Abstract
Bone marrow-derived mesenchymal stem cells (BM-MSC) can be differentiated into lung epithelial-like cells (MSC-EC) in vitro. The response of BM-MSC and MSC-EC to stimuli may vary because of their character and differentiation. We aimed to investigate the factors that may influence in vitro differentiation of BM-MSC to MSC-EC. We determined the response of BM-MSC, MSC-EC, bronchial epithelial cells, and alveolar epithelial cells to tumor necrosis factor (TNF)-α stimulation. We also investigated the changes in micro(mi)RNA-146a, miRNA-155, and TNF receptor 1 (TNFR1) expression after stimulation. Our results demonstrate that the addition of transforming growth factor-β1 and extracellular matrix collagen are required to facilitate such differentiation. After 3 weeks of culture, the morphological appearance and expression of airway epithelial markers, cytokeratin and Clara cell secretory protein, in MSC-EC were characteristics of lung epithelial cells. In response to TNF-α stimulation, the maximal interleukin (IL)-8 production by BM-MSC at the 24-h time point was 4.8 times greater compared with MSC-EC. TNF-α induced a significant increase in the expression of miRNA-146a in BM-MSC as compared with MSC-EC. miRNA-155 expression remained unchanged after stimulation. TNFR1 mRNA also significantly increased in BM-MSC after TNF-α stimulation. This was not observed in MSC-EC. Transfection with miRNA-146a mimics resulted in a significant increase of miRNA-146a expression and IL-8 production in both types of cells. In contrast, miRNA-146a inhibitors reduced miRNA-146a expression and IL-8 production. Overexpression of miRNA-146a, which positively regulates TNF-α-induced IL-8 release, may enhance the inflammatory response in both BM-MSC and MSC-EC. The expression of miRNA-146a and the response to stimuli may be modulated through mature differentiation of BM-MSC.
Introduction
Mesenchymal stem cells (MSC) in bone marrow are able to differentiate into osteoblasts, chondroblasts, adipocytes, and hepatocytes.1,2 Recent evidence has suggested the possibility that human embryonic stem cells and umbilical cord blood-derived stem cells can differentiate into alveolar type II cells.3–5 Such findings suggest a possible therapeutic role for stem cells in the treatment of several acute and chronic lung diseases, such as acute lung injury, emphysema, and pulmonary fibrosis.6
MicroRNAs (miRNAs) are single-stranded RNA molecules 21–23 nucleotides long that mediate RNA interference and are involved in the regulation of gene expression at the translational level.7 Increased expression of miRNAs has been demonstrated in myeloid cells activated through the Toll-like receptor 2, 4, or 5 by bacterial and fungal components or following exposure to tumor necrosis factor (TNF)-α or interleukin (IL)-1β.8–10 It is of interest that the miRNA-146a expression may negatively regulate inflammation during the innate immune response, especially in lung alveolar epithelial cells (AEC). IL-1β has been found to induce a time- and concentration-dependent increase in miRNA-146a, which negatively regulates the release of IL-8 and RANTES.11 In contrast, in human airway smooth muscle cells, IL-1β induced a dramatic increase in miRNA-146a expression, which was correlated with the release of IL-6 and IL-8.12 On the other hand, miRNA-155 is upregulated in macrophages in response to lipopolysaccharides and enhances TNF-α production.10 The miRNA-155 expression level is correlated with the degree of lung fibrosis.13 Knockdown of miRNA-155 suppresses transforming growth factor (TGF)-β-induced epithelial–mesenchymal transition, tight junction dissolution, cell migration, and invasion.14 The functions and mechanisms of miRNA vary in ways that may be dependent on the cell type.
TNF-α is a multifunctional cytokine that plays an active and key role in cell survival, apoptosis, immunity, and inflammation. The major cells producing TNF-α are activated macrophages, T-lymphocytes, and natural killer cells. TNF-α acts via two distinct receptors, TNF receptor 1 (TNFR1) and receptor 2 (TNFR2). TNFR1 is expressed in all cell types, while TNFR2 expression is mainly confined to immune cells.15 TNFR1 can be upregulated by IL-1β stimulation in airway epithelial cells, smooth muscle cells,16 or endothelial cells.17
Although bone-marrow-derived MSC (BM-MSC) can differentiate into lung epithelial cells, little is known about factors that influence such differentiation. The response to stimuli of BM-MSC, differentiated lung epithelial-like cells (MSC-EC) from BM-MSC, and primary lung epithelial cells may vary because of their differing character and maturity. In this study, we investigated factors that may influence in vitro differentiation of BM-MSC to lung epithelial cells. We determined the response to TNF-α stimulation of BM-MSC, MSC-EC, primary bronchial epithelial cells (PBEC), and AEC. We also investigated changes in miRNA-146a and miRNA-155 expression following TNF-α stimulation. We confirm that human BM-MSC can be differentiated into MSC-EC, a process influenced by TGF- β1 and collagen (as an extracellular matrix). TNF-α-induced IL-8 release was much higher in BM-MSC as compared with that in MSC-EC, PBEC, or AEC. An increase in TNFR1 mRNA was observed in BM-MSC following TNF-α stimulation, but did not occur in MSC-EC. The level of miRNA-146a after TNF-α stimulation differed in BM-MSC from that in other cell types. miRNA-146a was upregulated and was positively associated with IL-8 production in BM-MSC. The role of miRNA-146a in airway inflammation may vary and could be dependent on the cell type.
Materials and Methods
Isolation and characterization of BM-MSC
BM-MSC were isolated by negative immunodepletion of CD3, CD14, CD19, CD38, CD66b, and glycophorin-A-positive cells (RosetteSep; StemCell Technologies) using a commercially available kit, according to the manufacturer's instructions, followed by Ficoll-Paque density-gradient centrifugation (Amersham Biosciences), as previously described.1 Osteogenesis, chondrogenesis, and adipogenesis were assessed by alkaline phosphatase and von Kossa stainings, type II collagen staining, and Oil Red staining, respectively.
Modified air–liquid interface culture for PBEC
Human bronchus, obtained from surgical lobectomy for lung cancer, was used for bronchial epithelial cell culture. The standard procedures and specific culture medium for PBEC are described in the Supplementary Data (Supplementary Data available online at www.liebertpub.com/tea).
Enzymatic dissociation of AEC from lung parenchyma
Lung parenchyma was used for AEC culture. The standard procedures are available in the Supplementary Data.
