Abstract
Fibroblast growth factor-2 (FGF-2) is a well-characterized protein that is used in the treatment of healing-impaired wounds. We previously reported that fragmin/protamine microparticles (F/P MPs) are useful as biodegradable carriers for the controlled release of cytokines. We examined the ability of FGF-2-containing (FGF-2/) F/P MPs to prevent limb loss in an experimentally induced ischemic hindlimb model using adult Balb/c-nu/nu male mice. One day after inducing ischemia, intramuscular injections of 100 μL of FGF-2/F/P MPs turbid suspension (10 μg/mL FGF-2 and 6 mg/mL F/P MPs) were administered into eight sites of the ischemic hindlimb. A 100-μL suspension of each of the following—10 μg/mL FGF-2, 6 mg/mL F/P MPs, and phosphate-buffered saline (PBS; the control)—was similarly injected into the hindlimb. From 5 days onward after the injections, recovery from ischemia was observed in the FGF-2/F/P MP-treated group, but only partial recovery occurred in the FGF-2-treated group. The F/P MP-treated and PBS-treated groups (i.e., control) exhibited no recovery from the ischemia. The histological evaluations of the hindlimbs also confirmed that the capillary (i.e., mature vessels) density was significantly higher in the FGF-2/F/P MP-treated group than in the other groups. The mice injected with FGF-2/F/P MPs also recovered hindlimb blood flow, as reflected by oxygen saturation and surface temperature evaluation. Our present approach using FGF-2/F/P MPs could be considered a valuable option for the therapeutic treatment of peripheral ischemic diseases.
Introduction
Therapeutic angiogenesis and vasculogenesis are now recognized as promising approaches for improving impaired blood flow caused by ischemia. For example, several angiogenic factors such as fibroblast growth factor-2 (FGF-2),1 vascular endothelial growth factor (VEGF)2, and hepatocyte growth factor (HGF)3 have been proved to promote collateral vessel formation in animal models of limb and myocardial ischemia. Similar angiogenic activity is also noted with the transplantation of autologous cells such as bone-marrow stromal cells,4,5 adipose tissue-derived stromal cells,6 mesenchymal stem cells,7 and endothelial progenitor cells.8 Although a direct injection of naked plasmid DNA encoding angiogenic factors is another option to treat impaired blood flow,9–11 the efficiency of a target protein's expression appears to be variable among the experiments.
In various studies, FGF-2 has been used for its potency to induce vascular growth and to promote the recovery of blood flow after arterial occlusion.7,8 The difficulty in the use of growth factors lies in their low accumulation in the ischemic tissue and their rapid inactivation. For therapeutic angiogenesis, it is, therefore, necessary to enhance the in vivo activity of growth factors and enhance the capability of controlled release. Collagen,12 gelatin,13 fibrin,14 heparin, and alginate15 have been used as a controlled releasing matrix for FGF-2.
FGF-2 is a potent modulator of cell proliferation, motility, differentiation, and survival.16 Accumulated findings suggest that FGF-2 is essential for embryonic development,17 and it also plays an important role in regenerative processes such as angiogenesis,6 osteogenesis,17 chondrogenesis,18 and wound repair.19 FGF-2 is stored in various sites in vivo and interacts with heparin, heparan sulfate, and other heparin-like molecules that are collectively known as heparinoids.20 FGF-2 specifically binds to the heparinoids with a high affinity.21 Such an interaction protects FGF-2 from acid, heat inactivation, and protease-mediated degradation, which allows it to exert its effect on cellular function.6 Furthermore, heparin and heparan sulfate (i.e., heparinoids) serve as co-factors that enhance the activity of FGF-2.22,23
Heparin and low-molecular-weight heparin (LMWH) can interact with a variety of functional proteins such as growth factors, cytokines, extracellular matrix components, and cell adhesion molecules.24 Therefore, heparin has been used as a therapeutic agent for treating diseases caused by either a deficiency or an overexpression of these proteins, although the administration of high-dose heparin may increase the risk of bleeding.25 The LMWH (fragmin) has several pharmacological and practical advantages compared with heparin. For example, it exhibits a low, stable, and predictable anti-coagulant response, which obviates the need for laboratory monitoring to adjust the dosage. To maintain therapeutic concentrations in the blood, only one or two subcutaneous injections of fragmin are required each day because of its longer plasma half life.25
Protamine, a purified mixture of proteins obtained from fish sperm, neutralizes the activity of heparin and fragmin by forming a stable complex that lacks anti-coagulant activity.26 Therefore, protamine has been clinically used as a heparin antidote to reverse heparin's anti-coagulant activity after cardiopulmonary bypass and in heparin-induced bleeding.27
We previously demonstrated the usefulness of fragmin/protamine microparticles (F/P MPs) as carriers for the controlled release of heparin-binding growth factors such as FGF-2.6 The administration of FGF-2/F/P MPs results in substantial vascularization and fibrous tissue formation in vivo. By contrast, the administration of FGF-2 alone fails to generate such phenomena. It is easy to make F/P MPs, as fragmin and protamine are simply mixed just before use. Due to their microscale size (about 0.5 to 3.0 μm in diameter), the resulting microparticles can easily be taken up by a fine needle for injection to an affected area. In addition, since all components of the FGF-2/F/P MPs used in this research are in clinical use, they are likely safe when used in a clinical setting. Thus, FGF-2/F/P MPs have great potential for clinical use as a new biomaterial carrier to improve blood flow and improve impaired wound healing caused by ischemia. In this study, we tested the ability of FGF-2/F/P MPs to treat impaired blood flow by using an experimentally induced ischemic mouse model.
