Abstract
Cryopreservation is important for clinical translation of tissue-engineered constructs. With respect to a pancreatic substitute, encapsulated islets or beta cells have been widely studied for the treatment of insulin-dependent diabetes mellitus. Besides cell viability loss, cryopreservation may affect the function of the remaining viable cells in a pancreatic substitute by altering fundamental processes in glucose-stimulated insulin secretion, such as pathways associated with intermediary metabolism, potentially leading to insulin-secretion defects. In this study, we used 13C nuclear magnetic resonance (NMR) spectroscopy and isotopomer analysis to determine the effects of conventional freezing and ice-free cryopreservation (vitrification) on carbon flow through tricarboxylic acid (TCA) cycle–associated pathways in encapsulated murine insulinoma βTC-tet cells; the secretory function of the encapsulated cells postpreservation was also evaluated. Specifically, calcium alginate–encapsulated βTC-tet cells were frozen or vitrified with a cryoprotectant cocktail. Beads were warmed and 13C labeling and extraction were performed. Insulin secretion rates were determined during basal and labeling periods and during small-scale glucose stimulation and K+-induced depolarization. Relative metabolic fluxes were determined from 13C NMR spectra using a modified single pyruvate pool model with the tcaCALC modeling program. Treatments were compared with nonpreserved controls. Results showed that relative carbon flow through TCA-cycle-associated pathways was not affected by conventional freezing or vitrification. However, vitrification, but not freezing, led to impaired insulin secretion on a per viable cell basis. The reduced secretion from the Vitrified group occurred irrespective of scale and was present whether secretion was stimulated by glucose or K+-induced depolarization, indicating that it might be due to a defect in late-stage secretion events.
Introduction
Encapsulated islets or beta cells have been studied extensively as tissue-engineered pancreatic substitutes, as they may provide more physiologic control of blood glucose levels compared to exogenous insulin therapy.1–10 For future clinical translation, long-term storage of encapsulated cells for off-the-shelf availability is essential, and cryopreservation is a promising means toward achieving this objective. The two main methods of cryopreservation—vitrification, or ice-free cryopreservation, and conventional freezing—have both been studied in preserving encapsulated islets11–15 or beta cells.16,17 Vitrification is promising since it may maintain biomaterial integrity better than conventional freezing, as indicated in previous studies with encapsulated islets12 and insulinoma cells.16
In terms of construct function postpreservation, recent studies have indicated that insulin secretion is often impaired after cryopreservation, specifically after conventional freezing of encapsulated insulinoma cells16 and conventional freezing12 or vitrification11,12 of encapsulated islets. Although a reduction in cell viability may be an important component of this decreased secretion, the function of the remaining viable cells may also be compromised. Thus, an understanding of cryopreservation effects on fundamental aspects of beta cell function is critical for elucidating cryopreservation-induced secretion anomalies; however, no studies have addressed the quality of the remaining viable cells in cryopreserved encapsulated islets or beta cells, besides their ability to secrete insulin.11–16 Evaluating intermediary metabolism after cryopreservation is especially important, as it is key to glucose-stimulated insulin secretion (GSIS) in beta cells,18–21 and cryopreservation has been shown to affect aspects of intermediary metabolism in other systems.22,23 Recently, 13C nuclear magnetic resonance (NMR) spectroscopy and isotopomer analysis were used to study mitochondrial metabolism in GSIS, by correlating certain metabolic fluxes with insulin secretion using specific models applied in the tcaCALC modeling program.20,24–28 Since the natural abundance of 13C is only 1.1%, it can be introduced into cells as an isotopic tracer, with its path being followed by the labeling patterns found in enriched metabolites.29 Adjacently labeled 13C carbons lead to the formation of isotopomer patterns, and analysis of steady state glutamate isotopomer patterns with models of metabolism can estimate relative carbon flow through tricarboxylic acid (TCA) cycle–associated pathways, thus allowing for a quantitative assessment of intermediary metabolism.30
In this work, we applied 13C NMR and isotopomer analysis to elucidate the intermediary metabolism of calcium alginate–encapsulated murine insulinoma βTC-tet cells postcryopreservation by conventional freezing and vitrification. The secretory function of the encapsulated cells was also characterized. Cell-derived 13C-glutamate isotopomers were analyzed using a previously published model of glucose metabolism24,25,28 with tcaCALC to determine relative metabolic fluxes through TCA-cycle-associated pathways, and the corresponding insulin secretion was measured postpreservation. To gain further insight into effects on secretion, the latter was measured in response to both glucose and high K+ exposure. The ramifications of our findings toward identifying specific cellular processes affected by cryopreservation are discussed.