In vitro differentiation
Fifth-to-seventh-passage BM-MSC were cultured with an expansion medium for 3 days, which was then switched to the differentiation medium PBEC culture medium with varying concentrations of TGF-β1 (0.1–10 ng/mL). The medium was changed twice weekly. We observed the resulting cell morphology under inverted microscopy.
Transmission electron microscopy
Details of procedures for transmission electron microscopy are listed in the Supplementary Data.
Immunostaining
Cells were cultured at 80%–90% confluence, washed, and fixed with freshly made 4% paraformaldehyde at 4°C for 30 min. Cells were permeabilized with 0.1% Triton X-100, then washed, and blocked with 2% bovine serum albumin. Next, we incubated the cells overnight at 4°C with primary antibodies cytokeratin 5 (CK-5) and nonselenium glutathione peroxidase (NSGP, for detection of Clara cell secretory protein 26) (Abcam), and then conjugated corresponding secondary antibodies with FITC (green). The cells were counterstained with propidium iodide (red). Examination of expression levels of CK-5 and NSGP in cells positive for immunostaining was performed using an image analysis system (Image-Pro Plus 4.5; Media Cybernetics).
Western blots
Specific protein expression was determined by Western blotting. To confirm lung epithelial differentiation and membrane-bound receptor expression, we applied the anti-cytokeratin 19 (CK-19) monoclonal antibody (Chemicon International), anti-Clara cell secretory protein 26 (anti-CCSP 26, Chemicon International), and anti-TNFR1 (R&D System). To determine the TNF-α-induced signaling pathway, anti-phospho-p46/54 (SAPK/JNK, Thr183/Tyr185), anti-phospho-p44/42 (Thr202/Tyr204) antibody, anti-phospho-p38 (Thr180/Tyr182) antibody, or anti-Akt antibody (Cell Signaling Technology) was applied. After washing three times with Tris-buffered saline, blots were incubated for 1 h with a 1/2000 dilution of a horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology). The protein bands were viewed using enhanced chemiluminescence (Amersham Pharmacia Biotech) and autoradiography with a Kodak X-ray film.
Reverse transcription polymerase chain reaction
Total RNA was extracted and reverse transcribed into cDNA using the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems; P/N 4366597). The cDNA underwent polymerase chain reacton (PCR) for detection and quantitation of mRNA for ciliated bronchial epithelium 1 (CBE 1), surfactant protein (SP) A, SP-C, ICAM-1, and MUC5AC, one of the major mucins in the respiratory epithelium. The procedures, primer sequence, and products generated are listed in the Supplementary Data.
TNF-α stimulation and IL-8 release
We seeded cells in 24-well culture inserts at a density of 1×105 cells/mL (100 μL/insert) and grew them in the culture medium. At confluence, TNF-α (Sigma) was added to the apical compartment at varying concentrations. After incubating for 24 h, we collected the supernatants and stored them at −80°C until they were assayed for mediators. Levels of IL-8 were assayed using the ELISA kit (R&D Systems).
Real-time quantitative PCR for IL-8, miRNA-146a, miRNA-155, and TNFR1 mRNA expression
The mRNA expression of IL-8 was determined by real-time quantitative PCR (qPCR). Standard procedures and primers for IL-8, miRNA-146a, miRNA-155, miRNA-U6 (internal control for miRNA), TNFR1, and GAPDH (internal control for IL-8) are listed in the Supplementary Data.
Transfection with miRNA-146a inhibitor or mimic
BM-MSC were transfected with the 100 nM miRNA-146a inhibitor (Cat #: IH-300630-05; Dharmacon) according to the manufacturer's instructions. In addition, to confirm that miRNA-146a upregulates TNF-α-induced IL-8 production, MSC-EC were transfected with 100 nM miRNA-146a mimic (Cat #: IH-300630-03; Dharmacon). We evaluated miRNA expression levels at 24 h post-transfection, and cells given the same treatments were then exposed to TNF-α for IL-8 assay. Transfection control was determined by measuring GAPDH mRNA expression after transfection with GAPDH siRNA. Transfection efficiency was approximately 75% before each experiment.
Statistical analysis
Data are expressed as means±SEM. Statistical analysis for multiple comparisons was performed using analysis of variance (ANOVA), and the post hoc test applied for pairwise comparison following ANOVA was the LSD (least significance difference) test. A difference of p<0.05 was considered significant.
Results
Effect of TGF-β1, collagen, and cell density on morphological change of BM-MSC
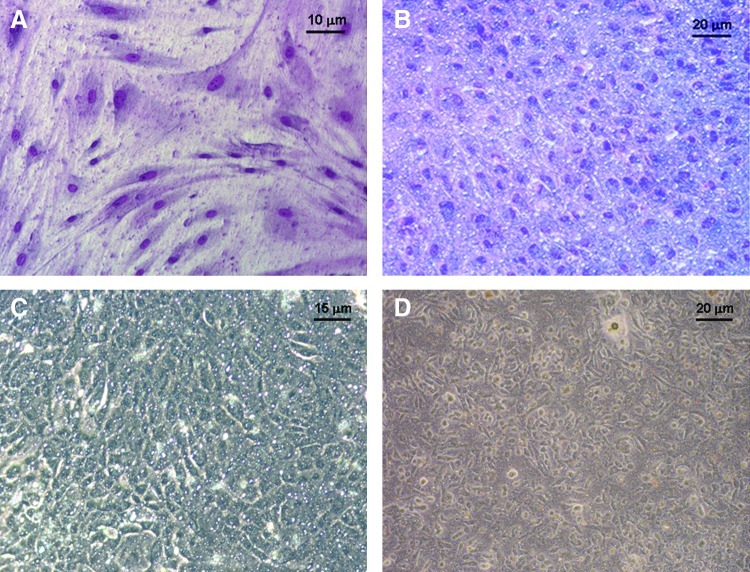
BM-MSC (passage 2–5) were differentiated into MSC-EC in an air–liquid interface culture system with a specific culture medium for PBEC.18 BM-MSC initially displayed a fibroblast spindle-shaped feature (Fig. 1A). TGF-β1 at a concentration of ≥10 ng/mL induced morphological change, which presented a cobblestone appearance after 10–14 days of culture (Fig. 1B). At a TGF-β1 concentration of 0–10 ng/mL, varying degrees of morphological change of stem cells were observed, from spindle-shaped to cobblestone appearance. The morphology of MSC-EC under light microscopy was found to be similar to that of explant PBEC (Fig. 1C) and isolated AEC (Fig. 1D). MSC-EC revealed a thin and flattened epithelial sheet (Fig. 2A) without cilia or microvilli formation, which were observed in PBEC (Fig. 2B) under transmission electron microscopy.
