:2102–2114. [PMC free article] [PubMed] [Google Scholar]
- 68.Kubosaki A, Gross S, Miura J, et al. Targeted disruption of the IA-2beta gene causes glucose intolerance and impairs insulin secretion but does not prevent the development of diabetes in NOD mice. Diabetes. 2004;53:1684–1691. doi: 10.2337/diabetes.53.7.1684. [DOI] [PubMed] [Google Scholar]
- 69.Saeki K, Zhu M, Kubosaki A, et al. Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes. 2002;51:1842–1850. doi: 10.2337/diabetes.51.6.1842. [DOI] [PubMed] [Google Scholar]
- 70.Kubosaki A, Nakamura S, Clark A, et al. Disruption of the transmembrane dense core vesicle proteins IA-2 and IA-2beta causes female infertility. Endocrinology. 2006;147:811–815. doi: 10.1210/en.2005-0638. [DOI] [PubMed] [Google Scholar]
- 71.Kim SM, Theilig F, Qin Y, et al. Dense-core vesicle proteins IA-2 and IA-2{beta} affect renin synthesis and secretion through the {beta}-adrenergic pathway. Am J Physiol Renal Physiol. 2009;296:F382–389. doi: 10.1152/ajprenal.90543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Churchill PC, Churchill MC. A1 and A2 adenosine receptor activation inhibits and stimulates renin secretion of rat renal cortical slices. J Pharmacol Exp Ther. 1985;232:589–594. [PubMed] [Google Scholar]
- 73.Kurtz A, Della Bruna R, Pfeilschifter J, et al. Role of cGMP as second messenger of adenosine in the inhibition of renin release. Kidney Int. 1988;33:798–803. doi: 10.1038/ki.1988.70. [DOI] [PubMed] [Google Scholar]
- 74.Brown R, Ollerstam A, Johansson B, et al. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 75.Brown RD, Thoren P, Steege A, et al. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1324–1329. doi: 10.1152/ajpregu.00313.2005. [DOI] [PubMed] [Google Scholar]
- 76.Schweda F, Segerer F, Castrop H, et al. Blood pressure-dependent inhibition of Renin secretion requires A1 adenosine receptors. Hypertension. 2005;46:780–786. doi: 10.1161/01.HYP.0000183963.07801.65. [DOI] [PubMed] [Google Scholar]
- 77.Kim SM, Mizel D, Huang YG, et al. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol. 2006;290:F1016–1023. doi: 10.1152/ajprenal.00367.2005. [DOI] [PubMed] [Google Scholar]
- 78.Schweda F, Wagner C, Kramer BK, et al. Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol. 2003;284:F770–777. doi: 10.1152/ajprenal.00280.2002. [DOI] [PubMed] [Google Scholar]
- 79.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol. 1989;256:H838–845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- 80.Gustafsson F, Holstein-Rathlou N. Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand. 1999;167:11–21. doi: 10.1046/j.1365-201x.1999.00603.x. [DOI] [PubMed] [Google Scholar]
- 81.Krattinger N, Capponi A, Mazzolai L, et al. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 82.Wagner C, de Wit C, Kurtz L, et al. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 83.Kurtz L, Schweda F, de Wit C, et al. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 84.Wagner C, Jobs A, Schweda F, et al. Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int. 2010;78:762–768. doi: 10.1038/ki.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chadjichristos CE, Scheckenbach KE, van Veen TA, et al. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation. 2010;121:123–131. doi: 10.1161/CIRCULATIONAHA.109.867176. [DOI] [PubMed] [Google Scholar]
- 86.Gollob MH, Jones DL, Krahn AD, et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354:2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 87.Lubkemeier I, Machura K, Kurtz L, et al. The Connexin40 A96S Mutation Causes Renin-Dependent Hypertension. J Am Soc Nephrol. 2011;22:1031–1040. doi: 10.1681/ASN.2010101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krattinger N, Alonso F, Capponi A, et al. Increased expression of renal cyclooxygenase-2 and neuronal nitric oxide synthase in hypertensive Cx40-deficient mice. J Vasc Res. 2009;46:188–198. doi: 10.1159/000156704. [DOI] [PubMed] [Google Scholar]
- 89.Alonso F, Boittin FX, Beny JL, et al. Loss of connexin40 is associated with decreased endothelium-dependent relaxations and eNOS levels in the mouse aorta. Am J Physiol Heart Circ Physiol. 2010;299:H1365–1373. doi: 10.1152/ajpheart.00029.2010. [DOI] [PubMed] [Google Scholar]
- 90.Schweda F, Kurtz L, de Wit C, et al. Substitution of connexin40 with connexin45 prevents hyperreninemia and attenuates hypertension. Kidney Int. 2009;75:482–489. doi: 10.1038/ki.2008.637. [DOI] [PubMed] [Google Scholar]