Materials and Methods
Cell culture and encapsulation
All chemicals were from Sigma (St. Louis, MO) unless otherwise noted. The βTC-tet cell line was obtained from S. Efrat (Albert Einstein College of Medicine, Bronx, NY).31 Cells were cultured as monolayers in Dulbecco's modified Eagle's medium (DMEM) (pH 7.4) containing 25 mM glucose and 4 mM L-glutamine, supplemented with 10% (v/v) fetal bovine serum (FBS) (Gemini Bioproducts, West Sacramento, CA), 1% (v/v) penicillin (10,000 U/mL)–streptomycin (10,000 μg/mL) (Mediatech, Manassas, VA), and 1% L-glutamine (Mediatech) to a final concentration of 6 mM. This medium is henceforth referred to as complete DMEM. Cells were cultured in a humidified incubator at 37°C with 5% CO2/95% air, and used between passages 37 and 42.
Cells were detached from monolayers using 0.25% trypsin (Mediatech) and encapsulated at 7×107 cells/mL alginate in 2% PRONOVA ultrapure LVM sodium alginate (FMC Biopolymer, Philadelphia, PA), with droplets crosslinked in 1.1% (w/v) CaCl2, as described previously,16 but without poly-L-lysine coating. Beads were 500–600 μm in diameter and were incubated overnight on a platform rocker (Stovall, Greensboro, NC) in complete DMEM. All salt solutions used in the encapsulation and subsequent procedures were adjusted to 300 mOsm to ensure isotonicity.
Vitrification
Cryopreservation was performed 1 day postencapsulation. Beads were vitrified using the cryoprotectant (CPA) cocktail DPS comprised of 3 M dimethylsulfoxide (DMSO), 3 M 1,2 propanediol (PD), and 0.5 M sucrose (Fisher Chemical, Fisher Scientific, Pittsburgh, PA) in a modified version of the EuroCollins carrier solution. The EuroCollins component of the CPA solution contained 34.95 g/L glucose, 0.84 g/L NaHCO3 (Fisher Chemical), 1.12 g/L KCl, and 1.68 g/L NaCl. Initial studies indicated that a volume of 6 mL Fresh beads was adequate to obtain sufficient signal-to-noise ratio in the 13C NMR spectra. As Vitrified/warmed beads had a minimum metabolically active cell number of 50% that of Fresh beads, a 13 mL of bead volume was vitrified to obtain a sufficient signal-to-noise ratio. Although similar metabolically active cell numbers were used for all groups to obtain sufficient signal-to-noise ratios, at isotopomeric steady state, isotopomer analysis is cell-number independent. CPAs were added to beads on ice stepwise according to the protocol in Table 1. This protocol was developed using a mathematical model that minimized cell osmotic excursions while ensuring CPA equilibration at each step.32 One milliliter of beads was placed in a 100 μm cell strainer (BD Biosciences, Bedford, MA) and sequentially transferred through CPA solutions of increasing concentration (Table 1) at 4°C in a six-well plate (BD Biosciences). Following CPA addition, beads plus CPA were transferred to 20-mL glass scintillation vials (Fisherbrand/Fisher Scientific), and isopentane (EMD Chemicals, Gibbstown, NJ) was added on top of the CPA/bead mixture. Vials were transferred to a rack and the rack was placed in an isopentane bath in a mechanical freezer (Sanyo North America, San Diego, CA) set at −135°C for fast cooling to ∼−100°C at a rate of ∼64°C/min. Vials were then transferred to a shelf in the freezer for slow cooling at ∼2°C/min to −130°C, after which they were transferred to freezer storage boxes. During cooling, temperature was tracked with a thermocouple in a sample containing only CPA and isopentane. For each 13C extraction, vitrification was performed on 1 mL of bead aliquots 13 separate times. Vials were kept in the freezer overnight, and the next day 1 mL of beads was warmed at a time by agitating vials in a room-temperature 30% (v/v) DMSO bath. Vitrification in each vial was verified by visual observation after cooling and immediately prior to warming.33 Additionally, no crystallization/devitrification was visible upon warming. CPAs were then removed stepwise at room temperature in a similar fashion as CPA addition (Table 1). After CPA removal, beads were incubated in complete DMEM at 37°C until all 13 mL of beads were warmed and CPA removed. Beads were then combined and a 12-mL volume was withdrawn for labeling and extraction.
Table 1.