FIG. 1.
Morphologies of bone marrow-derived mesenchymal stem cells (BM-MSC), lung epithelial-like cells (MSC-EC), primary bronchial epithelial cells (PBEC) and alveolar epithelial cells (AEC). BM-MSC (A) and MSC-EC (B) in an air–liquid interface culture with a specific culture medium and the addition of transforming growth factor-β (10 ng/mL) after 14 days culture (May-Grunwald-Giemsa stain), in comparison with (C) PBEC from explant culture and (D) AEC from lung parenchyma. Color images available online at www.liebertpub.com/tea
FIG. 2.
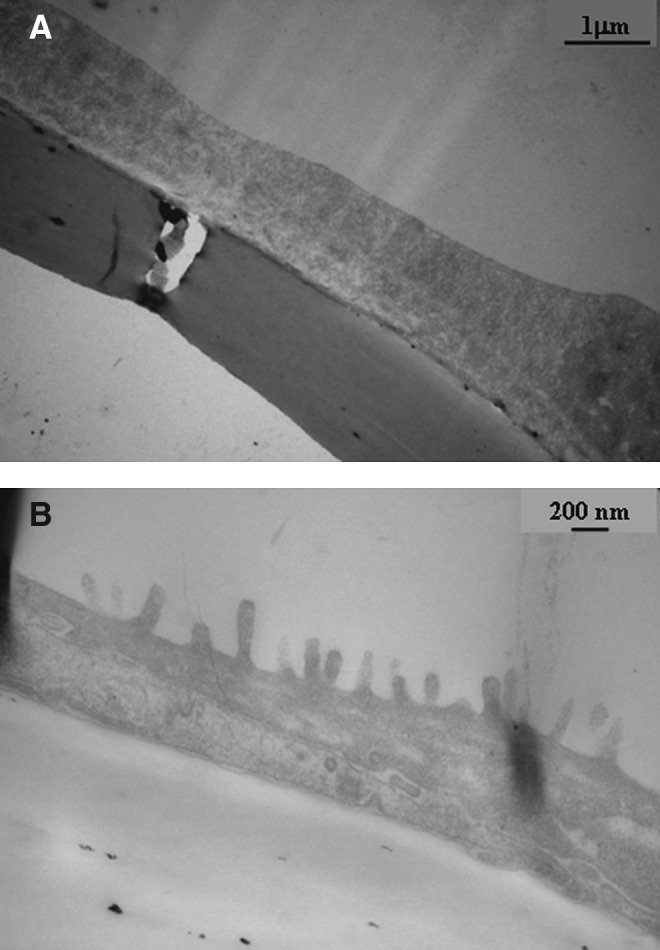
MSC-EC revealed a thin and flattened epithelial sheet (A) without cilia or microvilli formation, which was noticed in (B) PBEC under transmission electron microscopy.
Type IV collagen at a concentration of 50 μg/cm2 is suitable for airway epithelial cell adherence and growth in an air–liquid interface culture, as described in our previous study.18 BM-MSC added to culture inserts without a collagen coating were not differentiated into epithelial-like cells and remained spindle-shaped in appearance. Cells added to culture inserts coated with type IV collagen (50 μg/cm2) were able to differentiate into MSC-EC. BM-MSC at a density of 1×105/well (12-well inserts; growth area, 0.9 cm2) could reach 70%–80% confluence after 5–7 days of culture. A morphological change occurred between 10 to 14 days. To determine the effect of cell density on differentiation, 0.75, 0.5, and 0.25×105 cells/well were added to culture inserts. Cell differentiation was not affected by cell density.
Lung epithelial markers
BM-MSC were cultured for 1, 2, or 3 weeks and examined for phenotypic markers of airway epithelial cells. CK-19 and CCSP 26 (Fig. 3A) were expressed in MSC-EC only after 3 weeks of culture, although a morphological change occurred within 10–14 days. These proteins were hardly detected in BM-MSC. Reverse transcription-PCR was used to detect the mRNA expression of CBE 1, SP-A, SP-C, MUC5AC, and ICAM-1 (Table 1). However, mRNA expression of CBE 1, SP-A, SP-C, and MUC5AC was not detected in MSC-EC, in contrast with PBEC or AEC (data not shown). Increased mRNA expression of ICAM-1 was observed in MSC-EC in comparison with BM-MSC (data not shown). Immunofluorostaining of BM-MSC and MSC-EC showed that MSC-EC expressed lung epithelial markers, CK-5 and CCSP 26 (Fig. 3B), in contrast to BM-MSC, which did not exhibit epithelial marker expression (data not shown).
FIG. 3.
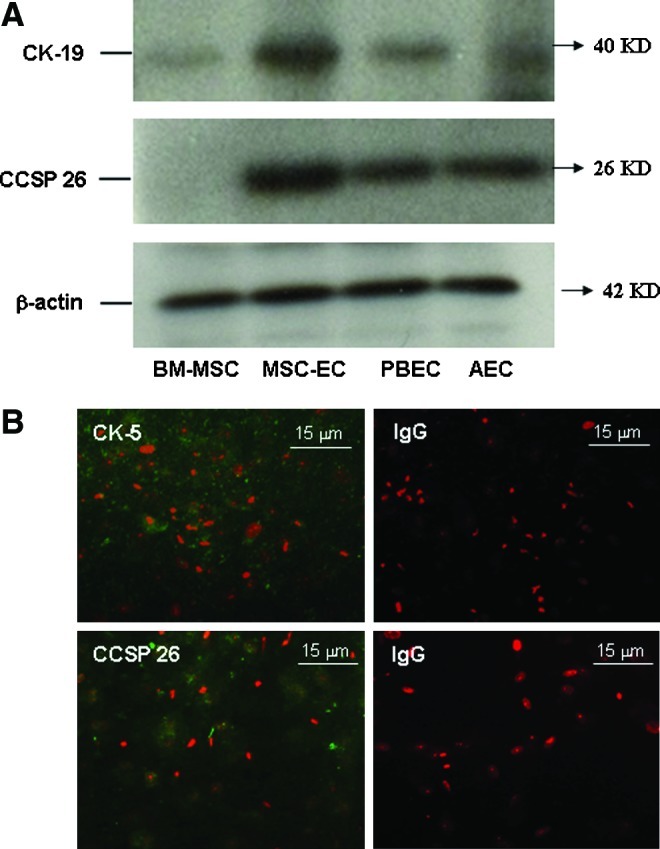
Lung epithelial markers. (A) Increased expression of cytokeratin-19 (CK-19) and Clara cell secretory protein 26 (CCSP 26) were observed in MSC-EC after 3 weeks of culture. The blots presented represent three separate experiments. (B) Immunostaining of CK-5 (green) and CCSP 26 (green) in combination with nuclear PI staining (red) in MSC-EC and their relevant IgG control samples. Positive staining was found in the cytoplasm of MSC-EC. Color images available online at www.liebertpub.com/tea
Table 1.