Materials and Methods
Preparation of the FGF-2/F/P MPs
The F/P MPs were prepared, as previously described.6 Briefly, protamine solution (10 mg/mL; Mochida Pharmaceutical Co., Tokyo, Japan) was added drop by drop to fragmin solution (6.4 mg/mL; Kissei Pharmaceutical Co., Tokyo, Japan), while vortexing for 2 min. A good yield of F/P MPs was obtained when 300 μL of protamine was mixed with 700 μL of fragmin (ratio=3:7 [v/v]). Using a high-speed centrifuge (MX-300; TOMY SEIKO Co. Ltd., Tokyo, Japan), the mixture was centrifuged at 8000 rpm for 5 min at 4°C to remove unreacted materials. After removing the supernatant, the pellet of microparticles was resuspended in 900 μL of Dulbecco's modified phosphate-buffered saline (PBS) with Ca2+ and Mg2+. About 100 μL of a suspension containing FGF-2 (1 mg/mL; Fiblast; Kaken Pharmaceutical Co., Tokyo, Japan) in PBS was then added to the turbid suspension of resuspended F/P MPs on ice. It was kept at 4°C just before use.
Animals and the surgical procedure
Ten-week-old adult male Balb/c-nu/nu mice (Japan SLC Co. Ltd., Hamamatsu, Shizuoka, Japan) were used in this study. Experimentally induced hindlimb ischemic model mice were produced, in accordance with the method described by Couffinhal et al.28 After an intraperitoneal injection of sodium pentobarbital (Nembutal; Dainippon Sumitomo Pharma Co. Ltd., Tokyo, Japan) and under sufficient anesthesia, the entire left deep femoral artery and the vein of a hindlimb were ligated at two points by a surgical 5-0 silk suture (Johnson & Johnson K.K., Tokyo, Japan). The vessels were cut between those two points and removed by forceps, as shown in Figure 1A. The remaining hindlimb was similarly treated. After the femoral artery ligation and closure of the incision, the mice were randomly divided into five groups: FGF-2/F/P MP-treated group (n=10), FGF-2-treated group (n=10), F/P MP-treated group (n=10), and two types of control groups [PBS-injected group (n=6) and nonadministration group (n=6)].
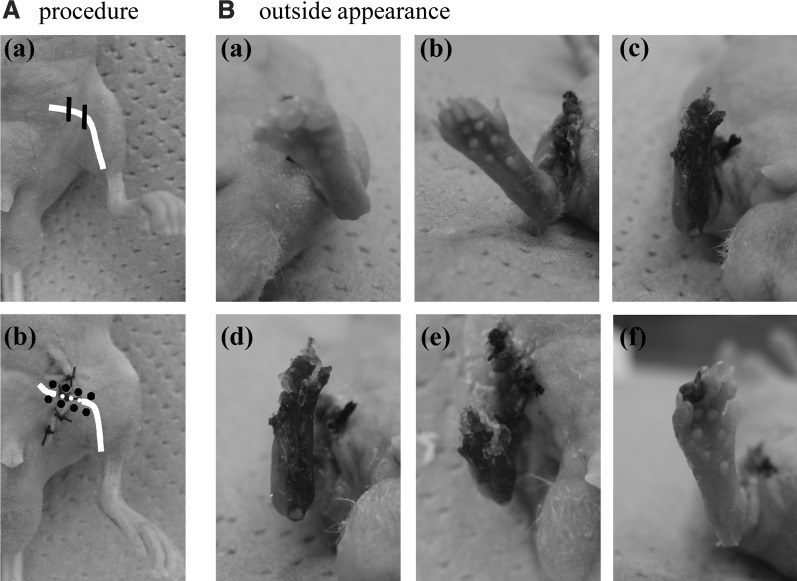
FIG. 1.
(A) The procedure of the study. (a) The ischemic hindlimb is experimentally induced, as described in Materials and Methods. The white line indicates the femoral artery and vein. (b) One day after surgery, a suspension of either FGF-2/F/P MPs, FGF-2, F/P MPs, or PBS (i.e., control) is injected into the femoris muscle (centering on sartorius muscle and adductor muscle) at 8 sites (black circles) in the femoral portion of the hindlimb. (B) Salvage of the ischemic limb after the injection of either FGF-2/F/P MPs, FGF-2 alone, or F/P MPs alone. Representative photographs of the ischemic hindlimbs 7 days after the injection of (a) FGF-2/F/P MPs (n=8), (b) FGF-2 (n=8), (c) F/P MPs alone (n=8), and (d) PBS (n=6). The ischemic hindlimbs on day 28 after the injection of (e) FGF-2 and (f) FGF-2/F/P MPs. FGF-2, fibroblast growth factor-2; F/P MPs, fragmin/protamine microparticles; PBS, phosphate-buffered saline.
One day after surgery, the femoris muscle (centering on sartorius muscle and adductor muscle) was injected (using a 27-gauge needle) into each site with 100 μL of each of the following suspensions: 10 μg/mL FGF-2 and 6 mg/mL F/P MPs in PBS (FGF-2/F/P MPs), 10 μg/mL FGF-2 in PBS (FGF-2), 6 mg/mL F/P MPs in PBS (F/P MPs), or PBS alone (i.e., the control). Eight sites (which were roughly separated by 3 mm) were injected, as shown in Figure 1A.
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals at National Defense Medical College (Saitama, Japan). All efforts were made to minimize the number of animals used and minimize their suffering.
Assessment of hindlimb survival
The hindlimb survival was evaluated after the administration of angiogenic factors to the ischemic limb model mice, in accordance with the criteria proposed by Cho et al.29 Briefly, after the intramuscular (i.e., femoris muscle) injection of the angiogenic factor into the ischemic hindlimb, the morphology of the treated limbs was visually inspected on days 3, 7, 10, 14, 17, 21, 24, and 28. The mice were classified into three groups: the limb salvage group (i.e., limbs with normal appearance), the necrosis group (i.e., the foot or digits exhibit darkening or loss of digits), and the limb loss group (i.e., autoamputation). The ratio (by percent) of limb salvage was expressed as the number in the limb salvage group divided by the total number of limbs in each group.
