Abstract
Background
Retrospective analysis of patients undergoing cancer surgery suggests the use of regional anesthesia may reduce cancer recurrence and improve survival. Amide-linked local anesthetics have anti-inflammatory properties, although the mechanism of action in this regard is unclear. As inflammatory processes involving Src tyrosine protein kinase and intercellular adhesion molecule-1 are important in tumor growth and metastasis, we hypothesized that amide-linked local anesthetics may inhibit inflammatory Src-signaling involved in migration of adenocarcinoma cells.
Methods
NCI-H838 lung cancer cells were incubated with Tumor Necrosis Factor-α in absence/presence of ropivacaine, lidocaine, or chloroprocaine (1nM-100μM). Cell migration and total cell lysate Src-activation and Intercellular Adhesion Molecule-1 phosphorylation were assessed. The role of voltage-gated sodium-channels in the mechanism of local anesthetic effects was also evaluated.
Results
Ropivacaine treatment (100μM) of H838 cells for 20 minutes decreased basal Src activity by 62% (p=0.003), and both ropivacaine and lidocaine co-administered with Tumor Necrosis Factor-α statistically significantly decreased Src-activation and Intercellular Adhesion Molecule-1 phosphorylation, whereas chloroprocaine had no such effect. Migration of these cells at 4 hours was inhibited by 26% (p=0.005) in presence of 1μM ropivacaine and 21% by 1μM lidocaine (p=0.004). These effects of ropivacaine and lidocaine were independent of voltage-gated sodium-channel inhibition.
Conclusions
This study indicates that amide-, but not ester-linked local anesthetics may provide beneficial anti-metastatic effects. The observed inhibition of NCI-H838 cell migration by lidocaine and ropivacaine was associated with the inhibition of Tumor Necrosis Factor-α-induced Src-activation and Intercellular Adhesion Molecule-1 phosphorylation, providing the first evidence of a molecular mechanism which appears to be independent of their known role as sodium-channel blockers.
Introduction
Lung cancer is the leading cause of death from cancer in males and the second leading cause of death in females1; effective treatment remains a challenge. Even if patients undergo complete tumor resection including lymphadenectomy, there is still a high rate of relapse due to undetected micrometastasis2–4. Regional anesthesia with local anesthetics in combination with general anesthesia is commonly used in patients undergoing tumor resection of the lung5.
It is well known that amide-linked local anesthetics – as opposed to the ester-linked compounds – can exert inhibitory effects on inflammatory processes6, e.g., as ropivacaine has been shown to possess anti-inflammatory properties in a model of pulmonary inflammation7. Also, the systemic use of lidocaine in patients undergoing colo-rectal surgery led to a decrease in inflammatory cytokine release as well as a shortened hospital stay8.
There is increasing evidence that inflammatory mechanisms may play an important role in the development and growth of cancer metastasis9. Cytokines such as Tumor Necrosis Factor-α (TNF-α) increase the expression of Intercellular Adhesion Molecule-1 (ICAM-1), a cell surface receptor required for leukocyte adhesion10 and tumor invasion11, in the H838 non-small cell lung cancer cell line12. ICAM-1 also facilitates tumor cell extravasation via the binding of neutrophils13. TNF-α is known to induce activation of Src protein tyrosine kinase, which functions as a regulator of endothelial permeability14. Src mediated phosphorylation of ICAM-1 is necessary for neutrophil adhesion to the endothelium during acute lung inflammation and injury15, a process considered to induce vascular hyperpermeability10. Additionally, Src is involved in signaling epithelial-to-mesenchymal transformation and extravasation of cancer cells16,17, processes that are necessary for solid tumor metastasis18.
Recently published retrospective analysis of patients undergoing cancer surgery showed a possible benefit of the perioperative use of regional anesthesia. Long-term survival after colon cancer surgery19 and cancer recurrence after radical prostatectomy for prostate cancer20 or breast cancer21 were significantly improved by epidural anesthesia/analgesia compared to patient-controlled analgesia with opioids. The mechanism by which regional anesthesia might prove to be beneficial at the molecular level in this context is not yet known. We hypothesized that this effect might be related to the anti-inflammatory properties of local anesthetics.
The primary goal of this study was to determine whether amide-linked local anesthetics (ropivacaine and lidocaine) attenuate TNFα-induced Src activation and ICAM-1 phosphorylation in H838 human lung cancer cells and whether these effects are due to the inhibition of voltage-gated sodium channels (VGSC). Secondly, we examined whether lidocaine, ropivacaine, or chloroprocaine inhibit migration of this cancer cell line in vitro.
Materials and Methods
Cell culture
The human NCI-H838 lung cancer cell line (CRL-5844, ATCC, Rockville, MD), isolated from an 80-year old patient with stage 3B non-small cell lung cancer, was cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin and 1% L-glutamine (all from Gibco Invitrogen, Carlsbad, CA). Experiments were performed with 70 – 100% confluent cells from passages 49 – 55. Prior to the experiments, cells were grown in reduced-serum (RPMI+1% FBS) for 16 hours. All cells were maintained in 5% CO2 - 95% room air in a water-jacketed 37°C incubator.
Experimental procedure
Cell monolayers were incubated either with Escherichia coli serotype 055:B5 lipopolysaccharide (Sigma-Aldrich) at a concentration of 4 μg/ml diluted in RPMI+1% FBS medium for 4 hours, or with TNF-α Gibco Invitrogen) at a concentration of 20 ng/ml, also diluted in RPMI-1% FBS, for 20 minutes for Western Blot or 4 hours for assessment of cell migration and cytotoxic effects.
Ropivacaine 0.5 % (Naropin®, APP Pharmaceuticals, Schaumburg, IL), Lidocaine 2% (APP Pharmaceuticals) or Chloroprocaine 3% (Bedford Laboratories, Bedford, OH) were diluted with RPMI+1% FBS medium to achieve the concentrations tested (1 nM – 100 μM) for the treatment of H838 cells in presence or absence of TNF-α or lipopolysaccharide. For some experiments, cells were pretreated with the VGSC agonist veratridine (Sigma-Aldrich) for 30 minutes or with the VGSC antagonist tetrodotoxin (Sigma-Aldrich) for 10 minutes.
Cytotoxicity Assay
Cytotoxicity was measured using the Cytotoxicity Detection KitPlus (Roche, Indianapolis, IN) following the instructions by the manufacturer. The assay measures the activity of lactate dehydrogenase as a marker for cytotoxicity in cell culture supernatants. NCI-H838 cells were incubated for 4 hours in low serum RPMI-1640 medium (1 % FBS, 1 % penicillin/streptomycin, 1 % L-glutamine) with different concentrations of ropivacaine, lidocaine or chloroprocaine (1 nM – 100 μM) in presence or absence of TNF-α at a concentration of 20 ng/ml. 30 minutes prior to the end of the experiment, some cells were lysed by the addition of 10% Triton-X 100 (Sigma-Aldrich) to measure the maximal release of lactate dehydrogenase. Then the supernatant was collected and centrifuged for 5 minutes at 700 g to remove all cellular debris. Lactate dehydrogenase content was determined by the measurement of red formazan, derived from the yellow tetrazolium salt INT (2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride) by a catalyst after reduction of NAD+ to NADH+H+ by lactate dehydrogenase. Cytotoxicity was then calculated according to the following formula:
Cell harvest and lysis
After the end of the experiment, cells were washed once with ice-cold Dulbecco’s phosphate buffered saline (Cellgro, Manassas, VA) and lysed with radioimmunoprecipitation buffer (Boston Bioproducts, Ashland, MA) supplemented with protease inhibitor cocktail, 200 mM phenylmethylsulfonylfluoride (PMSF), 1 mM EDTA, 1 mM sodium-fluoride (NaF) and 1 mM sodium-orthovanadate (Na3VO4, all from Sigma-Aldrich). After a brief sonification and 10 minute centrifugation at 4°C and 13,200 rpm in an Eppendorf 5415R microcentrifuge (Eppendorf AG, Hamburg, Germany), the supernatant was collected and stored at −20°C until further use. Total protein concentration was determined using a BCA protein assay kit (Pierce Thermo Fisher Scientific, Rockford, IL) according to the manufacturer’s instructions using albumin to generate a standard curve.