Characteristics of Lung Epithelial Markers
| Name | Pulmonary producing cells |
|---|---|
| Cytokeratin (CK-5, CK-9) | Cells of epithelial origin |
| Clara cell secretory protein (CCSP) | Clara, bronchial cells |
| Ciliated bronchial epithelium 1 (CBE1) | Ciliated bronchial epithelial cells |
| Surfactant protein (SP-A, SP-C) | Type II pneumonocyte, Clara cells |
| MUC5AC | Tracheobronchial and gastric epithelium, pancreas and gallbladder |
TNF-α-induced IL-8 production
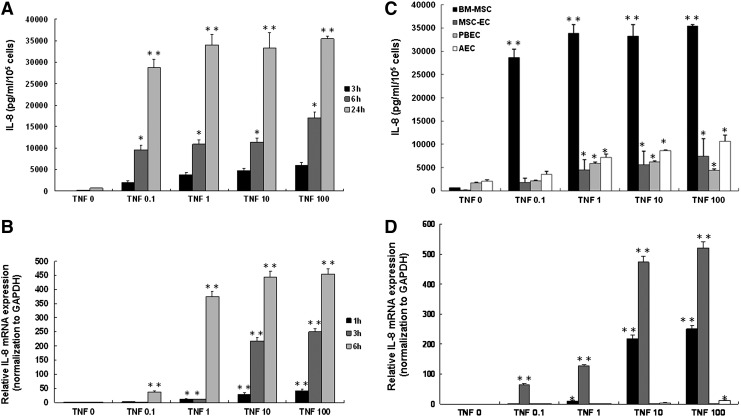
To evaluate the response of BM-MSC to inflammatory cytokine stimulation, we exposed cells to TNF-α at varying concentrations (0–100 ng/mL) and measured IL-8 production at 3, 6, and 24 h after stimulation (Fig. 4A). IL-8 production showed a time- and dose-dependent pattern. The mRNA expression of IL-8, confirmed by real-time qPCR, revealed a similar pattern (Fig. 4B). The response of BM-MSC to TNF-α stimulation differed significantly from that in MSC-EC, PBEC, and AEC (Fig. 4C). Cells were stimulated by TNF-α at varying concentrations (0–100 ng/mL) and IL-8 production was measured at 24 h (Fig. 5C). At a TNF-α concentration of 100 ng/mL, the maximal IL-8 production of BM-MSC observed at 24 h increased 4.8-fold in comparison with that of MSC-EC. MSC-EC showed a dose-dependent pattern in response to TNF-α stimulation. We observed increases of more than 250- and 520-fold of IL-8 mRNA in BM-MSC and MSC-EC following TNF-α (100 ng/mL) stimulation (Fig. 5D). The increase of IL-8 mRNA in PBEC and AEC was only 2.8-fold and 11.5-fold, respectively.
FIG. 4.
Effect of tumor necrosis factor (TNF)-α on IL-8 generation and mRNA expression in BM-MSC, MSC-EC, PBEC, and AEC. (A) IL-8 level in supernatants of BM-MSC incubated for 3, 6, and 24 h with a buffer alone or varying concentrations of TNF-α (0–100 ng/mL). (B) Quantitative polymerase chain reaction (qPCR) analysis of the expression of IL-8 mRNA and the housekeeping gene GAPDH following incubation of BM-MSC for 1, 3, and 6 h with varying concentrations of TNF-α. (C) IL-8 level in supernatants of different type of cells incubated for 24 h with buffer alone or varying concentrations of TNF-α (0–100 ng/mL). (D) qPCR analysis of the expression of IL-8 mRNA and the housekeeping gene GAPDH following incubation of different type of cells for 3 h with varying concentrations of TNF-α. Mean IL-8 values (±SEM) of four experiments performed in duplicate are shown. Data for mRNA expression are shown for three experiments performed in duplicate and expressed as fold increase in comparison with cells without TNF-α stimulation. *p<0.05, **p<0.001 compared with cells incubated with buffer alone.
FIG. 5.
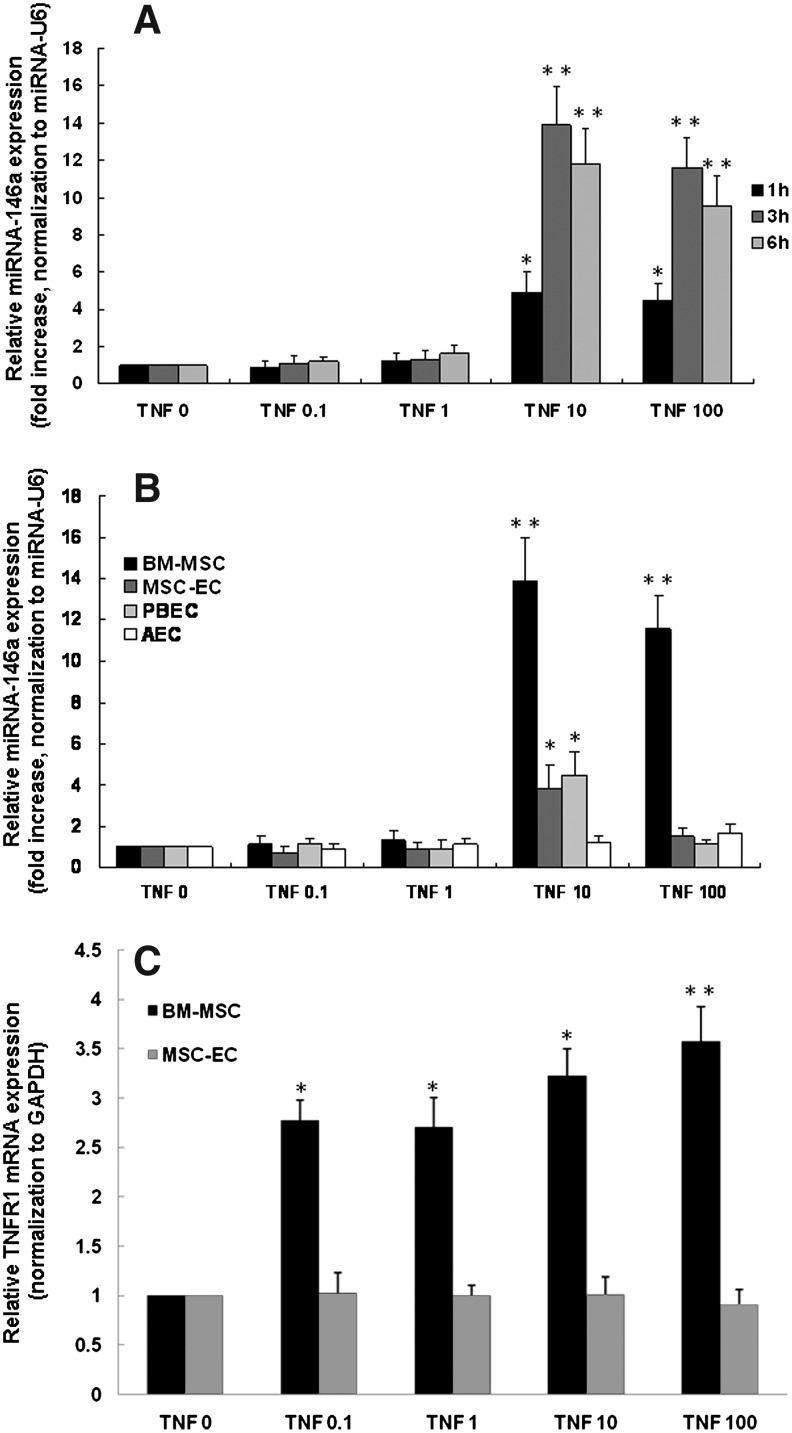