Quantification of angiogenesis in the mouse hindlimb
The experimentally induced ischemic hindlimb mice were anesthetized by sodium pentobarbital a day before the injection of the FGF-2/F/P MPs, and then, on days 1, 3, 5, 7, 10, and 14 after the injection (i.e., postinjection). They were then allowed to breathe room air for at least 20 min. The oxygen saturation of the limb in the femoral area and at the top of the foot was measured using a pulse oximeter (Nellcor N-395; Mallinckrodt, Inc., St. Louis, MO), as previously reported by Fujita et al.30 The value of the normal limb (i.e., without femoral artery ligation) was defined as 100%. The recovery of oxygen saturation indicated an improvement in blood perfusion, as oxygen saturation correlated well with blood flow.31
Thermographic monitoring of hindlimb blood flow
Based on the principle that tissues with normal blood supply exhibit an increase in regional surface temperature and tissues with reduced blood supply do not, the surface temperature was determined by thermographically scanning the normal and ischemia-induced hindlimbs. In accordance with the method described by Ring,31 infrared thermography (MobIR M4; Wuhan Guide Infrated Co., Ltd., Wuhan, China) was used to scan the surface temperature so that the level of hindlimb blood flow could be monitored in the mice. Briefly, the room temperature was maintained at 25°C throughout the analysis. The animals were kept at room temperature for 2 h before the analysis. The average surface temperature in the ischemic and the normal hindlimbs was calculated, on the basis of the scanned image, by a computer installed with the IrAnalyser standard software (Wuhan Guide Infrated Co., Ltd.). The rate of reduction in the surface temperature in the ischemic hindlimb was determined. The surface temperature in normal hindlimb is defined as 100%. The analyses were performed a day before the surgery to induce ischemia, and then on days 0, 1, 3, 5, 7, 10, and 14 after those injections.
Histological analysis of neovascularization
On days 3, 7, 14, and 21 after the injection of the FGF-2/F/P MP-suspension or other suspensions into the femoris muscle of the hindlimb in the ischemic model mice, the animals were sacrificed. The femoris muscle (centering on sartorius muscle and adductor muscle) surrounding the injection sites were dissected. The samples were then fixed with 10% formaldehyde in PBS, and subjected to standard histological processing by using hematoxylin and eosin (H&E) staining. Several areas in each section that had been injected with those suspensions were randomly photographed (microscopic field, ×100). The number of capillary lumens per microphotograph was counted. In this study, only mature vessels containing erythrocytes were counted.6,32
Statistical analyses
Statistical analysis was carried out by repeated-measures analysis of variance using the means and standard deviations for each experimental group. Scheffe's post hoc test was used for multiple comparisons, and p<0.05 was considered statistically significant. For survival analysis, the survival rate (as reflected by the hindlimb salvage rate) was analyzed by using the Kaplan–Meier method. The statistical significance of the hindlimb survival experiments was determined using the log-rank test; a p<0.05 was considered statistically significant.
Results
Ischemic limb salvage after FGF-2/F/P MP-treatment
Experimentally induced hindlimb ischemia models were used to evaluate the angiogenic potential of FGF-2/F/P MPs. One week after the intramuscular injection of PBS (i.e., the control), remarkably extensive ischemia-induced necrosis of the operated hindlimb was noted on visual examination (Fig. 1B-d). The necrosis resulted in hindlimb loss by autoamputation. Similar injuries were also observed when F/P MPs alone were injected (Fig. 1B-c). The limbs of 4 of 10 mice injected with FGF-2 suspension failed to be salvaged. The limbs of the remaining six mice were salvageable, and some of these mice had areas of tissue darkening (a symptom of necrosis) that was not large (Fig. 1B-b). By contrast, tissue darkening was suppressed through the entire hindlimb of 8 of the 10 mice treated with FGF-2/F/P MPs, which effectively resulted in limb salvage (Fig. 1B-a).
Four weeks after the intramuscular injection, all mice suffered hindlimb loss that had been injected with a suspension containing F/P MPs alone (10 mice) or PBS alone (6 mice), (data not shown). The administration of FGF-2 prevented hindlimb loss in only 1 of the 10 mice tested, while the remaining 9 mice suffered limb loss (Figs. 1B-e and 2). By contrast, 3 of the 10 mice injected with FGF-2/F/P MPs exhibited complete hindlimb salvage, and one of the salvaged mice had mild limb necrosis with the loss of digits (Figs. 1B-f and 2).
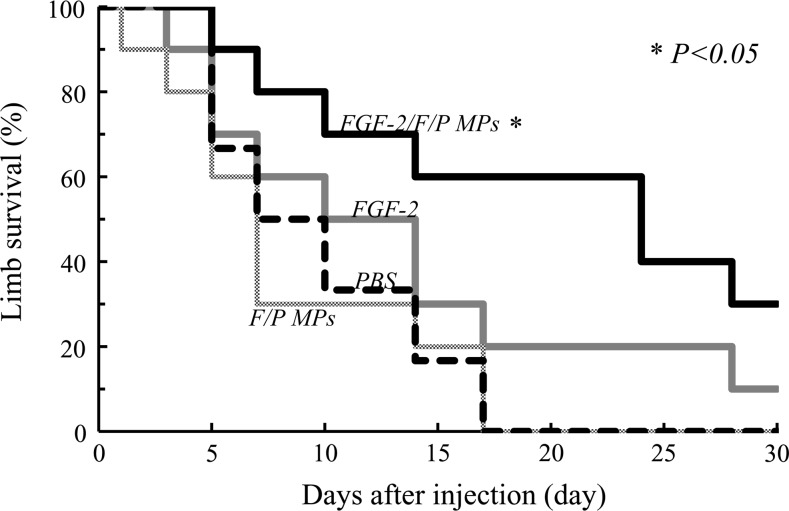
FIG. 2.
The hindlimb survival curves, based on the hindlimb salvage score, indicate the therapeutic effects after the injection of FGF-2/F/P MPs or the other suspensions. The curve was obtained by using the Kaplan–Meier method. The data were analyzed using the log-rank test. The injection of FGF-2/F/P MPs significantly attenuates limb loss (p<0.05; vs. FGF-2).
Prolonged survival of the ischemic hindlimb after the administration of FGF-2/F/P MPs
After the administration of the different suspensions, the survival of the ischemic hindlimbs and the number of hindlimb losses were checked daily and recorded (Fig. 2). The median hindlimb survival time of the mice injected with FGF-2/F/P MPs was 21 days; FGF-2, 8.5 days; F/P MPs, 5 days; and PBS, 6 days. The hindlimb survival was significantly higher in the FGF-2/F/P MP-treated group than in the groups treated with FGF-2, F/P MPs, or PBS (p<0.05).