Western Blot
Before boiling the lysates for 5 minutes, 6x Laemmli sample buffer (Boston Bioproducts) was added at a final concentration of 1x as well as 30 mM Dithiothreitol (DTT, Biorad, Hercules, CA). Equal amounts of protein (10 μg) were loaded in each lane of a 10% polyacrylamide gel for Sodium Dodecyl Sulfate Poly-Acrylamide Gel Electrophoresis (SDS-PAGE). After SDS-PAGE, the proteins were transferred to a nitrocellulose membrane (Biorad) with 5% blotting grade non-fat dry milk (Biorad) dissolved in Tris buffered saline with 0.05% TWEEN-20 (TBST) (Sigma-Aldrich) for 30 minutes. The membranes were then probed with primary antibodies in TBST for 2 hours on a shaker at room temperature or at 4°C overnight. Three washes with TBST were followed by incubation of the membrane with horseradish-peroxidase-conjugated secondary antibody (KPL, Gaithersburg, MD) in 5% milk in TBST for 1 hour on a shaker at room temperature. After four washes with TBST, enhanced chemiluminescence solution (Thermo Scientific) was used to visualize the bands on HyBlot CL film (Denville, South Plainfield, NJ). To detect the total amount of ICAM-1 or Src on the same membranes after detection of the phosphorylated forms of the proteins, the antibodies were removed from the membranes using Restore Western Blot Stripping buffer (Thermo Scientific). Subsequently, the membranes were blocked and re-probed with primary and secondary antibodies as stated above.
Primary antibodies for pY419 Src and (total) Src were purchased from Cell Signaling Technologies (Danvers, MA) and for pY512 ICAM-1 and (total) ICAM-1 from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell migration assay
NCI-H838 lung cancer cells were suspended at a concentration of 106/ml and were stained with fluorescent dye (Cytoselect®, Cell Biolabs, San Diego, CA). Then, 105 NCI-H838 cells were resuspended in 300 μl RPMI-1640 medium with 0% FBS and no other supplements or with different concentrations of ropivacaine, lidocaine or chloroprocaine (1 pM – 100 μM) and were incubated for 15 minutes at room temperature and placed into the upper chamber of a 6.5 mm Transwell® polycarbonate membrane insert with 8 μm pores (Corning Life Sciences, Lowell, MA). The inserts were then placed into 500 μl complete medium (RPMI-1640, 10 % FBS, 1 % penicillin/streptomycin, 1 % L-glutamine plus the same concentration of local anesthetic as present in the upper chamber) in a 24-well plate. To assess the reversibility of the inhibition of cell migration, cells were washed three times after incubation with ropivacaine before resuspending in 300 μl RPMI-1640 medium and placement into the upper chamber of a Transwell® insert. After 4 hours, all inserts were removed from the well. The remaining fluid in the upper chamber was removed and the inserts were placed into fresh wells filled with cell detachment and lysis solution. A Wallac Victor2® microplate reader (Perkin Elmer, Waltham, MA) was used to detect fluorescence at 485/535 nm (Excitation/Emission).
Statistical analysis
Normal distribution of all data was first assessed with a Shapiro-Wilk test. Normally distributed data (cytotoxicity) are presented as column scatter plots with the mean of each group indicated by a horizontal line. These results were analyzed using three-way ANOVA with the local anesthetics, their concentration, and the presence or absence of TNF-α as factors to be tested. All other data (densitometry, migration), also presented as column scatter plots with a horizontal line indicating the mean, were analyzed using non-parametric testing methods (Kruskal-Wallis and Mann-Whitney U-test). In order to maintain the family wise error rate below 0.05, the result of multiple comparisons (Mann-Whitney U-tests) following a statistically significant Kruskal-Wallis test were evaluated for significance by the Simes-Hochberg method. All tests were performed in a non-blinded and two-tailed manner with the SPSS Statistics Software Version 19 (IBM, Zurich, Switzerland). Graphs were generated using GraphPad Prism for Mac, Version 5 (GraphPad Software, La Jolla, CA). All experiments were performed at least three times. A p-value of <0.05 was considered statistically significant.
Results
Effect of ropivacaine, lidocaine and chloroprocaine on cytotoxicity
First the cytotoxic effect of different local anesthetics was evaluated by measuring the activity of lactate dehydrogenase in cell culture supernatants after incubation of NCI-H838 lung cancer cells with different concentrations (1 nM, 1 μM, 10μM, 100 μM) of ropivacaine, lidocaine, and chloroprocaine in the absence or presence of TNF-α at a concentration of 20 ng/ml for 4 hours. Untreated cells served as control. The overall data analysis was conducted using three-way ANOVA. Cytotoxicity was the dependent factor and the type of local anesthetic, its concentration, and presence or absence of TNF-α the factors to be investigated. The analysis revealed that only the presence of TNF-α significantly increased cytotoxicity (p=<0.001). Neither the application of the different local anesthetics (p=0.264), nor their different concentrations (p=0.919) had any influence on cytotoxicity measures. An analysis of the interaction of the local anesthetics with their different concentrations (p=0.908) or with TNF-α (p=0.358) did not reach statistical significance. The same result could be observed for the combined analysis of TNF-α together with the different concentrations of the local anesthetics (p=0.979) or a combination of all three factors (p=0.992).
Effect of ropivacaine on the phosphorylation status of Src and ICAM-1 in NCI-H838 lung cancer cells
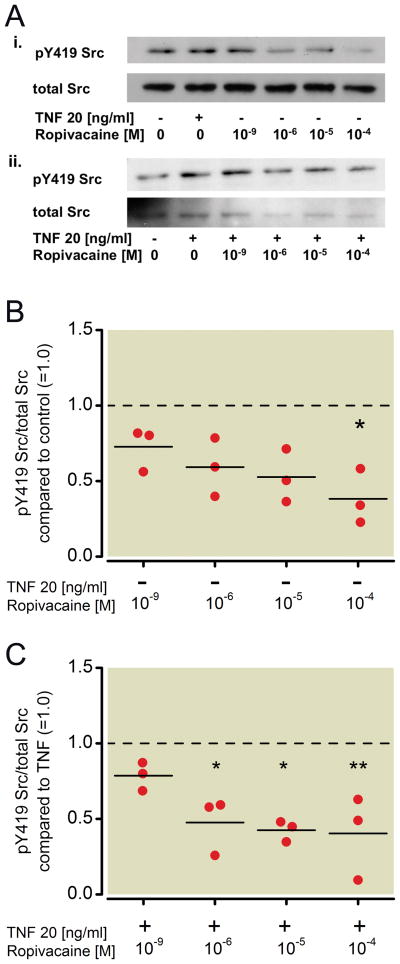
NCI-838 lung cancer cells were first incubated for 20 minutes with TNF-α at a concentration of 20 ng/ml in the presence or absence of different concentrations of ropivacaine (1 nm, 1 μM, 10 μM, 100 μM). Whole cell lysates were analyzed by Western blot analysis, probing for Src, phosphorylated at tyrosine 419 (pY419 Src) and total Src (Figure 1A).