miRNA-146a and TNF receptor 1 (TNFR1) mRNA expression after TNF-α stimulation. qPCR analysis of the expression of miRNA-146a (internal control miRNA-U6) at 1, 3, and 6 h for BM-MSC (A), 3 h for BM-MSC, MSC-EC, PBEC, and AEC (B) and at 3 h for TNFR1 (internal control GAPDH) (C) following incubation of cells with varying concentrations of TNF-α (0–100 ng/mL). Data are shown for three separate experiments performed in duplicate and expressed as fold increase in comparison with cells without TNF-α stimulation. *p<0.05, **p<0.001 compared with cells incubated with buffer alone.
Upregulation of miRNA-146a and TNFR1 by TNF-α stimulation
To determine the roles of miRNA-146a and miRNA-155 in the inflammatory response, the expression levels of both were examined using real-time qPCR. The miRNA-146a expression in BM-MSC after TNF-α stimulation at 1, 3, and 6 h is shown (Fig. 6A). At higher concentrations (10 and 100 ng/mL), TNF-α stimulated a significant increase in the expression of miRNA-146a in BM-MSC as compared with MSC-EC (Fig. 6B). The expression of miRNA-146a in PBEC and AEC reached the highest levels at a TNF-α concentration of 10 ng/mL. miRNA-155 expression remained stable in the four different types of cells, even in the presence of TNF-α at various concentrations (data not shown). TNFR1 mRNA expression was also determined 3 h after TNF-α stimulation. We observed a dose-dependent increase in TNFR1 mRNA expression in BM-MSC, starting to increase at a TNF-α concentration of 0.1 ng/mL and reaching a 3.7-fold increase at 100 ng/mL. This alteration in TNFR1 mRNA expression was not observed in MSC-EC (Fig. 6C). To further confirm the TNFR1 protein expression, Western blotting was performed. Without stimulation, TNFR1 in BM-MSC and MSC-EC was hardly detectable in comparison to that of PBEC (Fig. 6A). After stimulation with TNF-α for 24 h, TNFR1 expression significantly increased in BM-MSC compared with MSC-EC (Fig. 6B). Again, this result is compatible with mRNA expression of TNFR1 in both cell types. We further investigated whether TNF-α-induced IL-8 production in BM-MSC occurred through mitogen-activated protein kinase (MAPK) phosphorylation. p44/42 MAPK phosphorylation peaked at 10 min and declined at 60 min (Fig. 6C). There were no observable differences in p38, p46/54 (SAPK/JNK), and Akt phosphorylation, in either the presence or absence of TNF-α stimulation (data not shown).
FIG. 6.
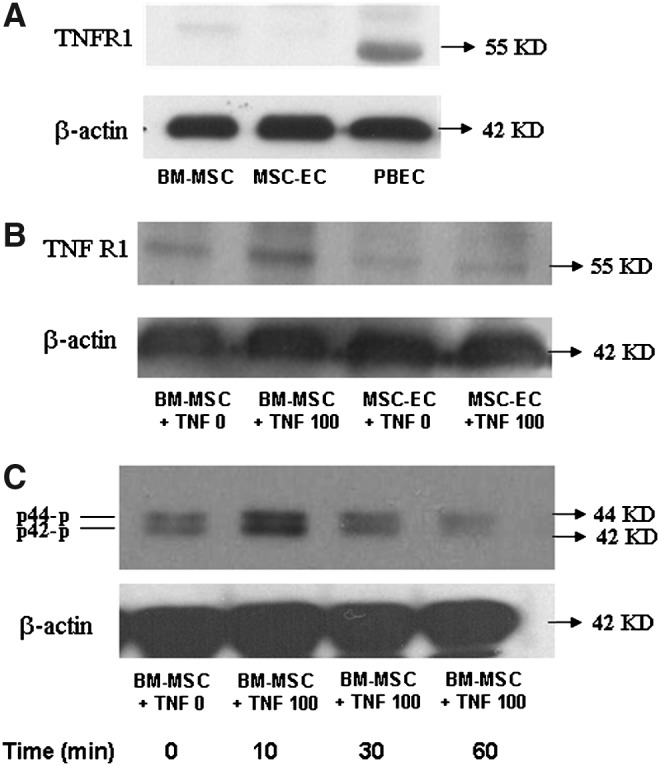
TNFR1 expression and p44/42 phosphorylation after TNF-α stimulation. TNFR1 expression is shown in BM-MSC, MSC-EC, and PBEC at basal condition (A) and in BM-MSC (B) which were treated with TNF-α (100 ng/mL) for 24 h and harvested for Western blotting. Immunoblots presented are representative of three separate experiments each for TNFR1 (B) and p44/42 (C).
