Abstract
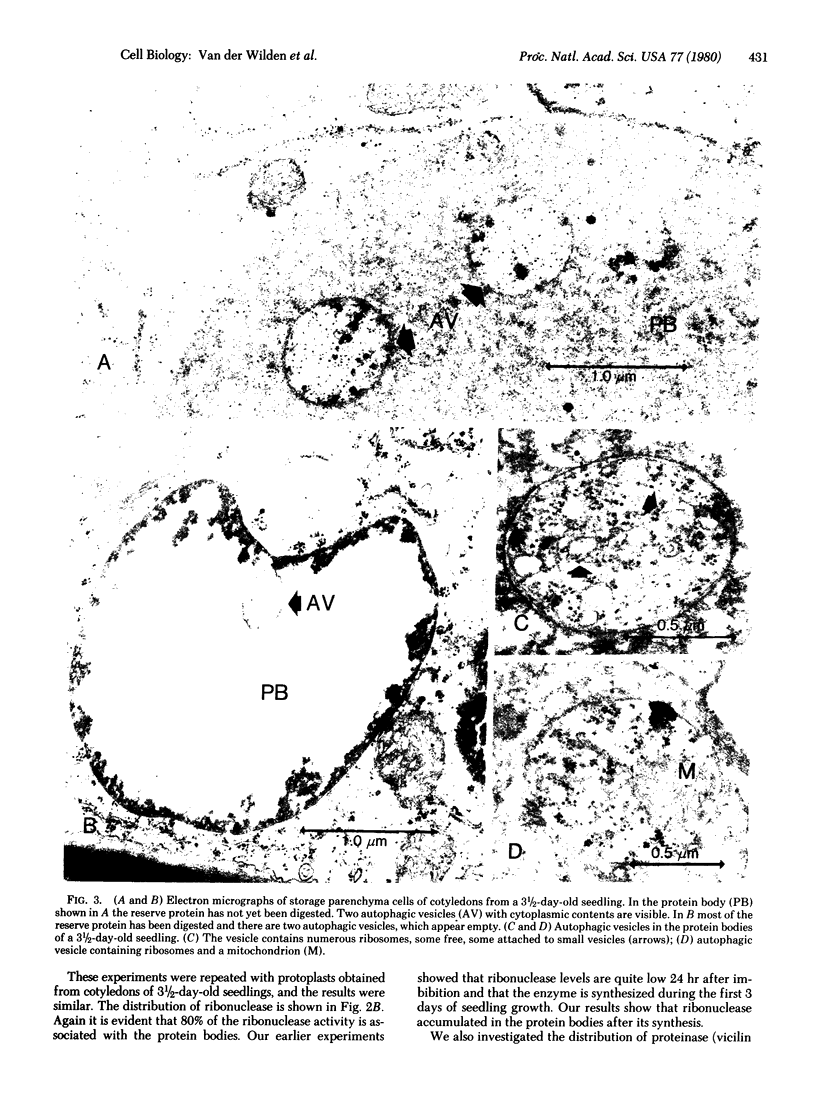
We present evidence that protein bodies constitute the principal lytic compartment in storage parenchyma cells of mung bean cotyledons and propose that they play a role in cellular autophagy. We developed a method to isolate protein bodies by incubating tissue slices with cell wall-degrading enzymes and fractionating the cellular organelles on a Ficoll gradient. About 75-80% of the protein bodies present in the protoplasts were recovered intact in a band at the 5/25% Ficoll interface. This band contained a similar proportion of the cellular α-mannosidase, N-acetyl-β-glucosaminidase, ribonuclease, acid phosphatase, phosphodiesterase, and phospholipase D. β-Amylase was present in the cells but not in the protein bodies. Ultrastructural observations showed that on the 3rd day of seedling growth protein bodies contain small vesicles (0.3-1.0 μm) with a cytoplasmic content (ribosomes, membrane vesicles, mitochondria). Later in seedling growth these vesicles appeared empty. We believe that these are autophagic vesicles resulting from invaginations of the protein body membrane and that their cytoplasmic contents are digested by the acid hydrolases present in the protein bodies.