Improvement of oxygen saturation in the ischemic hindlimb model after the administration of FGF-2/F/P MPs
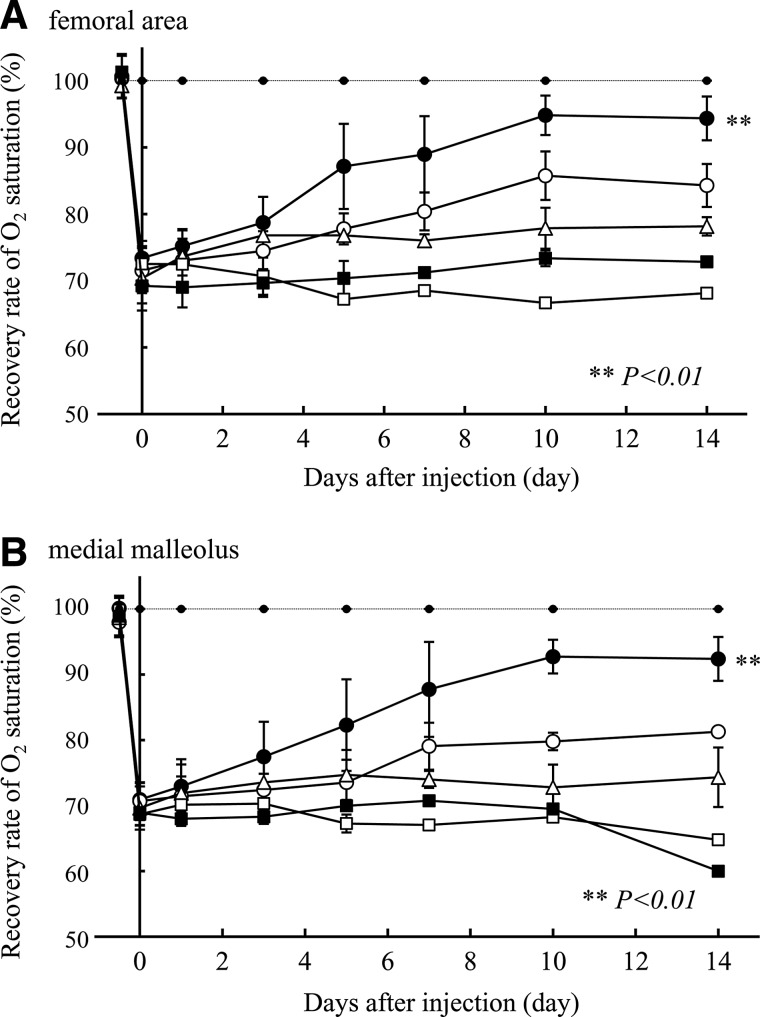
Immediately after femoral artery ligation, the oxygen saturation level in the femoral area and the medial malleolus of the ischemic hindlimb decreased to ∼70% of the oxygen saturation level of the normal hindlimb without the femoral artery ligation (Fig. 3). During 2 weeks after the injection, the reduced oxygen saturation levels remained almost unchanged in the surviving hindlimbs of the F/P MP-treated and control groups. However, the oxygen saturation level in the medial malleolus tended to be lower than the oxygen saturation level in the femoral area. In the surviving hindlimbs of the FGF-2/F/P MP-treated group, the oxygen saturation level in the femoral area and medial malleolus recovered to nearly normal levels by 10 days after the injection, and the recovered levels remained for at least 17 days. The oxygen saturation level in the FGF-2-treated group recovered slowly, but the oxygen saturation level was always lower than that of the FGF-2/F/P MP-treated group.
FIG. 3.
The time course of the recovery of oxygen saturation in (A) the femoral area and (B) the medial malleolus after the injection of FGF-2/F/P MPs (●), FGF-2 (◯), F/P MPs (△), PBS (i.e., control) (■), or nonadministration group (i.e., control) (□). All values are the means±the SD up to the fifth postinjection day. The oxygen saturations of the surviving limbs in each group were measured 7, 10, and 14 days after the injection, as some of the target limbs were lost during this period. The dashed line represents the group in which the skin was only incised (i.e., there was no ligation, cutting, and removal of blood vessels). Repeated-measures ANOVA was used for comparisons of the FGF-2/F/P MP-treated group and the other groups on postinjection days 10 and 14. ANOVA, analysis of variance; SD, standard deviations.
Perfusion measurement using thermography
Thermography was used to evaluate skin blood flow a day before the injection, and then on days 1, 3, 5, 7, 10, 14, and 21 after the injection (Fig. 4). In the ischemia-induced hindlimb model, the surface temperature of the ischemic hindlimb (in which the femoral artery had been ligated) was reduced to 85% of the surface temperature of the normal hindlimb. Since the surface temperature of a body reflects the state of blood perfusion, a reduction in the surface temperature in the ischemic hindlimb suggests reduced hindlimb blood flow.
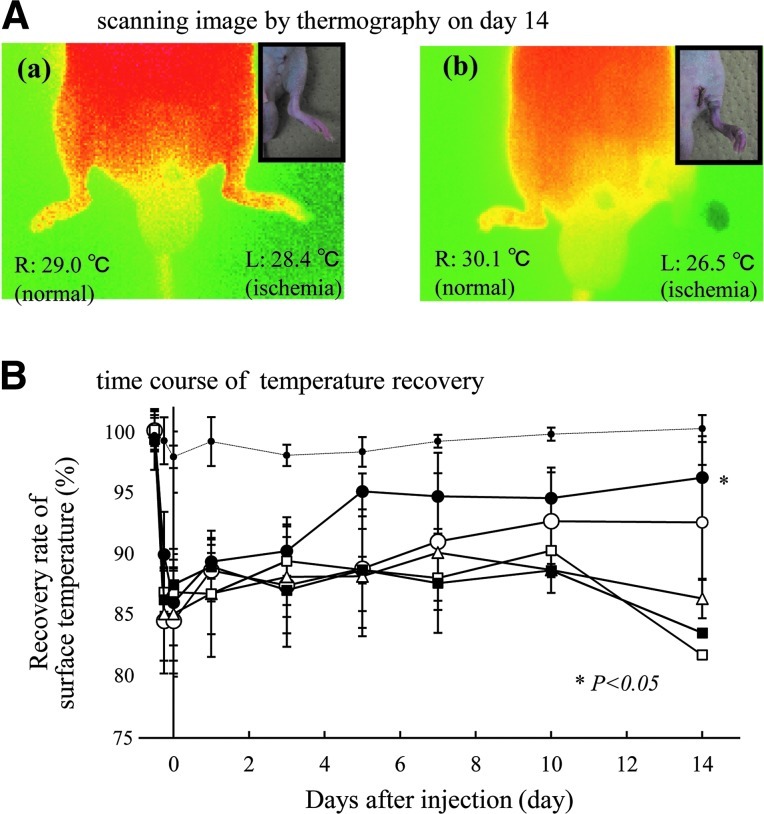
FIG. 4.