Expression of miRNA-146a is associated with TNF-α-induced IL-8 production
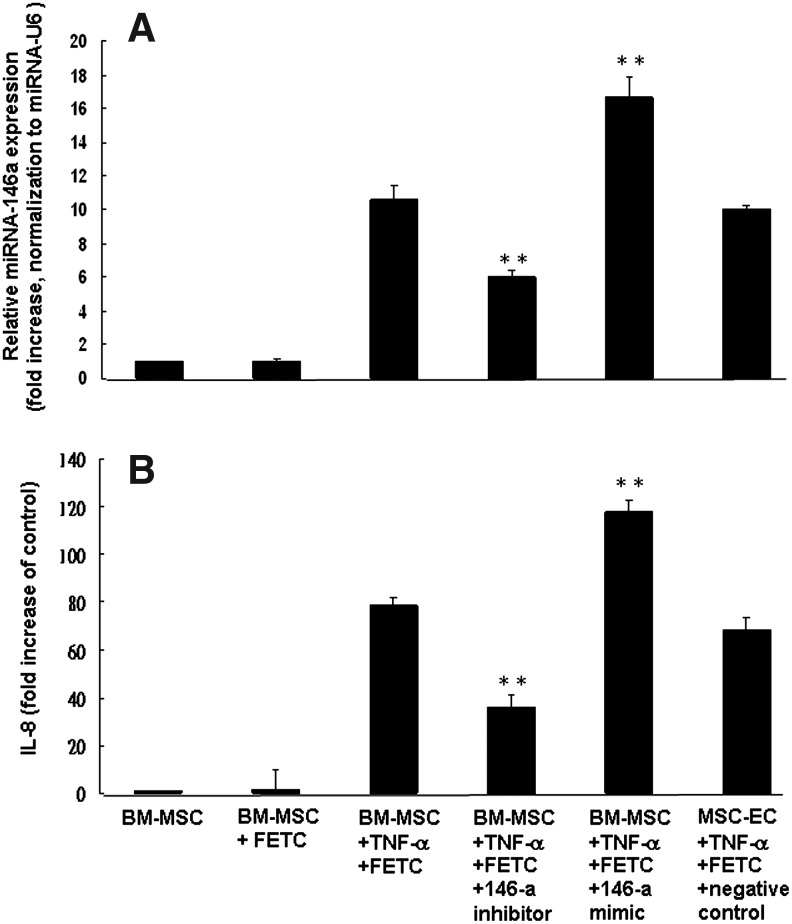
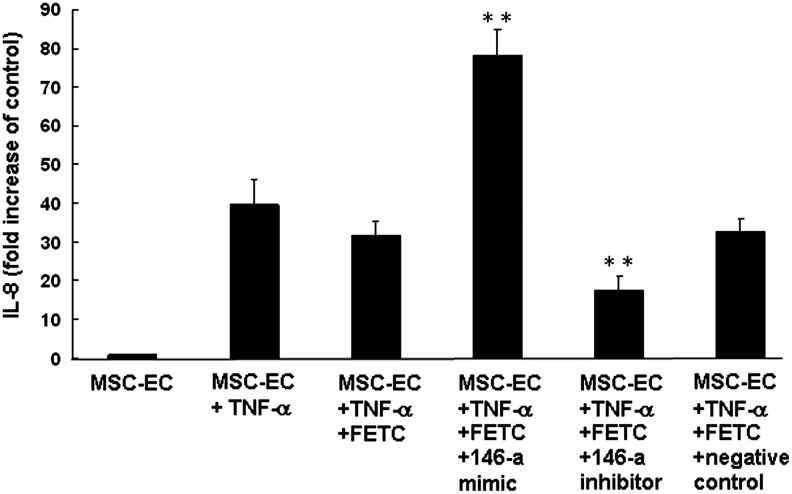
We assessed the functional relevance of changes in miRNA-146a expression during TNF-α-induced IL-8 production using transfection with miRNA-146a inhibitors and mimics. BM-MSC were transfected with miRNA-146a inhibitors and the expression of miRNA-146a following TNF-α stimulation (100 ng/mL) was significantly decreased (45% decrease, p<0.001) (Fig. 7A). The miRNA-146a inhibitors produced a 55% reduction in TNF-α-induced IL-8 release (Fig. 7B). In contrast, miRNA-146a mimics increased expression of miRNA-146a 58% and production of TNF-α-induced IL-8 release 51% in BM-MSC (Fig. 7B). To examine the association between miRNA-146a expression and TNF-α-induced IL-8 production in MSC-EC, we transfected cells with miRNA-146a mimics and inhibitors and measured IL-8 release. miRNA-146a mimics increased TNF-α-induced IL-8 production by approximately 95%. On the contrary, transfection with miRNA-146a inhibitors resulted in a 55% reduction in TNF-α-induced IL-8 release in MSC-EC (Fig. 8).
FIG. 7.
Effect of miRNA-146a inhibitor and mimic on IL-8 production by BM-MSC after TNF-α stimulation. BM-MSC were transfected with miRNA-146a inhibitor, mimic (100 nM) or negative control in siRNA transfection reagent (FETC) and the miRNA-146a expression levels evaluated at 24 h post-transfection by qPCR (A). Cells given the same treatments were then exposed to TNF-α (100 ng/mL) and supernatants were collected and assayed for IL-8 concentration 24 h after stimulation (B). Data are shown for three experiments performed in duplicate and expressed as fold increase of miRNA-146a expression. Mean IL-8 values (±SEM) are shown for three experiments performed in duplicate. **p<0.001 compared with cells treated with transfection reagent FETC and TNF-α.
FIG. 8.
Effect of miRNA-146a mimic and inhibitor on IL-8 production by MSC-EC after TNF-α stimulation. MSC-EC were transfected with miRNA-146a mimic, inhibitor (100 nM), or a negative control in the reagent FETC. Cells were then exposed to TNF-α (100 ng/mL) and supernatants were collected and assayed for IL-8 concentration 24 h after stimulation. Mean IL-8 values (±SEM) are shown for three experiments performed in duplicate. **p<0.001 compared to cells treated with TNF-α alone, or with transfection reagent FETC, or with negative control for miRNA-146a mimic.