Cryoprotectant Addition/Removal Protocol for DPS Vitrification
| Steps | DMSO (M) | PD (M) | Sucrose (M) | Time (min) | Temperature (°C) |
|---|---|---|---|---|---|
| A1 | 1 | 1 | 0.15 | 2 | 4 |
| A2 | 2 | 2 | 0.3 | 2 | 4 |
| A3 | 3 | 3 | 0.5 | 2 | 4 |
| R1 | 2.25 | 2.25 | 0.3 | 2 | RT |
| R2 | 1.5 | 1.5 | 0.2 | 2 | RT |
| R3 | 0.75 | 0.75 | 0.1 | 2 | RT |
| R4 | 0 | 0 | 0 | 4 | RT |
A, addition; R, removal; RT, room temperature; PD, 1,2 propanediol; DMSO, dimethylsulfoxide.
Conventional freezing
Beads were frozen in complete DMEM containing 10% (v/v) DMSO. After incubation in this solution at 4°C for 10 min, beads were transferred to 2.0 mL cryogenic vials (Corning, Inc., Corning, NY) and placed in a Mr. Frosty isopropyl alcohol bath (Nalgene/Thermo Fisher Scientific, Rochester, NY) in a −80°C freezer (VWR International, Inc., Radnor, PA) for 1.5 h, followed by plunging into liquid nitrogen. As previous studies indicated a minimum metabolically active cell number of ∼90% that of Fresh beads postwarming, 8 mL of beads was frozen (in 1-mL batches) to obtain a sufficient signal-to-noise ratio in the NMR spectra. After overnight storage, 1 mL of beads at a time was warmed rapidly by agitating vials in a 37°C water bath until no ice was visible. Beads were next placed in complete DMEM at 37°C for 10 min. After a subsequent wash with complete DMEM, beads were incubated in complete DMEM at 37°C until all batches were warmed. Beads were then combined and a 7-mL volume was withdrawn for labeling and extraction.
13C labeling and perchloric acid extraction of encapsulated cells
Figure 1 shows the experimental procedure for 13C labeling and extraction. 13C labeling and extraction was performed 1 day postencapsulation for Fresh and 2 days postencapsulation for Cryopreserved beads. A 6-mL bead volume was used for the Fresh control. After washing beads four times with basal medium consisting of glutamine-free and glucose-free DMEM supplemented with 1.31 g/L bovine serum albumin (BSA) (Sigma) at pH 7.4, beads were incubated in the same type of medium for 1 h at 37°C. Beads were subsequently washed once with basal medium and once with labeling medium, containing 15 mM uniformly labeled 13C glucose (Cambridge Isotope Laboratories, Andover, MA) in glucose-free DMEM (pH 7.4) plus 10% (v/v) FBS (Gemini Bioproducts), 1% (v/v) L-glutamine (Mediatech), and 1% (v/v) penicillin–streptomycin (Mediatech), and incubated in the same type of medium for 6 h. This labeling period was determined in a previous isotopomeric steady-state study (data not shown). Subsequently, perchloric acid extraction was performed based on the protocol in Simpson et al.,28 with modifications. Beads were washed with glucose-free and glutamine-free DMEM and alginate was dissolved in 110 mM sodium citrate (Fisher Chemical/Fisher Scientific). Cells were pelleted at 200 g at 4°C for 10 min, and the pellet was washed with 0.85% (w/v) NaCl. Cells were then extracted twice in 0.5 M perchloric acid using an Omni GLH homogenizer (Omni International, Marietta, GA) with an Omni Tip™ plastic generator probe to prevent paramagnetic ion contamination. Supernatants were pooled and neutralized with 0.01–5 M KOH. This solution was then centrifuged at 10,000 g to remove precipitated salts and Chelex-100® resin (Sigma) was added to the supernatant to remove paramagnetic ions. After stirring the solution containing chelex resin for 1 h on ice, the resin was removed by filtration through ashless filter paper (Whatman, Inc., Piscataway, NJ) in a Nalgene® polysulfone filter holder (Thermo Fisher Scientific). Subsequently, the resin was chased with ultrapure water. The pH of the filtrate was adjusted to ∼7.4 with 0.01–1 M HCl. The final extract was frozen at −80°C and subsequently placed in a lyophilizer (Labconco Corporation, Kansas City, MO) for 2 days prior to reconstitution.
FIG. 1.
Overall experimental procedure for 13C labeling and extraction experiments of Fresh and Cryopreserved encapsulated βTC-tet cells.