(A) A thermographic scan of the hindlimb on day 14. The limbs of some mice injected with (a) FGF-2/F/P MPs or (b) FGF-2 have a similar external appearance as shown in insets. However, a difference in the skin temperature exists between the two groups. (B) The time course change of temperature recovery. Thermographic scanning shows that the surface temperature of the ischemic hindlimb decreases by about 15%, compared with the normal hindlimb. The reduction of skin temperature is partially recovered in the FGF-2/F/P MP-treated group (●) and the FGF-2-treated group (◯). In the F/P MP-treated group (△) and PBS group (i.e., control) (■), there is no recovery of the surface temperature in the ischemic hindlimb such as the nonadministration group (□). The dashed line represents the group in which the skin was only incised (i.e., there was no ligation, cutting, and removal of blood vessels). Repeated-measures ANOVA was used to compare the FGF-2/F/P MP-treated group and the other groups on postinjection day 14. Color images available online at www.liebertpub.com/tea
More than half of the FGF-2-treated group had lost their hindlimbs by 14 days after the injection (as shown in Fig. 2). However, some limbs of the FGF-2-treated group had a similar external appearance and recovery pattern as those of the FGF-2/F/P MP-treated group. Despite this, there was a significant difference between the FGF-2/F/P MP-treated and FGF-2-treated groups with regard to the skin temperature of the surviving hindlimbs 14 days after the injection (Fig. 4A). There was no recovery of surface temperature in the ischemic hindlimbs of the F/P MP-treated and control groups (Fig. 4B). These findings reflected a significant improvement of blood flow in the ischemic hindlimb after the administration of FGF-2/F/P MPs.
Reduction of muscle damage in the ischemic hindlimb after the administration of FGF-2/F/P MP
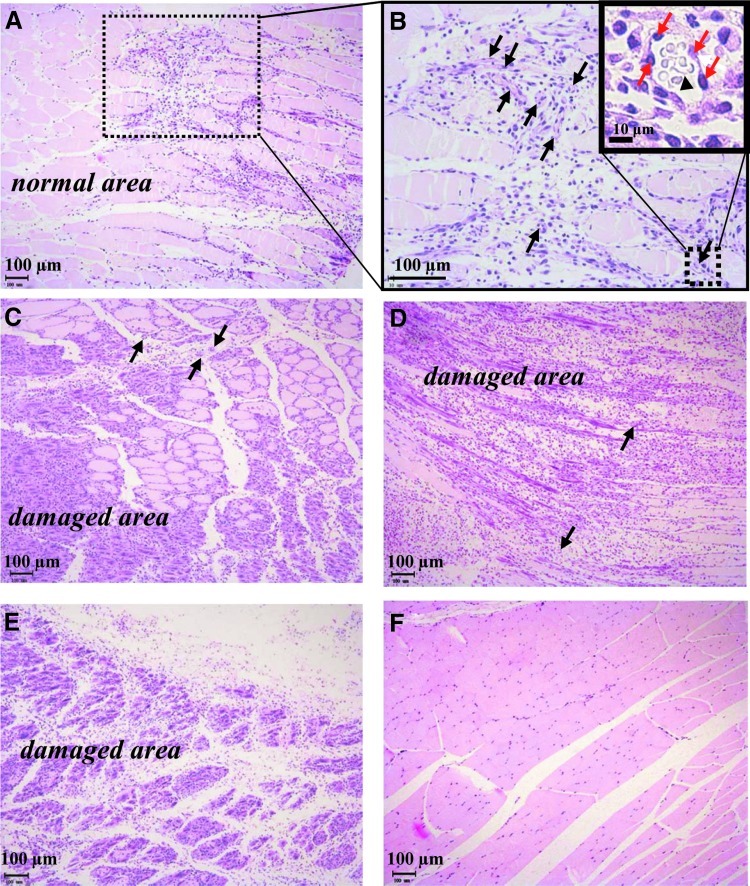
A pathological examination of the H&E-stained femoris muscle specimens, which were dissected 14 days after the injection, was performed to determine whether ischemia-induced necrotic damage is reduced when FGF-2/F/P MPs are administered (Fig. 5). The muscles in the ischemic hindlimb of FGF-2/F/P MP-treated group were, as expected, protected from muscle degeneration (Fig. 5A, B). Fourteen days after the injection, the FGF-2/F/P MPs remained notably present at the injection sites (data not shown). Furthermore, the generation of fibrous tissue with numerous neutrophils was observed in the injected area (Fig. 5B). The administration of FGF-2 alone resulted in partial muscle degeneration (Fig. 5C). By contrast, massive muscle degeneration was accompanied by lysis in the ischemic regions of F/P MP-treated group (Fig. 5D) and the PBS group (Fig. 5E).
FIG. 5.