Discussion
We have demonstrated that BM-MSC can be differentiated into MSC-EC in an air–liquid interface culture under a specific culture medium. The addition of growth factor TGF-β1 and extracellular matrix collagen is required to facilitate such differentiation. The morphological appearance and expression of airway epithelial markers in MSC-EC pertain to lung epithelial cells. However, the MSC-EC was not well-differentiated and did not display the characteristics of cilial formation in bronchial epithelial cells or SP-A/SP-C in alveolar cells. The differentiation of MSC-EC may be a key factor influencing the response to TNF-α stimulation. BM-MSC greatly differed from MSC-EC in miRNA-146a expression, TNFR1 induction, and IL-8 production in response to TNF-α stimulation. On the other hand, both type of cells were regulated by miRNA-146a during TNF-α-induced IL-8 release. In both types of cells, transfection with miRNA-146a mimics resulted in a significant increase of miRNA-146a expression and IL-8 production. In contrast, miRNA-146a inhibitors reduced miRNA-146a expression and IL-8 production. These data suggest that miRNA-146a expression positively modulates TNF-α-induced IL-8 release in both MSC and differentiated MSC-EC.
It is debatable whether expression of miRNA-146a is protective or harmful as well as proinflammatory or anti-inflammatory. In A549 cancer cells, expression of miRNA-146a negatively regulated the release of IL-1β-induced IL-8 and RANTES. In contrast, in human airway smooth muscle cells, IL-1β induced a 100-fold increase in miRNA-146a expression, which was positively correlated with the release of IL-6 and IL-8. These results suggest that the function and mechanism of miRNA-146a may be cell-type dependent.12 On the other hand, different kinds of stimuli result in different types of miRNA expression. Under basal conditions, miRNAs were differentially expressed in cord blood and adult peripheral blood-derived cells. Upon interferon-γ stimulation, seven miRNAs (miR-29a, miR-29b, miR-34c-5p, miR-132, miR-146a, miR-146b-5p, and miR-155) changed in expression in CD14+cord blood (CB) cells, whereas upon lipopolysaccharide stimulation only two miRNAs (miR-18a and miR-155) changed in expression in CD14+CB cells.19 Of particular note, MSC is pluripotent and is able to differentiate into almost all cells of the three germ layers. The timing and microenvironment may influence the differentiation and function of MSC. In an animal model, MSC injected immediately after lung injury were shown to differentiate into functional lung epithelial or endothelial cells, while those injected at a later stage mostly appeared as myofibrocytes.20 In addition, stem cells may contribute to the pathogenesis of several adult lung diseases either through their hyperproliferative response, or negatively via an impairment or depletion of local regenerative cells.21 Overexpression of miRNA-146a, which positively regulates TNF-α-induced IL-8 release, may enhance the inflammatory response in both BM-MSC and MSC-EC. The expression of miRNA-146a and response to TNF-α stimuli may be modulated through mature differentiation.
TNF-α was employed in this study because it plays an important role in the initiation and maintenance of acute and chronic lung inflammation. TNF-α is mainly produced by macrophages and its expression is enhanced by bacterial or viral infection or allergen challenge. TNF-α may stimulate airway epithelial cells to produce cytokines, such as IL-8.22 The response of BM-MSC and MSC-EC to TNF-α stimulation was quite different. We found that in BM-MSC, TNFR1 was upregulated at both mRNA and protein levels after TNF-α stimulation. This did not occur in MSC-EC. This may explain, at least a part of the mechanism why TNF-α-induced IL-8 production was much higher in BM-MSC than in MSC-EC. TNF-α induced p44/42 MAPK and Akt phosphorylation in a time- and dose-dependent manner in ameloblastoma cells.23 Cyclic stretch can induce p38 and p44/42 MAPK activation and subsequent IL-8 production in human bronchial epithelial cells. In human airway smooth muscle cells, IL-1 β-induced IL6 and IL-8 release was associated with increased expression of miRNA-146a. The post-transcriptional processing of primary miR-146a to mature miR-146a was regulated by MEK-1/2 and JNK.12 However, in this study, TNF-α-induced IL-8 production in BM-MSC was regulated by expression of miRNA-146-a and was associated with activation of p44/42 MAPK, but not p38, JNK, or Akt.
The growth factor TGF-β1 and extracellular matrix collagen are key factors in the differentiation of BM-MSC to MSC-EC. TGF-β1 can induce differentiation of MSC into smooth muscle cells,24 cardiomyocytes,25 and myofibroblasts.26 TGF-β1 signaling is involved in the transdifferentiation of type II pneumocytes to type I pneumocytes.27 In our study, BM-MSC were not differentiated into MSC-EC without the addition of TGF-β1; however, with the addition of TGF-β1 (≥10 ng/mL) to the culture medium, BM-MSC were differentiated into MSC-EC with epithelial markers of CK-19, the intermediate filaments that make up the major part of the cytoskeleton in the epithelia; CK-5, found in most basal cells in the normal airway, and CCSP 26, a specific marker of bronchiolar Clara cells. Clara cells have been suggested to function as airway progenitors and stem cells of the distal airways.28–30 One study has shown that extracellular matrix laminin can greatly affect the differentiation of embryonic stem cells into pneumonocytes with enhanced gene expression of SP-C.31 In this study, a type IV collagen coating on the tissue culture inserts was found to be critical for the differentiation of BM-MSC to MSC-EC, as well as for the maintenance of primary human bronchial epithelium in vitro.
Several limitations of this study need to be highlighted. First, although we generated MSC-EC, the mechanisms underlying the changes in gene expression during differentiation are not yet clear. Second, we investigated only two miRNAs with respect to modulation of inflammation in BM-MSC and MSC-EC. Third, the capacities of MSC-EC in terms of proliferation and engraftment in injured airway epithelium are unknown, and it remains to be shown whether or not MSC-EC possesses anti-inflammatory and antifibrotic properties to attenuate inflammation, subsequent damage, and fibrosis. The question likewise remains as to which cells, BM-MSC or MSC-EC, are more appropriate to use as a material for cell-based therapy for lung diseases.
In conclusion, we have demonstrated that BM-MSC, cultured in a specific medium with the addition of TGF-β1 and grown on type IV collagen-coated inserts, can be differentiated into MSC-EC, and we found these two types of cells differed greatly in response to TNF-α stimulation. The expression of miRNA-146a and response to TNF-α stimuli may be modulated through mature differentiation of BM-MSC. Overexpression of miRNA-146a, which positively regulates TNF-α-induced IL-8 release, may enhance the inflammatory response in both BM-MSC and MSC-EC. The differences in biological function between BM-MSC, MSC-EC, and primary lung epithelial cells merit further investigation.
Supplementary Material
Acknowledgments
This study was supported by Taiwan's National Science Council research grants NSC 97-2314-B-075-059-MY3.