13C extract reconstitution and NMR spectroscopy
Lyophilized extracts were reconstituted in 200 μL of 99.99% D2O and subsequently spun at 18,300 g at room temperature for 10 min. The supernatant was placed on ice for 30 min to precipitate out KClO4 salts prior to being centrifuged at 18,000 g for 10 min at 0°C to pellet out additional precipitated salts. The supernatant was allowed to warm to room temperature before being placed in a 5-mm symmetrical NMR microtube (Shigemi, Inc., Allison Park, PA) that was magnetic susceptibility matched to D2O. NMR data were acquired with a 5-mm broadband probe in an 11.7 T vertical bore Bruker DRX 500 magnet with an Avance Console (Bruker, Billerica, MA) with previously described parameters.24,28
13C NMR spectral analysis and tcaCALC modeling
13C NMR spectra were processed using NUTS NMR software (Acorn NMR, Fremont, CA). A 1 Hz line-broadening factor and polynomial baseline correction were applied to spectra prior to relative peak area determination. Peaks were referenced to the lactate C3 peak at 21.0 ppm. Relative multiplet peak areas of isotopomer patterns of glutamate C2, C3, and C4 were determined using a line-fitting routine in NUTS as described previously.24 Relative multiplet peak areas were input into tcaCALC30,34,35 to determine metabolic fluxes relative to the flux through citrate synthase, defined as 1.
To determine the appropriate model of glucose metabolism to use with tcaCALC, %C3/C4 error24,25,28 and the residual sum of squares28 were determined for a standard model of metabolism and a previously published modified single pyruvate pool model containing a second nonpyruvate carboxylase anaplerotic entrance to the TCA cycle.24,25,28 Parameters examined in tcaCALC included relative fluxes through glycolysis (lactate dehydrogenase [LDH]), pyruvate dehydrogenase (PDH), pyruvate carboxylase (YPC), phosphoenolpyruvate carboxykinase/pyruvate kinase or malic enzyme (PK), acetyl-CoA synthetase (ACS), nonpyruvate carboxylase anaplerosis (YS), and the average relative flux of pyruvate carboxylase and pyruvate kinase-pyruvate cycling [(YPC+PK)/2].26,27 To ensure that local minima were not reached during modeling, convergence to the same output was confirmed using multiple initial parameter estimates, and simulated spectra were generated based on the tcaCALC output using tcaSIM. Conditions of complete scrambling between oxaloacetate and fumarate and no conserved orientation transfer between succinyl-CoA and malate were assumed. Relative metabolic fluxes were converted to percent carbon entry into the TCA cycle from different metabolic pathways relative to the total carbon entry into the TCA cycle, which is defined as follows:
 |
(1) |
Similarly, relative fluxes were converted to percent carbon entering and leaving the pyruvate pool using the following equations.
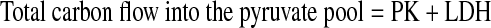 |
(2) |
 |
(3) |
Insulin secretion during 13C labeling and extraction experiments
The average insulin secretion rate (ISR), calculated as (total insulin accumulation up to a given time point)/(time of incubation), was measured during basal and labeling periods and normalized to the viable cell number determined at the end of the 6-h labeling period. Medium samples were taken at the following times, where t=0 h represents the beginning of the basal incubation period: 0, 1, 1.5, 4, and 7 h (end of labeling period).
Small-scale GSIS experiment
Fresh, Frozen, and DPS-vitrified beads were exposed to the same basal and high glucose incubation conditions as during the 13C labeling and extraction experiments, except on a smaller scale, with 0.3 mL beads per group in duplicate wells. At this scale, cryopreservation was performed in a single batch, as opposed to the multiple batches used in the scaled-up experiments. Stimulation index was determined by dividing the high-glucose ISR measured during the initial 0.5 h by the basal ISR. Medium samples were removed at the same time points as the scaled-up 13C experiments for later assay of insulin, and the viable cell number was measured at the end of the experiment.
K+ depolarization experiment
Fresh, Frozen, and DPS-vitrified beads were exposed to high K+ to induce insulin secretion by membrane depolarization using a protocol adapted from Fleischer et al.36 Cryopreservation was performed on a small scale in a single batch. Briefly, 0.3 mL of encapsulated cells from each group in duplicate wells was exposed for 1 h to HEPES buffered Krebs-Ringer buffer (KRB) containing 4.7 mM KCl (pH 7.4) supplemented with 1.31 g/L BSA (basal incubation), followed by 1 h in HEPES buffered KRB containing 47 mM KCl (pH 7.4) also supplemented with 1.31 g/L BSA (high K+ incubation). The NaCl concentration in the high K+ KRB was reduced to maintain isotonicity. Medium samples were taken at the beginning and end of the basal incubation, and at the beginning, 0.5 h, and end of the high K+ incubation period. Samples were stored at −80°C for later assay for insulin. At the end of the basal period, beads from a parallel well containing 0.2 mL of beads were solubilized, and cells were pelleted and extracted to measure intracellular insulin. Intracellular and secreted insulin was normalized to the viable cell number measured at the end of the experiment.