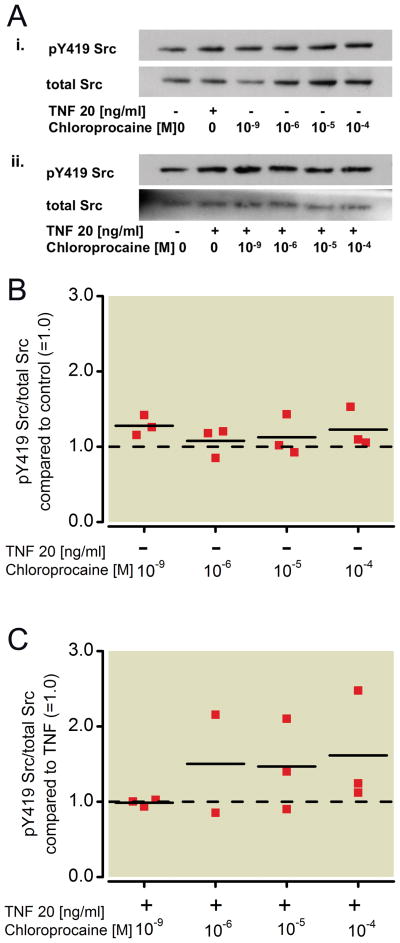
Figure 1. Effect of ropivacaine on the phosphorylation status of Src in NCI-H838 lung cancer cells.
(A) (i) Representative Western blot of NCI-H838 cell Src, phosphorylated at tyrosine 419 (pY419 Src, row 1) and total Src (row 2) after treatment with either TNF-α (20 ng/ml) or with different concentrations of ropivacaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (ii) Representative blot of NCI-H838 lung cancer cell pY419 Src (row 1) and total Src (row 2) after treatment with TNF-α (20 ng/ml) with or without different concentrations of ropivacaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 minutes. (B) Relative density of the Western blot bands of pY419 Src normalized to the densitometry values of total Src compared to control (=1.0, dashed line) in the absence of TNF-α expressed. Data from three independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group; * p < 0.05 vs. control. (C) Relative density of the Western blot bands of pY419 Src normalized to the densitometry values of total Src after coincubation of ropivacaine with TNF-α (20 ng/ml) compared to TNF-α alone (=1.0, dashed line). Data from 3 independent experiments shown as scatter plot (n=3). Horizontal line indicates mean for each group; * p < 0.05 vs. TNF-α, ** p < 0.01 vs. TNF-α. TNF-α = Tumor necrosis factor-α.
Incubation of cells with ropivacaine alone for 20 minutes led to a significant decrease in phosphorylation of Src compared to untreated cells (=control) of 62 % by 100 μM (p=0.003, Figure 1B). Ten μM ropivacaine led to a 48 % decrease, which was not considered statiscally significant (p=0.022, Figure 1B). Co-incubation of TNF-α with different concentrations of ropivacaine showed that ropivacaine also decreased the phosphorylation of Src at tyrosine 419 in a significant manner compared to TNF-α alone even at 1 μM by 52% (p=0.022, Figure 1C). Higher concentrations of ropivacaine led to an even greater attenuation of TNF-α-induced Src phosphorylation (58 % by 10 μM, p=0.006 and 60 % by 100 μM, p=0.017, Figure 1C).
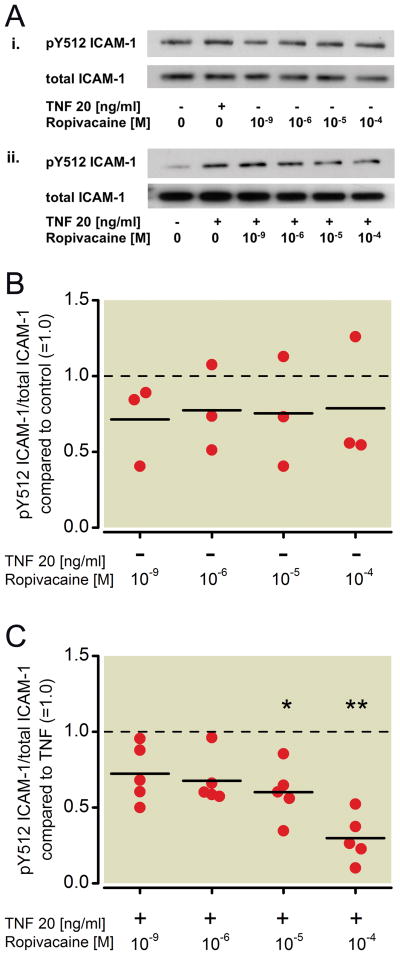
The same whole cell lysates were also analyzed via Western blot probing for ICAM-1, phosphorylated at tyrosine 512 (pY512 ICAM-1) and total ICAM-1 (Figure 2A). Increasing concentrations of ropivacaine did not alter the phosphorylation status of ICAM-1 (Kruskal-Wallis test, p=0.99, Figure 2B), but co-incubation with ropivacaine resulted in a significant attenuation of TNF-α-induced phosphorylation of ICAM-1 at tyrosine 512 compared to treatment with TNF-α alone, with significant inhibition beginning at 10μM (40 % decrease, p=0.01, Figure 2C). A higher dose of ropivacaine further decreased TNF-α-induced ICAM-1 phosphorylation (71 % by 100 μM, p<0.001, Figure 2C). A 32 % decrease by 1 μM was also observed but not considered statistically significant (p=0.031, Figure 2C).
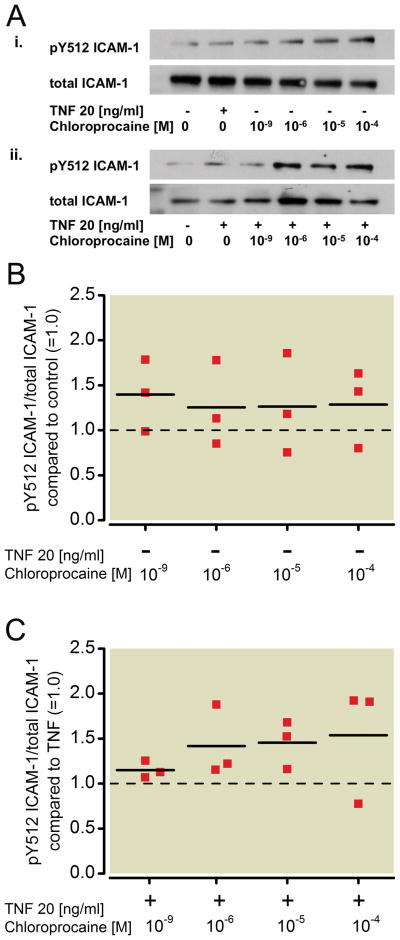
Figure 2. Effect of ropivacaine on the phosphorylation status of ICAM-1 in NCI-H838 lung cancer cells.