Keywords: acid hydrolase, autophagy, lysosome
Full text
PDF
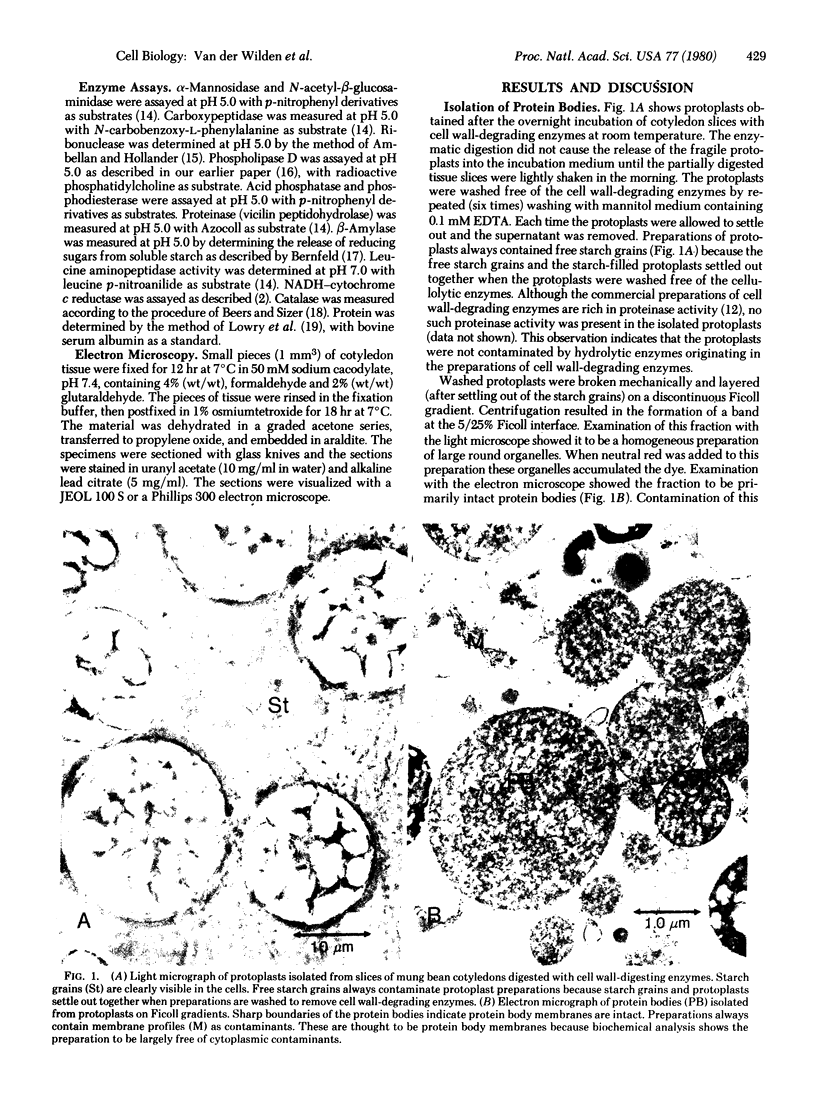
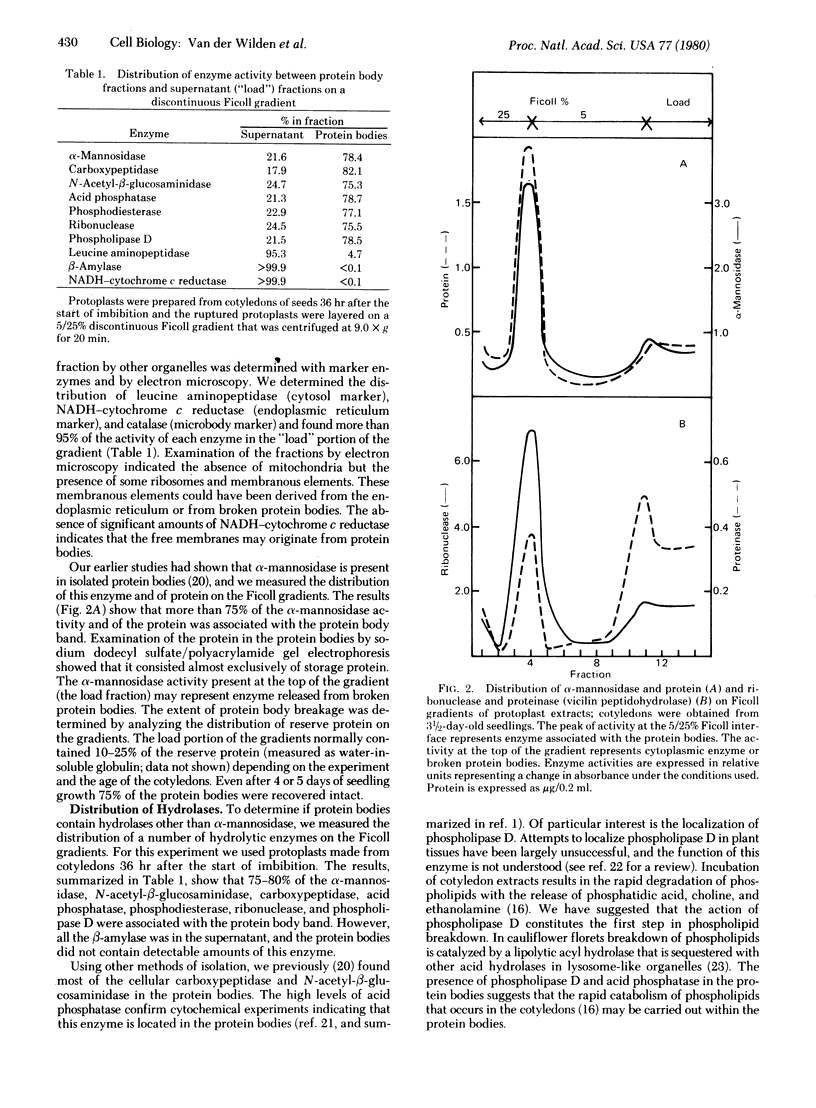

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambellan E., Hollander V. P. A simplified assay for RNase activity in crude tissue extracts. Anal Biochem. 1966 Dec;17(3):474–484. doi: 10.1016/0003-2697(66)90182-5. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Baumgartner B., Tokuyasu K. T., Chrispeels M. J. Localization of vicilin peptidohydrolase in the cotyledons of mung bean seedlings by immunofluorescence microscopy. J Cell Biol. 1978 Oct;79(1):10–19. doi: 10.1083/jcb.79.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher H. C., Wagner G. J., Siegelman H. W. Localization of Acid hydrolases in protoplasts: examination of the proposed lysosomal function of the mature vacuole. Plant Physiol. 1977 Jun;59(6):1098–1103. doi: 10.1104/pp.59.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Baumgartner B., Harris N. Regulation of reserve protein metabolism in the cotyledons of mung bean seedlings. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3168–3172. doi: 10.1073/pnas.73.9.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes N. R., Herman E. M., Chrispeels M. J. Rapid degradation and limited synthesis of phospholipids in the cotyledons of mung bean seedlings. Plant Physiol. 1979 Jul;64(1):38–42. doi: 10.1104/pp.64.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N., Chrispeels M. J. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975 Aug;56(2):292–299. doi: 10.1104/pp.56.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M. Phospholipase D. Adv Lipid Res. 1978;16:267–326. doi: 10.1016/b978-0-12-024916-9.50011-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Hydrolases in vacuoles from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]