Histological examination of samples obtained 14 days after the injection from the sites injected with (A, B) FGF-2/F/P MPs, (C) FGF-2, (D) F/P MPs, and (E) PBS. Image (F) shows the femoral site of the normal healthy mouse. In (B), (C), and (D), the black arrows indicate blood vessels. In (B), the magnification of the sample injected with FGF-2/F/P MPs shows vessels containing endothelial cells (red arrows) and erythrocytes (black triangle). Ischemia-induced necrotic damage is observed in (C) partial muscle and (D, E) massive muscle. Color images available online at www.liebertpub.com/tea
Increased capillary number in the ischemic hindlimb after the administration of FGF-2/F/P MPs
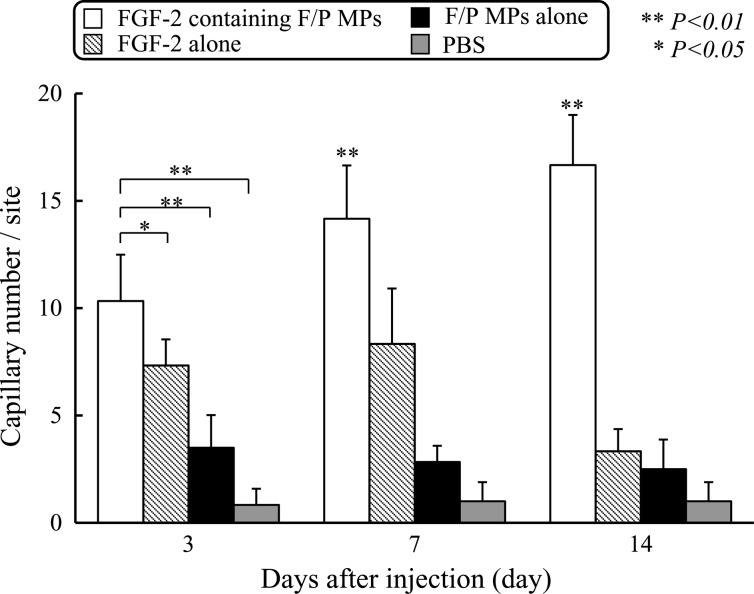
The number of microcapillaries in tissues near the injection sites was measured on postinjection days 3, 7, 14, and 21. As shown in Figure 6, 7 days after the injection, numerous mature vessels containing erythrocytes around the sites were injected with FGF-2/F/P MPs. Notable neovascularization commenced as early as 3 days after the injection. The degree of neovascularization was significantly higher in the FGF-2/F/P MP-treated group than in the other groups (p<0.01). Thus, the increase in the number of microcapillaries appeared to be correlated with improved blood flow.
FIG. 6.
The effect of FGF-2/F/P MPs injection on neovascularization in vivo. The number of capillaries is counted by using a microphotograph (Fig. 5) of each section (n=6) exhibiting a high mature capillary density. All values are the means±the SD. Repeated-measures ANOVA was used for comparisons.
Discussion
It is important to know which strains of mice should be chosen to create an ischemic hindlimb model, as the degree of perfusion recovery in the model after treatment with angiogenic factors is affected by the genetic background of the mouse strain. Fukino et al33 suggest that the Balb/c-nu/nu mouse is suitable for the study of angiogenesis, as this mouse strain has a greater tendency for impaired collateral vessel formation than other mouse strains. The impaired collateral vessel formation results from the reduced intramuscular expression of the VEGF receptor. As a result, impaired blood flow is more profound in ischemic Balb/c-nu/nu mice than in other mouse strains. Based on this background, we chose Balb/c-nu/nu mice to create an ischemic hindlimb model.
In our previous report, we found that F/P MPs consist of LMWH (i.e., fragmin) and protamine, which serve as a very useful carrier for the controlled release of heparin-binding growth factors such as FGF-2.6,34 FGF-2 is gradually released from the FGF-2/F/P MPs. The local delivery of the FGF-2/F/P MPs induces local angiogenesis and fibrous tissue formation in normal mice. Heparin and heparan sulfate (i.e., heparinoids) are co-factors that enhance FGF-2 activity.22,23 Since fragmin is a component of F/P MPs, the synergistic effects of FGF-2/F/P MPs would be expected to result in a high level of recovery of blood flow after ischemia. We recently also published a paper that described efficacy to induce collateral vessels in a rabbit model of hindlimb ischemia.35 The purpose of the previous study was to evaluate the ability of FGF-2/F/P MPs to induce arteriogenesis and larger collateral arteries (more than 200 micro-meters in diameter) in vivo. Therefore, the study used a mild rabbit ischemic model that could recover to normal blood flow with time by spontaneous cure and performed angiography for monitoring the diameter of blood vessels.35 On the other hand, the present study examined the ability of FGF-2/F/P MPs to improve impaired blood flow by using an experimentally induced severe ischemic mouse model. The impaired blood flow was more profound than other similar models, and all nontreated ischemic limbs were lost within 17 days. Thus, the present study (not the previous study) suggests that FGF-2/F/P MPs-treatment may be effective for the creation of collateral vessels as an alternative approach for the management of severe ischemia, with the hope of preventing amputation from nonreconstructable vascular diseases.
The injection of FGF-2 alone caused neovascularization, which peaked from 3 to 7 days, in the ischemic hindlimb model. This effect was better in the FGF-2-treated group than in the F/P MP-treated and control groups (Fig. 6). The results were correlated with the gradual recovery of oxygen saturation (Fig. 3) and surface temperature (Fig. 4), after neovascularization improved the blood flow. However, in terms of recovery of surface temperature, oxygen saturation level, and the rate of hindlimb survival, these effects of neovascularization were significantly greater in the FGF-2/F/P MP-treated group than the FGF-2-treated group (Fig. 2). In the present study, we observed that the single administration of 10 μg of FGF-2 coupled with F/P MPs was sufficient to optimally induce neovascularization in the ischemic hindlimb model. For the future practical use of the F/P MP-mediated delivery of FGF-2 in vivo as a therapeutic drug, the optimal dose of the protein and the mixing rate between FGF-2 and F/P MPs would need to be determined to achieve maximal therapeutic results.
In ischemic mice receiving FGF-2/F/P MPs, the induction of neovascularization commenced near the injected sites as early as 3 days after the drug was administered and reached maximal levels at 2 weeks (Fig. 6). The number of newly formed blood vessels remained unchanged from 14 to 21 days after the injection of FGF-2/F/P MPs, but decreased by day 28 onward (data not shown). This pattern of generation and disappearance of newly formed blood vessels appears to be correlated with the time course of the controlled release of FGF-2, as well as the degradation of F/P MPs, as previously described.6 Furthermore, the reduced oxygen saturation level in the femoral area and medial malleolus of the ischemic hindlimb recovered to nearly normal levels 3 to 10 days after the injection of FGF-2/F/P MPs. The recovered levels were maintained for at least 21 days (Fig. 3). Those results collectively demonstrate a correlation between FGF-2/F/P MP-induced neovascularization in the hindlimb and improved blood perfusion.