(A) (i) Representative Western blot of NCI-H838 lung cancer cell ICAM-1, phosphorylated at tyrosine 512 (pY512 ICAM-1, row 1) and total ICAM-1 (row 2) after treatment with either TNF-α (20 ng/ml) or different concentrations of ropivacaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (ii) Representative blot of NCI-H838 cell pY512 ICAM-1 (row 1) and total ICAM-1 (row 2) after treatment with TNF-α (20 ng/ml) in the presence or absence of different concentrations of ropivacaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (B) Relative density of the Western blot bands of pY512 ICAM-1 normalized to the densitometry values of total ICAM-1 compared to control (=1.0, dashed line) in the absence of TNF-α. Data from 3 independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group. (C) Relative density of the Western blot bands of pY512 ICAM-1 normalized to the densitometry values of total ICAM-1 after coincubation of ropivacaine with TNF-α (20 ng/ml) compared to TNF-α alone (=1.0, dashed line). Data from five independent experiments shown as scatter plot (n = 5). Horizontal line indicates mean for each group; * p < 0.05 vs. TNF-α, ** p < 0.01 vs. TNF-α. TNF-α = Tumor necrosis factor-α.
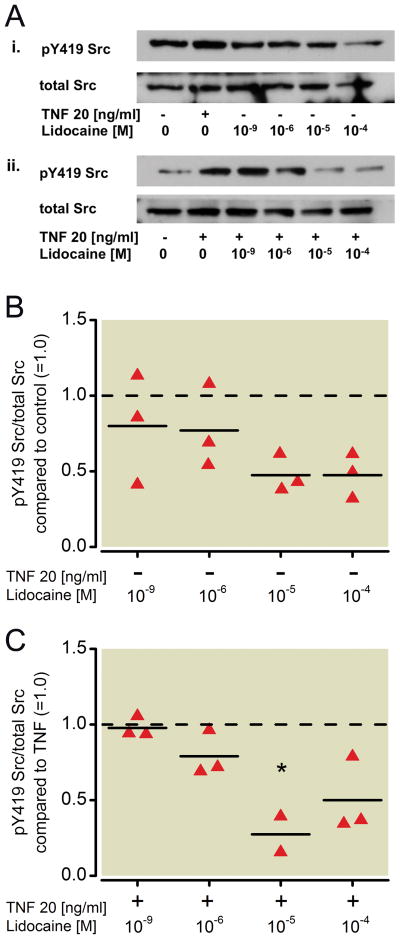
Effect of lidocaine on the phosphorylation status of Src and ICAM-1 in NCI-H838 lung cancer cells
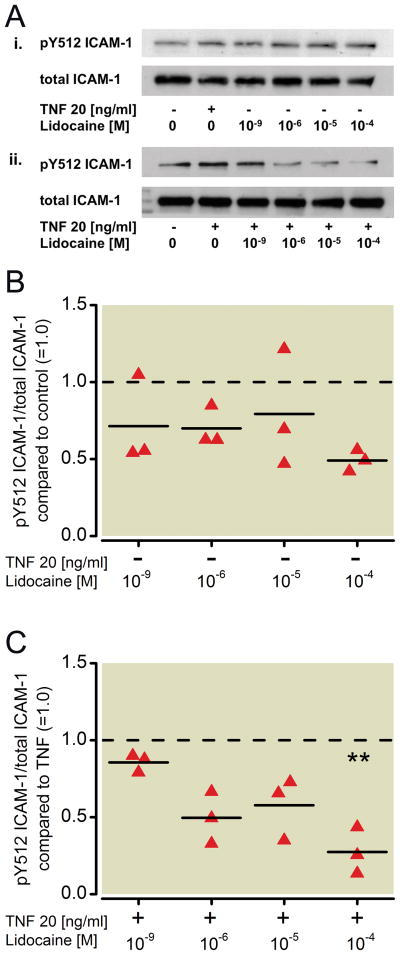
To evaluate the effect of lidocaine on NCI-H838 lung cancer cell Src signaling, cells were treated with increasing concentrations of lidocaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min and again analyzed for Src phosphorylation via Western blot (Figure 3A). Although a dose-dependent decrease in Src phosphorylation at tyrosine 419 was observed after incubation of the cells with lidocaine for 20 minutes, this decrease did not reach statistical significance (Kruskal-Wallis test, p=0.146, Figure 3B). However, a significant decrease in TNF-α-induced Src phosphorylation of 73 % was observed after co-incubation of cells with TNF-α and 10 μM (p=0.012) of lidocaine (Figure 3C). ICAM-1 phosphorylation in the Western blot (Figure 4A) was not affected by lidocaine alone (Kruskal-Wallis test, p=0.624, Figure 4B), but when co-incubated with TNF-α, lidocaine reduced ICAM-1 phosphorylation compared to TNF-α alone in a significant manner (73 % decrease by 100 μM, p=0.002, Figure 4C). An attenuation of 50 % was observed after co-incubation of 1 μM lidocaine with TNF-α, but did not reach statistical significance (p=0.022, Figure 4C).
Figure 3. Effect of lidocaine on the phosphorylation status of Src in NCI-H838 lung cancer cells.
(A) (i) Representative Western blot of NCI-H838 cell Src, phosphorylated at tyrosine 419 (pY419 Src, row 1) and total Src (row 2) after treatment with either TNF-α (20 ng/ml) or with different concentrations of lidocaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (ii) Representative blot of NCI-H838 lung cancer cell pY419 Src (row 1) and total Src (row 2) after treatment with TNF-α (20 ng/ml) with or without different concentrations of lidocaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (B) Relative density of the Western blot bands of pY419 Src normalized to the densitometry values of total Src compared to control (=1.0, dashed line) in the absence of TNF-α. Data from three independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group. (C) Relative density of the Western blot bands of pY419 Src normalized to the densitometry values of total Src after coincubation of lidocaine with TNF-α (20 ng/ml) compared to TNF-α alone (=1.0, dashed line). Data from three independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group; * p < 0.05 vs. TNF-α. TNF-α = Tumor necrosis factor-α.
Figure 4. Effect of lidocaine on the phosphorylation status of ICAM-1 in NCI-H838 lung cancer cells.
(A) (i) Representative Western blot of NCI-H838 lung cancer cell ICAM-1, phosphorylated at tyrosine 512 (pY512 ICAM-1, row 1) and total ICAM-1 (row 2) after treatment with either TNF-α (20 ng/ml) or different concentrations of lidocaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (ii) Representative blot of NCI-H838 cell pY512 ICAM-1 (row 1) and total ICAM-1 (row 2) after treatment with TNF-α (20 ng/ml) in the presence or absence of different concentrations of lidocaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (B) Relative density of the Western blot bands of pY512 ICAM-1 normalized to the densitometry values of total ICAM-1 compared to control (=1.0, dashed line) in the absence of TNF-α. Data from three independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group. (C) Relative density of the Western blot bands of pY512 ICAM-1 normalized to the densitometry values of total ICAM-1 after coincubation of lidocaine with TNF-α (20 ng/ml) compared to TNF-α alone (=1.0, dashed line). Data from three independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group; ** p < 0.01 vs. TNF-α. TNF-α = Tumor necrosis factor-α.