Authors' Contributions
Diahn-warng Perng: Conception and design, financial support, data analysis and interpretation, writing of manuscript. Tina Lo: Conception and design, provision of study materials, data analysis and interpretation. Yi-han Hsiao: Conception and design, provision of study materials, data analysis and interpretation. Oscar Kuang-sheng Lee: Conception and design, provision of study materials, data analysis and interpretation. De-ming Yang: Provision of study materials, collection and assembly of data. Mo-tzu Wu: Provision of study materials, collection and assembly of data. Yu-chung Wu: Provision of study materials. Yu-chin Lee: Administrative support, collection and assembly of data.
Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.Lee K.D. Kuo T.K. Whang-Peng J. Chung Y.F. Lin C.T. Chou S.H., et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Berger M.J. Adams S.D. Tigges B.M. Sprague S.L. Wang X.J. Collins D.P., et al. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy. 2006;8:480. doi: 10.1080/14653240600941549. [DOI] [PubMed] [Google Scholar]
- 4.Samadikuchaksaraei A. Cohen S. Isaac K. Rippon H.J. Polak J.M. Bielby R.C., et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- 5.Van Haute L. De Block G. Liebaers I. Sermon K. De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths M.J. Bonnet D. Janes S.M. Stem cells of the alveolar epithelium. Lancet. 2005;366:249. doi: 10.1016/S0140-6736(05)66916-4. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.O'Connell R.M. Taganov K.D. Boldin M.P. Cheng G. Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taganov K.D. Boldin M.P. Chang K.J. Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tili E. Michaille J.J. Cimino A. Costinean S. Dumitru C.D. Adair B., et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 11.Perry M.M. Moschos S.A. Williams A.E. Shepherd N.J. Larner-Svensson H.M. Lindsay M.A. Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008;180:5689. doi: 10.4049/jimmunol.180.8.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larner-Svensson H.M. Williams A.E. Tsitsiou E. Perry M.M. Jiang X. Chung K.F., et al. Pharmacological studies of the mechanism and function of interleukin-1beta-induced miRNA-146a expression in primary human airway smooth muscle. Respir Res. 2010;11:68. doi: 10.1186/1465-9921-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pottier N. Maurin T. Chevalier B. Puissegur M.P. Lebrigand K. Robbe-Sermesant K., et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial-mesenchymal interactions. PLoS One. 2009;4:e6718. doi: 10.1371/journal.pone.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong W. Yang H. He L. Zhao J.J. Coppola D. Dalton W.S., et al. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggarwal B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 16.Cardell L.O. Uddman R. Zhang Y. Adner M. Interleukin-1beta up-regulates tumor necrosis factor receptors in the mouse airways. Pulm Pharmacol Ther. 2008;21:675. doi: 10.1016/j.pupt.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Berger A.C. Alexander H.R. Wu P.C. Tang G. Gnant M.F. Mixon A., et al. Tumour necrosis factor receptor I (p55) is upregulated on endothelial cells by exposure to the tumour-derived cytokine endothelial monocyte- activating polypeptide II (EMAP-II) Cytokine. 2000;12:992. doi: 10.1006/cyto.2000.0687. [DOI] [PubMed] [Google Scholar]
- 18.Perng D.W. Wu Y.C. Tsai M.C. Lin C.P. Hsu W.H. Perng R.P., et al. Neutrophil elastase stimulates human airway epithelial cells to produce PGE2 through activation of p44/42 MAPK and upregulation of cyclooxygenase-2. Am J Physiol Lung Cell Mol Physiol. 2003;285:L925. doi: 10.1152/ajplung.00182.2002. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi N. Nakaoka T. Yamashita N. Profiling of immune-related microRNA expression in human cord blood and adult peripheral blood cells upon proinflammatory stimulation. Eur J Haematol. 2011;88:31. doi: 10.1111/j.1600-0609.2011.01707.x. [DOI] [PubMed] [Google Scholar]
- 20.Yan X. Liu Y. Han Q. Jia M. Liao L. Qi M., et al. Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol. 2007;35:1466. doi: 10.1016/j.exphem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Majka S.M. Beutz M.A. Hagen M. Izzo A.A. Voelkel N. Helm K.M. Identification of novel resident pulmonary stem cells: form and function of the lung side population. Stem Cells. 2005;23:1073. doi: 10.1634/stemcells.2005-0039. [DOI] [PubMed] [Google Scholar]
- 22.Kwon O.J. Au B.T. Collins P.D. Adcock I.M. Mak J.C. Robbins R.R., et al. Tumor necrosis factor-induced interleukin-8 expression in cultured human airway epithelial cells. Am J Physiol. 1994;267:L398. doi: 10.1152/ajplung.1994.267.4.L398. [DOI] [PubMed] [Google Scholar]
- 23.Hendarmin L. Sandra F. Nakao Y. Ohishi M. Nakamura N. TNF-alpha played a role in induction of Akt and MAPK signals in ameloblastoma. Oral Oncol. 2005;41:375. doi: 10.1016/j.oraloncology.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Kurpinski K. Chu J. Wang D. Li S. Proteomic profiling of mesenchymal stem cell responses to mechanical strain and TGF-beta1. Cell Mol Bioeng. 2009;2:606. doi: 10.1007/s12195-009-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li T.S. Komota T. Ohshima M. Qin S.L. Kubo M. Ueda K., et al. TGF-beta induces the differentiation of bone marrow stem cells into immature cardiomyocytes. Biochem Biophys Res Commun. 2008;366:1074. doi: 10.1016/j.bbrc.2007.12.095. [DOI] [PubMed] [Google Scholar]
- 26.Popova A.P. Bozyk P.D. Goldsmith A.M. Linn M.J. Lei J. Bentley J.K., et al. Autocrine production of TGF-beta1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L735. doi: 10.1152/ajplung.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhaskaran M. Kolliputi N. Wang Y. Gou D. Chintagari N.R. Liu L. Trans-differentiation of alveolar epithelial type II cells to type I cells involves autocrine signaling by transforming growth factor beta 1 through the Smad pathway. J Biol Chem. 2007;282:3968. doi: 10.1074/jbc.M609060200. [DOI] [PubMed] [Google Scholar]
- 28.Giangreco A. Reynolds S.D. Stripp B.R. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong K.U. Reynolds S.D. Giangreco A. Hurley C.M. Stripp B.R. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 30.Kim C.F. Jackson E.L. Woolfenden A.E. Lawrence S. Babar I. Vogel S., et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y.M. Zhang A. Rippon H.J. Bismarck A. Bishop A.E. Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng Part A. 2010;16:1515. doi: 10.1089/ten.TEA.2009.0232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.