The intramuscular application of FGF-2/F/P MPs may, thus, provide new therapeutic options to enhance neovascularization in tissues where the blood flow is impaired by ischemia, accidental injuries, or disease. Preparation of FGF-2/F/P MPs is very simple, and the FGF-2/F/P MPs are ready to apply directly into the ischemic region. Furthermore, FGF-2/F/P MPs seem to be safe, as all of the components of FGF-2/F/P MPs are, in fact, in clinical use. However, since sufficient data are not yet available on the complete toxicity profile of the FGF-2/F/P MPs in humans, standard toxicological studies need to be completed before using the materials for human subjects.
Our preliminary studies demonstrated that platelet-rich plasma (which contains various concentrated angiogenic factors) or the combined use of angiogenic factors and anti-apoptotic factors coupled with F/P MPs effectively enhanced neovascularization in experimentally induced ischemic hindlimb models, compared with the single use of each factor (data unpublished). The co-administration of FGF-2 and HGF into an ischemic limb model similarly effectively enhances blood vessel formation.36 Thus, the combined use of several biologically active factors or the use of platelet-rich plasma coupled with F/P MPs would increase the therapeutic efficacy.
The option of repeatedly injecting FGF-2/F/P MPs may also increase therapeutic efficacy. The present study shows a decrease in formed blood vessels 14 days after the first injection of FGF-2/F/P MPs (Fig. 6). Our preliminary results showed that neovascularization induced by a re-injection of FGF-2/F/P MPs maintained blood flow and tended to prevent later hindlimb loss (data not shown).
In summary, the intramuscular injection of the FGF-2/F/P MPs into ischemic hindlimbs promotes neovascularization and attenuates limb loss associated with local ischemia. The present approach using FGF-2/F/P MPs could be considered a valuable option for therapeutic angiogenesis targeted for tissue regeneration and treating ischemic disease.
Acknowledgments
The authors thank Dr. Koichi Fukuda (Center for Laboratory Animal Science, National Defense Medical College) for his support in animal experiments, and Dr. Yoshihiro Tanaka (National Defense Medical College) for discussion. This work was supported by the Grant-in-Aid for Young Scientists (B) (Grant No. 22780274) from the Ministry of Education, Science, Sports, Culture, and Technology of Japan.
Disclosure Statement
No competing financial interests exist.
References
- 1.Abraham J.A. Mergia J. Whang L. Tumolo A. Friedman J. Hjerrild K.A. Gospodarowicz D. Fiddes J.C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986;234:545. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- 2.Takeshita S. Tsurumi Y. Couffinahal T. Asahara T. Bauters C. Symes J. Ferrara N. Isner J.M. Gene transfer of naked DNA encoding for three isoforms of vascular endothelial growth factor stimulates collateral development in vivo. Lab Invest. 1996;75:487. [PubMed] [Google Scholar]
- 3.VanBelle E. Witzenbichler B. Chen D. Silver M. Chang L. Schwall R. Isner J.M. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor. The case for paracrine amplification of angiogenesis. Circulation. 1998;97:381. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 4.Kocher A.A. Schuster M.D. Szabolcs M.J. Takuma S. Burkhoff D. Wang J. Homma S. Edwards N.M. Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs S. Baffour R. Zhou Y.F. Shou M. Pierre A. Tio F.O. Weissman N.J. Leon M.B. Epstein S.E. Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura S. Kanatani Y. Kishimoto S. Nakamura S.-I. Ohno C. Horio T. Fujita M. Hattori H. Tanaka Y. Kiyosawa T. Maehara T. Ishihbara M. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J Biomed Mater Res. 2009;91A:814. doi: 10.1002/jbm.a.32265. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger M.F. Martin B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 8.Kalka C. Masuda H. Takahashi T. Kalka-Moll W.M. Silver M. Kearney M. Li T. Isner J.M. Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shyu K.G. Manor O. Magner M. Yancopoulos G.D. Isner J.M. Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit ischemic hindlimb. Circulation. 1998;98:2081. doi: 10.1161/01.cir.98.19.2081. [DOI] [PubMed] [Google Scholar]
- 10.Taniyama Y. Morishita R. Aoki M. Nakagami H. Yamamoto K. Yamazaki K. Matsumoto K. Nakamura T. Kaneda Y. Ogihara T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hind limb ischemia models: preclinical study for treatment of peripheral arterial disease. Gene Ther. 2001;8:181. doi: 10.1038/sj.gt.3301379. [DOI] [PubMed] [Google Scholar]
- 11.Morishita R. Makino H. Aoki M. Hashiya N. Yamasaki K. Azuma J. Taniyama Y. Sawa Y. Kaneda Y. Ogihara T. Phase I/IIa clinical trial of therapeutic angiogenesis using hepatocyte growth factor gene transfer to treat critical limb ischemia. Arterioscler Thromb Vasc Biol. 2011;31:713. doi: 10.1161/ATVBAHA.110.219550. [DOI] [PubMed] [Google Scholar]