Effect of chloroprocaine on the phosphorylation status of Src and ICAM-1 in NCI-H838 lung cancer cells
To compare the results observed with the amide-linked local anesthetics (ropivacaine, lidocaine) to an ester-linked local anesthetic, NCI-H838 lung cancer cells were treated as above with increasing concentrations of the ester-linked chloroprocaine (1 nm, 1 μM, 10 μM, 100 μM). Analysis of Src phosphorylation (Figure 5A) in H838 cell lysates showed that chloroprocaine had no effect on Src activation, either alone (Kruskal-Wallis test, p=0.305, Figure 5B) or when co-incubated with TNF-α compared to TNF-α alone (p=0.443, Figure 5C). There was also no effect of chloroprocaine on ICAM-1 phosphorylation (Figure 6A) after incubation with chloroprocaine alone compared to control (Figure 6B, Kruskal-Wallis test, p=0.924), and after co-incubation with TNF-α (Figure 6C, p=0.217).
Figure 5. Effect of chloroprocaine on the phosphorylation status of Src in NCI-H838 lung cancer cells.
(A) (i) Representative Western blot of NCI-H838 cell Src, phosphorylated at tyrosine 419 (pY419 Src, row 1) and total Src (row 2) after treatment with either TNF-α (20 ng/ml) or with different concentrations of chloroprocaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (ii) Representative blot of NCI-H838 lung cancer cell pY419 Src (row 1) and total Src (row 2) after treatment with TNF-α (20 ng/ml) with or without different concentrations of chloroprocaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (B) Relative density of the Western blot bands of pY419 Src normalized to the densitometry values of total Src compared to control (=1.0, dashed line) in the absence of TNF-α. Data from three independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group. (C) Relative density of the Western blot bands of pY419 Src normalized to the densitometry values of total Src after coincubation of chloroprocaine with TNF-α (20 ng/ml) compared to TNF-α alone (=1.0, dashed line). Data from three independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group. TNF-α = Tumor necrosis factor-α.
Figure 6. Effect of chloroprocaine on the phosphorylation status of ICAM-1 in NCI-H838 lung cancer cells.
(A) (i) Representative Western blot of NCI-H838 lung cancer cell ICAM-1, phosphorylated at tyrosine 512 (pY512 ICAM-1, row 1) and total ICAM-1 (row 2) after treatment with either TNF-α (20 ng/ml) or different concentrations of chloroprocaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (ii) Representative blot of NCI-H838 cell pY512 ICAM-1 (row 1) and total ICAM-1 (row 2) after treatment with TNF-α (20 ng/ml) in the presence or absence of different concentrations of chloroprocaine (1 nm, 1 μM, 10 μM, 100 μM) for 20 min. (B) Relative density of the Western blot bands of pY512 ICAM-1 normalized to the densitometry values of total ICAM-1 compared to control (=1.0, dashed line) in the absence of TNF-α. Data from 3 independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group. (C) Relative density of the Western blot bands of pY512 ICAM-1 normalized to the densitometry values of total ICAM-1 after co-incubation of lidocaine with TNF-α (20 ng/ml) compared to TNF-α alone (=1.0, dashed line). Data from 3 independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group. TNF-α = Tumor necrosis factor-α.
Effect of sodium channel activator veratridine and sodium-channel blocker tetrodotoxin on phosphorylation status Src and ICAM-1 in NCI-H838 lung cancer cells
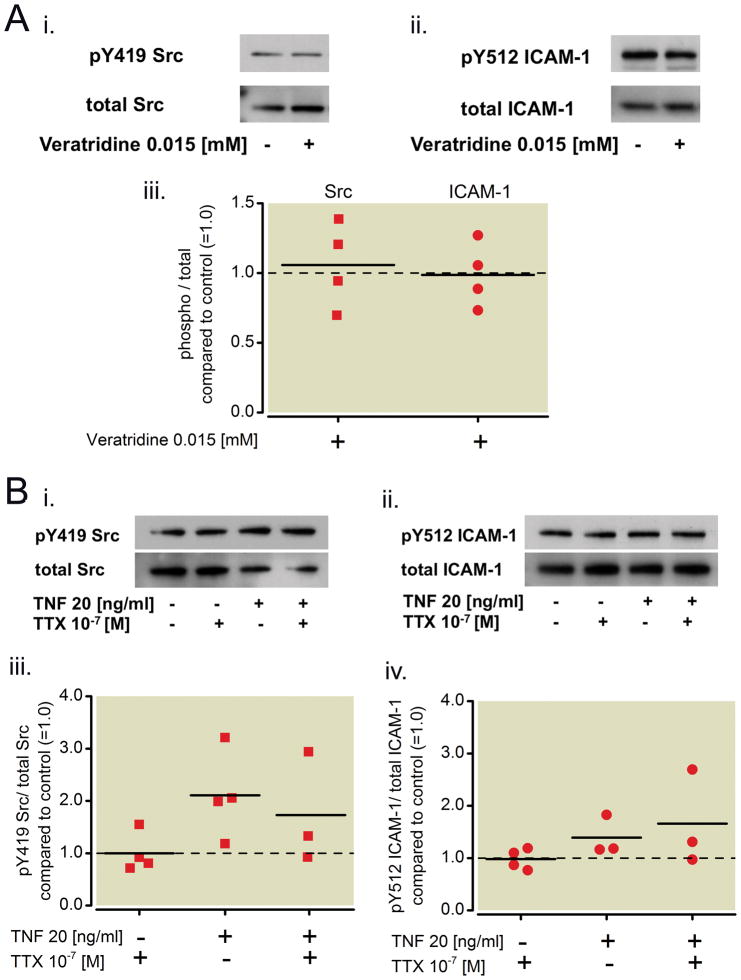
To investigate whether the VGSC plays a role in activation of Src and subsequent phosphorylation of ICAM-1, NCI-H838 lung cancer cells were treated with the alkaloid and VGSC agonist veratridine at a concentration of 0.015 mM for 45 minutes before the cells were harvested, lysed and prepared for Western Blot analysis of pY419 Src and pY512 ICAM-1 as well as the total amounts of each protein (Figure 7Ai and ii). This concentration was chosen, since it was shown that 7.4 μM veratridine abolishes lidocaine-induced membrane depolarization of intestinal cells22. For our experiments, we doubled this concentration. Densitometry analysis revealed that neither Src activation nor ICAM-1 phosphorylation was affected by treatment with veratridine compared to control (Kruskal-Wallis test, p=0.98, Figure 7Aiii).
Figure 7. Effect of a sodium channel activator and blocker on the phosphorylation status of Src and ICAM-1 in NCI-H838 lung cancer cells.
(A) (i) Representative Western blot of NCI-H838 cell Src, phosphorylated at tyrosine 419 (pY419 Src, row 1) and total Src (row 2) after treatment with Veratridine (0.015 mM) for 45 min. (ii) Representative blot of NCI-H838 lung cancer cell pY512 ICAM-1 (row 1) and total Src (row 2) after treatment with Veratridine (0.015 mM) for 45 min. (iii) Relative density of the Western blot bands of pY419 Src normalized to total Src (squares) and pY512 ICAM-1 normalized to total ICAM-1 (circles) compared to control (=1.0, dashed line) after treatment with Veratridine (0.015 mM) for 45 min. Data from four independent experiments shown as scatter plot (n = 4). Horizontal line indicates mean for each group. (B) (i) Representative Western blot of NCI-H838 cell Src, phosphorylated at tyrosine 419 (pY419 Src, row 1) and total Src (row 2) after pretreatment with tetrodotoxin (TTX, 100 nM) for 10 minutes and subsequent addition of TNF-α (20 ng/ml) for 20 min. (ii) Representative Western blot of NCI-H838 cell ICAM-1, phosphorylated at tyrosine 512 (pY512 ICAM-1, row 1) and total ICAM-1 (row 2) after pretreatment with TTX (100 nM) for 10 min and subsequent addition of TNF-α (20 ng/ml) for 20 min. (iii) Relative density of the Western blot bands of pY419 Src normalized to the densitometry values of total Src compared to control (=1.0, dashed line) after pre-treatment with tetrodotoxin (TTX, 100 nM) for 10 min and subsequent addition of TNF-α (20 ng/ml) for 20 minutes. Data from four independent experiments shown as scatter plot (n = 4). Horizontal line indicates mean for each group. (iv) Relative density of the Western blot bands of pY512 ICAM-1 normalized to the densitometry values of total ICAM-1 after pretreatment with tetrodotoxin (TTX, 100 nM) for 10 min and subsequent addition of TNF-α (20 ng/ml) for 20 min compared to control (=1.0, dashed line). Data from three independent experiments shown as scatter plot (n = 3). Horizontal line indicates mean for each group. TNF-α = Tumor necrosis factor-α.