- 91.Wagner C, Kurtz L, Schweda F, et al. Connexin 37 is dispensable for the control of the renin system and for positioning of renin-producing cells in the kidney. Pflugers Arch. 2009;459:151–158. doi: 10.1007/s00424-009-0707-6. [DOI] [PubMed] [Google Scholar]
- 92.Kurt B, Kurtz L, Sequeira-Lopez ML, et al. Reciprocal expression of connexin 40 and 45 during phenotypical changes in renin-secreting cells. Am J Physiol Renal Physiol. 2011;300:F743–748. doi: 10.1152/ajprenal.00647.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanner F, von Maltzahn J, Maxeiner S, et al. Connexin45 is expressed in the juxtaglomerular apparatus and is involved in the regulation of renin secretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2008;295:R371–380. doi: 10.1152/ajpregu.00468.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castrop H, Lorenz JN, Hansen PB, et al. Contribution of the basolateral isoform of the Na-K-2Cl- cotransporter (NKCC1/BSC2) to renin secretion. Am J Physiol Renal Physiol. 2005;289:F1185–1192. doi: 10.1152/ajprenal.00455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim SM, Eisner C, Faulhaber-Walter R, et al. Salt sensitivity of blood pressure in NKCC1-deficient mice. Am J Physiol Renal Physiol. 2008;295:F1230–1238. doi: 10.1152/ajprenal.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanner F, Chambrey R, Bourgeois S, et al. Increased renal renin content in mice lacking the Na+/H+ exchanger NHE2. Am J Physiol Renal Physiol. 2008;294:F937–944. doi: 10.1152/ajprenal.00591.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asico LD, Ladines C, Fuchs S, et al. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest. 1998;102:493–498. doi: 10.1172/JCI3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang MZ, Yao B, Fang X, et al. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53:564–570. doi: 10.1161/HYPERTENSIONAHA.108.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang MZ, Yao B, McKanna JA, et al. Cross talk between the intrarenal dopaminergic and cyclooxygenase-2 systems. Am J Physiol Renal Physiol. 2005;288:F840–845. doi: 10.1152/ajprenal.00240.2004. [DOI] [PubMed] [Google Scholar]
- 100.Zhang MZ, Yao B, Wang S, et al. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest. 2011 doi: 10.1172/JCI57324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robben JH, Fenton RA, Vargas SL, et al. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int. 2009;76:1258–1267. doi: 10.1038/ki.2009.360. [DOI] [PubMed] [Google Scholar]
- 102.Vargas SL, Toma I, Kang JJ, et al. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol. 2009;20:1002–1011. doi: 10.1681/ASN.2008070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pluznick JL, Zou DJ, Zhang X, et al. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci U S A. 2009;106:2059–2064. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clark AF, Sharp MGF, Morley SD, et al. Renin-1 is essential for normal renal juxtaglomerular cell granulation and macula densa morphology. J Biol Chem. 1997;272:18185–18190. doi: 10.1074/jbc.272.29.18185. [DOI] [PubMed] [Google Scholar]
- 105.Pentz ES, Lopez ML, Kim HS, et al. Ren1d and Ren2 cooperate to preserve homeostasis: evidence from mice expressing GFP in place of Ren1d. Physiol Genomics. 2001;6:45–55. doi: 10.1152/physiolgenomics.2001.6.1.45. [DOI] [PubMed] [Google Scholar]
- 106.Taugner R, Buhrle CP, Nobiling R, et al. Coexistence of renin and cathepsin B in epithelioid cell secretory granules. Histochemistry. 1985;83:103–108. doi: 10.1007/BF00495138. [DOI] [PubMed] [Google Scholar]
- 107.Mercure C, Lacombe MJ, Khazaie K, et al. Cathepsin B is not the processing enzyme for mouse prorenin. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1212–1216. doi: 10.1152/ajpregu.00830.2009. [DOI] [PubMed] [Google Scholar]
- 108.Friis UG, Madsen K, Svenningsen P, et al. Hypotonicity-induced Renin exocytosis from juxtaglomerular cells requires aquaporin-1 and cyclooxygenase-2. J Am Soc Nephrol. 2009;20:2154–2161. doi: 10.1681/ASN.2008090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moreno C, Hoffman M, Stodola TJ, et al. Creation and characterization of a renin knockout rat. Hypertension. 57:614–619. doi: 10.1161/HYPERTENSIONAHA.110.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]