- 12.Cote M.F. Laroche G. Gagnon E. Chevallier P. Doillon C.J. Denatured collagen as support for a FGF-2 delivery system: physicochemical characterizations and in vitro release kinetics and bioactivity. Biomaterials. 2004;25:3761. doi: 10.1016/j.biomaterials.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Young S. Wong M. Tabata Y. Mikos A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 14.DeBlois C. Cote M.F. Doillon C.J. Heparin-fibroblast growth factor-fibrin complex: in vitro and in vivo applications to collagen-based materials. Biomaterials. 1994;15:665. doi: 10.1016/0142-9612(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 15.Tanihara M. Suzuki Y. Yamamoto E. Noguchi A. Mizushima Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J Biomed Mater Res. 2001;56:216. doi: 10.1002/1097-4636(200108)56:2<216::aid-jbm1086>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Ornitz D.M. Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:3005.1. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canalis E. Centrella M. McCarthy T. Effects of basic fibroblast growth factor on bone formation in vitro. J Clin Invest. 1988;81:1572. doi: 10.1172/JCI113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima F. Ogasawara A. Goto K. Moriya H. Ninomiya Y. Einhorn T.A. Yamazaki M. Spatial and temporal gene expression in chondrogenesis during fracture healing and the effects of basic fibroblast growth factor. J Orthop Res. 2001;19:935. doi: 10.1016/S0736-0266(01)00024-9. [DOI] [PubMed] [Google Scholar]
- 19.Tsuboi R. Rifkin D.B. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impairing db/db mice. J Exp Med. 1990;172:245. doi: 10.1084/jem.172.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara M. Ono K. Structure and function of heparin and heparan sulfate: heparinoid library and modification of FGF-activities. Trend Glycosci Glycotech. 1998;10:223. [Google Scholar]
- 21.Ishihara M. Biosynthesis, structure, and biological activity of basic FGF binding domains of heparan sulfate. Trend Glycosci Glycotech. 1993;5:343. [Google Scholar]
- 22.Salmivirta M. Lidholt K. Lindahl U. Heparan sulfate: a piece of information. FASEB J. 1996;10:1270. doi: 10.1096/fasebj.10.11.8836040. [DOI] [PubMed] [Google Scholar]
- 23.Lindahl U. Lidholt K. Spillmann D. Kjellen L. More to “heparin” than anti-coagulation. Thromb Res. 1994;75:1. doi: 10.1016/0049-3848(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara M. Obara K. Ishizuka T. Fujita M. Sato M. Masuoka K. Saito Y. Yura H. Matsui T. Hattori H. Kikuchi M. Kurita A. Controlled release of fibroblast growth factors and heparin from photocrosslinked chitosan hydrogels and subsequent effect on in vivo vascularization. J Biomed Mater Res. 2003;64A:551. doi: 10.1002/jbm.a.10427. [DOI] [PubMed] [Google Scholar]
- 25.Hirsh J. Warkentin T.E. Shaughnessy S.G. Anand S.S. Halperin J.L. Raschke R. Granger C. Ohman E.M. Dalen J.E. Heparin and low-molecular-weight heparin, mechanisms of action, phormacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119:64S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- 26.Wolzt M. Weltermann A. Nieszpaur-Los M. Schneider B. Fassolt A. Lechner K. Eichler H.G. Kyrle P.A. Studies on the neutralizing effects of protamine on unfractionated and low molecular weight heparin (FragminR) at the site of activation of the coagulation system in man. Thromb Haemost. 1995;73:439. [PubMed] [Google Scholar]
- 27.Pan M. Suarez de Lezo J. Medina A. Romero M. Hernandez E. Segura J. Melian F. Wanguemert F. Landin M. Benitez F. Amat M. Velasco F. Torres A. In-laboratory removal of femoral sheath following protamine administration in patients having intracoronary stent implantation. Am J Cardiol. 1997;80:1336. doi: 10.1016/s0002-9149(97)00676-0. [DOI] [PubMed] [Google Scholar]
- 28.Couffinhal T. Silver M. Zheng L.P. Kearney M. Witzenbichler B. Isner J.M. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667. [PMC free article] [PubMed] [Google Scholar]
- 29.Cho S.W. Moon S.H. Lee S.H. Kang S.W. Kim J. Lim J.M. Kim H.S. Kim B.S. Chung H.M. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- 30.Fujita M. Ishihara M. Shimizu M. Obara K. Nakamura S. Kanatani Y. Morimoto Y. Takase B. Matsui T. Kikuchi M. Maehara T. Therapeutic angiogenesis induced by controlled release of fibroblast growth factor-2 from injectable chitosan/non-anticoagulant heparin hydrogel in a rat hindlimb ischemia model. Wound Repair Regen. 2007;15:58. doi: 10.1111/j.1524-475X.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- 31.Ring F.J. Thermal imaging today and its relevance to diabetes. Diabetes Sci Technol. 2010;4:857. doi: 10.1177/193229681000400414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura S. Kishimoto S. Nakamura S.-I. Nambu M. Fujita M. Tanaka Y. Mori Y. Tagawa M. Maehara T. Ishihara M. Fragmin/protamine microparticles as cell carriers to enhance viability of adipose-derived stromal cells and their subsequent effect on in vivo neovascularization. J Biomed Mater Res. 2010;92A:1614. doi: 10.1002/jbm.a.32506. [DOI] [PubMed] [Google Scholar]
- 33.Fukino K. Sata M. Seko Y. Hirata Y. Nagai R. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res Commun. 2003;310:143. doi: 10.1016/j.bbrc.2003.08.134. [DOI] [PubMed] [Google Scholar]
- 34.Kishimoto S. Nakamura S. Nakamura S.-I. Kanatani Y. Hattori H. Tanaka Y. Harada Y. Tagawa M. Mori Y. Maehara T. Ishihara M. Fragmin/protamine microparticle-coated matrix immobilized cytokines to stimulate various cell proliferations with low serum media. Artif Organs. 2009;33:431. doi: 10.1111/j.1525-1594.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 35.Horio T. Fujita M. Tanaka Y. Ishihara M. Kishimoto S. Nakamura S. Hase K. Maehara T. Efficacy of fragmin/protamine microparticles containing fibroblast growth factor-2 (F/P MPs/FGF-2) to induce collateral vessels in a rabbit model of hindlimb ischemia. J Vasc Surg. 2011;54:791. doi: 10.1016/j.jvs.2011.02.060. [DOI] [PubMed] [Google Scholar]
- 36.Marui A. Kanematsu A. Yamahara K. Doi K. Kushibiki T. Yamamoto M. Itoh H. Ikeda T. Tabata Y. Komeda M. Simultaneous application of basic fibroblast growth factor and hepatocyte growth factor to enhance the blood vessels formation. J Vasc Surg. 2005;41:82. doi: 10.1016/j.jvs.2004.10.029. [DOI] [PubMed] [Google Scholar]