The effect of tetrodotoxin, a non-local anesthetic VGSC antagonist, was investigated. Tetrodotoxin at a concentration of 100 nM (10−7 M) was used, as this concentration is known to inhibit all VGSCs in excitable membranes23. NCI-H838 lung cancer cells were pre-treated with tetrodotoxin for 10 minutes before TNF-α (20 ng/ml) was added. Cells were lysed after 20 minutes. Representative Western blots of phosphorylated Src and ICAM-1 as well as the total amount of the proteins are shown in Figure 7Bi and ii. Densitometry analysis r evealed no difference in Src and ICAM-1 phosphorylation after pre-treatment with tetrodotoxin and subsequent addition of TNF-α compared to TNF-α alone (Mann-Whitney U-test, p=0.48 for Src and p=0.827 for ICAM-1, Figure 7Biii and B iv).
Migratory ability of NCI-H838 lung cancer cells in the presence of ropivacaine, lidocaine and chloroprocaine
NCI-H838 cells, stained with a fluorescent dye, were allowed to migrate through an 8 μm pore polycarbonate membrane for 4 hours in the presence or absence of different concentrations of ropivacaine, lidocaine or chloroprocaine (1 pM, 1nM, 1 μM, 10 μM, 100 μM). Migrated cells were detached from the bottom of the membrane, and lysed. Fluorescence was measured at 485/535 nm (excitation/emission) (Figure 8).
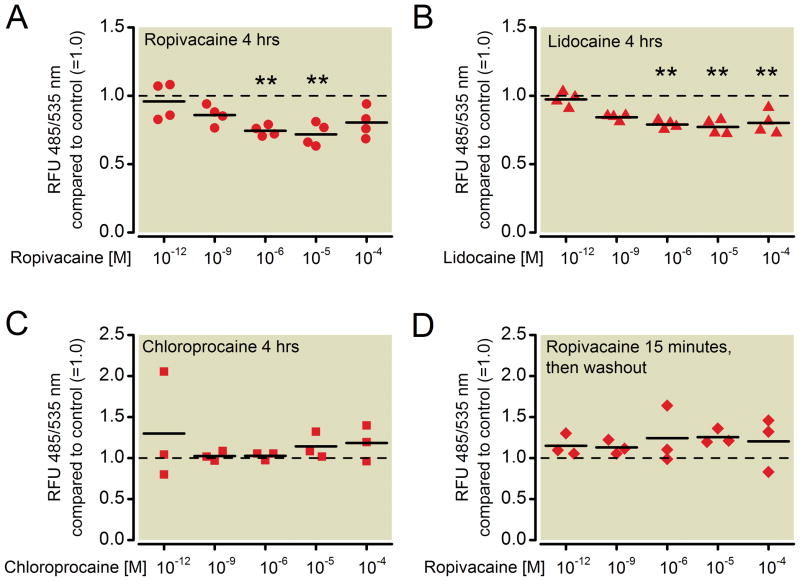
Figure 8. Migratory ability of NCI-H838 lung cancer cells in the presence of ropivacaine, lidocaine and chloroprocaine.
Determination of in vitro transmigration of NCI-H838 cells through a polycarbonate membrane after 4 h in the absence or presence of different concentrations (1 pm, 1 nM, 1 μM, 10 μM, 100 μM) of (A) ropivacaine, (B) lidocaine and (C) chloroprocaine. (D) Determination of in vitro transmigration of NCI-H838 cells through a polycarbonate membrane after 4 h in the absence or presence of different concentrations (1 pm, 1 nM, 1 μM, 10 μM, 100 μM) of ropivacaine for only 15 min followed by a wash of the cells with fresh medium right before the start of the assay. Cells were stained with a fluorescent dye before the start of the assay and lysed after migration through the membrane. Fluorescence was measured at 435/535 nm (excitation/emission). Values for untreated cells have been set as 1.0 (dashed line). Data from 4 (A and B) or 3 (C and D) independent experiments shown as scatter plot. Horizontal line indicates mean for each group; ** p < 0.01 vs. control, RFU = relative fluorescence units.
Compared to untreated cells (=control), ropivacaine induced a significant decrease in fluorescence – and therefore the number of cells migrated – by 26% (p=0.005) at 1 μM concentration. A higher concentrations decreased fluorescence by 28% (10 μM, p=0.003, Figure 8A). This effect was completely abolished by removal of ropivacaine after the initial 15 minutes of incubation (Kruskal-Wallis test, p=0.447, Figure 8D).
Treatment with lidocaine decreased fluorescence after 4 hours of migration (Kruskal-Wallis test, p=0.004). 1 μM of lidocaine attenuated migration by 21% compared to control (p=0.004), whereas 10μM and 100μM led to a decrease of 23% (p=0.002) and 20% (p=0.009), respectively (Figure 8B). In contrast, incubation with different concentrations of chloroprocaine had no effect on fluorescence measured after 4 hours (Kruskal-Wallis test, p=0.718, Figure 8C).
Discussion
The results of this study indicate that the amide-linked local anesthetics ropivacaine and lidocaine inhibit Src (auto)phosphorylation at tyrosine 419 induced by inflammatory stimuli such as TNF-α, and that ropivacaine inhibited Src phosphorylation (activity) even in the absence of inflammatory stimuli. In contrast, the ester-linked local anesthetic chloroprocaine had no such effect. In addition, we observed decreased Src-mediated phosphorylation of ICAM-1 at tyrosine 512 in presence of ropivacaine and lidocaine in NCI-H838 lung cancer cells treated with TNF-α and lipopolysaccharide). Moreover, ropivacaine and lidocaine were shown to inhibit the migration of H838 cancer cells.
Interestingly, the inhibitory effect on either the phosphorylation of Src and ICAM-1 or migration of lung cancer cells was observed with amide local anesthetics (ropivacaine and lidocaine), but not with ester local anesthetics (Chloroprocaine). Previous studies also demonstrated a selective protective effect of amide local anesthetics as compared to the ester-linked compounds. De Klaver et al demonstrated that lidocaine, ropivacaine and bupivacaine protected endothelial cells against lipopolysaccharide -induced cellular injury, whereas procaine and tetracaine did not24.
Recently, Src tyrosine protein kinase has become a key research focus of cancer development, growth and metastasis. Src is involved in signaling epithelial-to-mesenchymal transformation, e. g. via loss of E-cadherin and subsequently decreased cell-cell-adhesion16,17,25 necessary for solid tumor metastasis18. Src is activated either by (auto)phosphorylation at tyrosine 419 and/or by dephosphorylation at tyrosine 52926. This study showed that ropivacaine dose-dependently decreased Src activation in NCI-H838 lung cancer cells, even in the absence of an inflammatory stimulus (TNF-α or lipopolysaccharide), although stimulation was necessary to observe an effect of ropivacaine on Src-dependent ICAM-1 phosphorylation at tyrosine 512. This indicates that Src might be the primary target of ropivacaine and that phosphorylation of ICAM-1 is attenuated secondary to the inhibition of Src activity, as ICAM-1 was previously shown to be a Src substrate15.
Local anesthetics are known to block the VGSC27. The inhibitory effect of ropivacaine and lidocaine on Src activation and ICAM-1 phosphorylation, as observed in this study, is independent from the inhibition of the VGSC. No change in the phosphorylation status of the two proteins after incubation with the sodium channel agonist veratridine was observed. Neither Src nor ICAM-1 phosphorylation induced by TNF-α was altered by pretreatment with the sodium channel inhibitor tetrodotoxin. These results are in accordance with those of another study that demonstrated VGSC-independent inhibition of TNF-α-induced epithelial chemokine secretion28. However, there is some evidence that VGSCs linked with Fyn, another member of the Src protein tyrosine kinase family, might play a role in the migration of neuronal cells and metastasis29. Also, an inhibition of VGSCs in other human non-small-cell lung cancer cell lines led to a reduction of in vitro invasion of these malignant cells30.
The identification of Src as a key enzyme in tumor growth and metastasis has led to the development of several “targeted therapies”, such as Src-inhibitors or combined Bcr/Abl and Src-inhibitors (e. g. Dasatinib®, Bristol-Myers Squibb, New York, NY)31. Most of these compounds act as adenosine triphosphate-competitive inhibitors at the adenosine triphosphate-binding pocket of Src32. It would therefore be interesting to determine in future studies how ropivacaine inhibits Src activity, assessing for example whether it competes with adenosine triphosphate for binding within the Src kinase domain33.
ICAM-1, a member of the immunoglobulin superfamily of genes that play key roles in the binding of leukocytes to the vascular endothelium, is also involved in growth and metastasis of cancer. TNF-α stimulation of NCI-H838 cells (those used in the current study) for 16 hours increased ICAM-1 expression12. Elevated expression of ICAM-1 could be linked to a more aggressive tumor phenotype34,35 and to enhanced leukocyte infiltration and binding to tumor cells36. Additionally, it was shown that A549 lung cancer cells overexpressing ICAM-1 exhibited enhanced in vitro cell migration and in vivo metastasis, an effect that could be inhibited by an anti-ICAM-1 antibody35.
This study focused on the phosphorylation of ICAM-1, which is necessary for rapid TNF-α-induced clustering of ICAM-1 resulting in enhanced neutrophil binding15. The fact that ropivacaine and lidocaine inhibited this phosphorylation might be beneficial in the setting of metastasis. They might also attenuate the binding of circulating cancer cells to the vascular endothelium and therefore reduce transmigration and metastasis.
The well-established cytotoxic effect of local anesthetics, e.g. on neuronal cells37, is not considered in the interpretation of our results. The concentrations of ropivacaine, lidocaine and chloroprocaine used in this study showed that they did not induce cytotoxic effects after 4 hours of treatment. Additionally, an alteration in TNF-α-induced cytotoxicity in our lung cancer cell line was not observed.
Another finding was the inhibition of tumor cell migration by ropivacaine and lidocaine at 1 μM. Src is known to play a key role in cell migration, which is necessary for cancer cells to metastasize18. It regulates cytoskeletal changes required for cell migration by phosphorylating proteins associated with focal adhesions and actin bundling which control cell membrane protrusions38,39. Src is also an upstream regulator of Rho family GTPases such as Rac and Rho which together regulate dynamic changes in the cytoskeleton and control the disassembly of actin-based cytoskeletal structures and cell-matrix adhesions17. We therefore hypothesize that the inhibition of tumor cell migration by amide local anesthetics is due to the inhibition of Src. The fact that the observed effect of inhibition of migration could be abolished by washout of the local anesthetic after 15 minutes (as shown for ropivacaine) also indicates first that the observed effect is not due to a cytotoxic effect of the local anesthetic and second, that this effect is reversible.
It is well known that hematogenous dissemination of cancer cells occurs during the surgical resection of a tumor40. Circulating tumor cells, detected in the blood 24 hours post-operatively, are an independent prognostic marker for cancer recurrence because of the ability of these cells to extravasate and metastasize41. In accordance with the results of our study, we suggest that the perioperative administration of local anesthetics may have the potential added benefit of attenuating extravasation and metastasis of circulating tumor cells prior to initiation of systemic treatment with cytotoxic agents.
Thus far, the potential beneficial effect of regional anesthesia to improve long-term outcome after cancer surgery has been attributed to inhibition of the surgical stress response and to the decrease in opioid requirements. It has been shown, that opioids even at clinically useful concentrations might promote migration and proliferation of tumor cells, e. g. in breast cancer42,43. This work reveals to the best of our knowledge a possible mechanism by which local anesthetics might be beneficial in patients with cancer and/or undergoing cancer surgery and that this mechanism may be due, at least in part, to inhibition of Src tyrosine kinase, a key enzyme in cancer growth and metastasis. Future studies will focus on establishing the molecular mechanism of Src inhibition and the influence of this inhibition on ICAM-1 phosphorylation and function with comparison to established Src inhibitors.
What we already know about this topic
Regional anesthesia is associated in some clinical studies with reduced risk of metastasis or late mortality in patients undergoing cancer surgery
Local anesthetics have antiinflammatory effects which may participate in a reduced risk of metastasis, but their mechanisms are unknown
What this article tells us that is new
In cellular studies in vitro with lung cancer cells, amide-but not ester-local anesthetics reduced tumorcell migration and reduced signaling pathways important to tumor growth and metastases
Regional anesthesia might reduce cancer metastasis by direct effects of absorbed amide local anesthetics on tumor cells
Acknowledgments
Support was provided from institutional sources, National Institutes of Health (Bethesda, MD) grants HL60678 and HL71626, The lung Zurich, Switzerland and the Swiss Society of Anesthesiology and Resuscitation, Bern, Switzerland.
Footnotes
Sent from the Division of Anesthesiology, Balgrist University Hospital Zurich, Switzerland
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Izbicki JR, Passlick B, Pantel K, Pichlmeier U, Hosch SB, Karg O, Thetter O. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: Results of a prospective randomized trial. Ann Surg. 1998;227:138–44. doi: 10.1097/00000658-199801000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen ZL, Perez S, Holmes EC, Wang HJ, Coulson WF, Wen DR, Cochran AJ. Frequency and distribution of occult micrometastases in lymph nodes of patients with non-small-cell lung carcinoma. J Natl Cancer Inst. 1993;85:493–8. doi: 10.1093/jnci/85.6.493. [DOI] [PubMed] [Google Scholar]
- 4.Kim AW. Lymph node drainage patterns and micrometastasis in lung cancer. Semin Thorac Cardiovasc Surg. 2009;21:298–308. doi: 10.1053/j.semtcvs.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Senturk M. New concepts of the management of one-lung ventilation. Curr Opin Anesthesiol. 2006;19:1–4. doi: 10.1097/01.aco.0000192778.17151.2c. [DOI] [PubMed] [Google Scholar]
- 6.Hollmann MW, Gross A, Jelacin N, Durieux ME. Local anesthetic effects on priming and activation of human neutrophils. Anesthesiology. 2001;95:113–22. doi: 10.1097/00000542-200107000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal S, Borgeat A, Pasch T, Reyes L, Booy C, Lambert M, Schimmer RC, Beck-Schimmer B. Ropivacaine decreases inflammation in experimental endotoxin-induced lung injury. Anesthesiology. 2006;104:961–9. doi: 10.1097/00000542-200605000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Herroeder S, Pecher S, Schonherr ME, Kaulitz G, Hahnenkamp K, Friess H, Bottiger BW, Bauer H, Dijkgraaf MG, Durieux ME, Hollmann MW. Systemic lidocaine shortens length of hospital stay after colorectal surgery: A double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007;246:192–200. doi: 10.1097/SLA.0b013e31805dac11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staudt LM. Oncogenic activation of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102:e120–31. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roland CL, Harken AH, Sarr MG, Barnett CC., Jr ICAM-1 expression determines malignant potential of cancer. Surgery. 2007;141:705–7. doi: 10.1016/j.surg.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Melis M, Spatafora M, Melodia A, Pace E, Gjomarkaj M, Merendino AM, Bonsignore G. ICAM-1 expression by lung cancer cell lines: Effects of upregulation by cytokines on the interaction with LAK cells. Eur Respir J. 1996;9:1831–8. doi: 10.1183/09031936.96.09091831. [DOI] [PubMed] [Google Scholar]
- 13.Wu QD, Wang JH, Condron C, Bouchier-Hayes D, Redmond HP. Human neutrophils facilitate tumor cell transendothelial migration. AmJ Physiol Cell Physiol. 2001;280:C814–22. doi: 10.1152/ajpcell.2001.280.4.C814. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Minshall RD. Regulation of transendothelial permeability by Src kinase. Microvasc Res. 2009;77:21–5. doi: 10.1016/j.mvr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, Vogel SM, Gao X, Javaid K, Hu G, Danilov SM, Malik AB, Minshall RD. Src phosphorylation of endothelial cell surface intercellular adhesion molecule-1 mediates neutrophil adhesion and contributes to the mechanism of lung inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1342–50. doi: 10.1161/ATVBAHA.110.222208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MP, Park SI, Kopetz S, Gallick GE. Src family kinases as mediators of endothelial permeability: effects on inflammation and metastasis. Cell Tissue Res. 2009;335:249–59. doi: 10.1007/s00441-008-0682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 18.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 19.Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: A variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–32. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 20.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology. 2008;109:180–7. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 21.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barshack I, Levite M, Lang A, Fudim E, Picard O, Ben Horin S, Chowers Y. Functional voltage-gated sodium channels are expressed in human intestinal epithelial cells. Digestion. 2008;77:108–17. doi: 10.1159/000123840. [DOI] [PubMed] [Google Scholar]
- 23.Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- 24.de Klaver MJ, Weingart GS, Obrig TG, Rich GF. Local anesthetic-induced protection against lipopolysaccharide-induced injury in endothelial cells: The role of mitochondrial adenosine triphosphate-sensitive potassium channels. Anesth Analg. 2006;102:1108–13. doi: 10.1213/01.ane.0000200310.39031.1f. [DOI] [PubMed] [Google Scholar]
- 25.Aleshin A, Finn RS. SRC: A century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 27.Owen MD, Dean LS. Ropivacaine. Expert Opin Pharmacother. 2000;1:325–36. doi: 10.1517/14656566.1.2.325. [DOI] [PubMed] [Google Scholar]
- 28.Lang A, Ben Horin S, Picard O, Fudim E, Amariglio N, Chowers Y. Lidocaine inhibits epithelial chemokine secretion via inhibition of nuclear factor kappa B activation. Immunobiology. 2010;215:304–13. doi: 10.1016/j.imbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Brackenbury WJ, Djamgoz MB, Isom LL. An emerging role for voltage-gated Na+ channels in cellular migration: Regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist. 2008;14:571–83. doi: 10.1177/1073858408320293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roger S, Rollin J, Barascu A, Besson P, Raynal PI, Iochmann S, Lei M, Bougnoux P, Gruel Y, Le Guennec JY. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol. 2007;39:774–86. doi: 10.1016/j.biocel.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, Pang S, Shen DR, Fang Q, de Fex HF, McIntyre KW, Shuster DJ, Gillooly KM, Behnia K, Schieven GL, Wityak J, Barrish JC. 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem. 2006;49:6819–32. doi: 10.1021/jm060727j. [DOI] [PubMed] [Google Scholar]
- 32.Schenone S, Bruno O, Radi M, Botta M. New insights into small-molecule inhibitors of Bcr-Abl. Med Res Rev. 2011;31:1–41. doi: 10.1002/med.20175. [DOI] [PubMed] [Google Scholar]
- 33.Breitenlechner CB, Kairies NA, Honold K, Scheiblich S, Koll H, Greiter E, Koch S, Schafer W, Huber R, Engh RA. Crystal structures of active SRC kinase domain complexes. J Mol Biol. 2005;353:222–31. doi: 10.1016/j.jmb.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Schroder C, Witzel I, Muller V, Krenkel S, Wirtz RM, Janicke F, Schumacher U, Milde-Langosch K. Prognostic value of intercellular adhesion molecule (ICAM)-1 expression in breast cancer. J Cancer Res Clin Oncol. 2011;137:1193–201. doi: 10.1007/s00432-011-0984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin YC, Shun CT, Wu MS, Chen CC. A novel anticancer effect of thalidomide: Inhibition of intercellular adhesion molecule-1-mediated cell invasion and metastasis through suppression of nuclear factor-kappaB. Clin Cancer Res. 2006;12:7165–73. doi: 10.1158/1078-0432.CCR-06-1393. [DOI] [PubMed] [Google Scholar]
- 36.Roland CL, Dineen SP, Toombs JE, Carbon JG, Smith CW, Brekken RA, Barnett CC., Jr Tumor-derived intercellular adhesion molecule-1 mediates tumor-associated leukocyte infiltration in orthotopic pancreatic xenografts. Ex Biol Med (Maywood) 2010;235:263–70. doi: 10.1258/ebm.2009.009215. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Castro R, Patel S, Garavito-Aguilar ZV, Rosenberg A, Recio-Pinto E, Zhang J, Blanck TJ, Xu F. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997–1007. doi: 10.1213/ane.0b013e31819385e1. [DOI] [PubMed] [Google Scholar]
- 38.Horwitz AR, Parsons JT. Cell migration--movin’ on. Science. 1999;286:1102–3. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- 39.Yeatman TJ. A renaissance for SRC. Nature Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 40.Ge MJ, Shi D, Wu QC, Wang M, Li LB. Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in peroperative period. J Cancer Res Clin Oncol. 2006;132:248–56. doi: 10.1007/s00432-005-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peach G, Kim C, Zacharakis E, Purkayastha S, Ziprin P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: A systematic review. Br J Cancer. 2010;102:1327–34. doi: 10.1038/sj.bjc.6605651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8. [PubMed] [Google Scholar]
- 43.Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: Role of receptor transactivation. Microvascular Res. 2006;72:3–11. doi: 10.1016/j.mvr.2006.04.004. [DOI] [PubMed] [Google Scholar]