Assays
Insulin concentrations in medium samples were determined using a mouse insulin ELISA kit (Mercodia, Uppsala, Sweden) according to manufacturer's instructions. Absorbance was read at 450 nm on a SpectraMax Plus microplate reader (Molecular Devices, Sunnyvale, CA). Intracellular insulin was extracted using a mammalian cell lysis kit (Sigma). Viable cell numbers were measured by solubilizing beads with sodium citrate and using trypan blue dye exclusion.
Statistical analysis
Data are reported as mean±standard deviation. Values were obtained from three independent experiments (n=3). Statistical analyses were performed with a two-tailed t-test or a one-way ANOVA using the General Linear Model in Minitab® (Minitab, Inc., State College, PA). Multiple pairwise comparisons were performed using Tukey's post hoc analysis. Values of p<0.05 were considered to be statistically significant.
Results
Metabolic fluxes from fresh and cryopreserved cells
Metabolic fluxes were determined using a modified single pyruvate pool model24,25,28 (Fig. 2), as it was shown to have lower %C3/C4 error and lower residual sum of square values compared with a standard model of metabolism without a second nonpyuruvate carboxylase anaplerotic entrance to the TCA cycle (data not shown). The similarity of the simulated and experimental glutamate spectra (Fig. 3) indicates that the metabolic model and parameters used provided a good representation of experimental results. As indicated in Figure 4A, there were no differences in the raw relative metabolic fluxes among the different treatment groups and the Fresh, noncryopreserved control group (p>0.05). Figure 4B indicates that when converting raw metabolic flux data into percent carbon flow into the TCA cycle, there were also no differences among groups for any of the pathways examined (p>0.05). Percent carbon entry via nonpyruvate carboxylase anaplerosis, YPC anaplerosis, and PDH was similar among groups, with 28%–36% of carbon entry via each pathway. In addition, there was negligible entry to the TCA cycle via the ACS pathway, with less than 1% entry in all groups. To gain a snapshot of metabolism upstream of the TCA cycle, flow into and out of the pyruvate pool was also examined. Figure 4C shows that no differences among the treatments and the Fresh, noncryopreserved group were identified. More pyruvate entered the pyruvate pool through malic enzyme or phosphoenolpyruvate carboxykinase/pyruvate kinase than through glycolysis for all groups (p<0.05).
FIG. 2.
A previously published modified single pyruvate pool model of glucose metabolism applied in tcaCALC to determine metabolic fluxes. Figure was adapted from Simpson et al.24 The tcaCALC fluxes represented above are PK (phosphoenol-pyruvate carboxykinase/pyruvate kinase or malic enzyme), LDH (glycolysis), PDH (pyruvate dehydrogenase), YPC (pyruvate carboxylase), ACS (acetyl-CoA synthetase), and YS (nonpyruvate carboxylase anaplerosis).
FIG. 3.
Representative isotopomer patterns from glutamate C2, C3, and C4 resonances from experimental spectra obtained from extracts of Fresh, Frozen, and DPS vitrified encapsulated βTC-tet cells and simulated spectra obtained from tcaSIM based on tcaCALC output. A modified single pyruvate pool model was used to determine the tcaCALC output. The different multiplet components are represented by Q (quartet), D (doublet), S (singlet), and T (triplet).
FIG. 4.
Cryopreservation effects on metabolism of encapsulated βTC-tet cells determined using a modified single pyruvate pool model applied in tcaCALC. (A) Relative metabolic fluxes, (B) Percent carbon flow into the tricarboxylic acid (TCA) cycle, and (C) Percent carbon flow into and out of the pyruvate pool. Data obtained from Fresh (white bars), Frozen (gray bars), and DPS vitrified (striped bars) groups. Data are presented as mean±standard deviation (n=3).
Insulin secretion during 13C labeling and extraction studies
Figure 5 shows that on a per viable cell basis, there was no difference in ISR between Fresh and Frozen groups during the basal period and at all time points assayed in the high-glucose labeling period. However, ISR per viable cell was significantly lower for the DPS group compared with the Fresh or Frozen groups at all time points during the high-glucose labeling period (p<0.05).
FIG. 5.
Insulin secretion rate (ISR) measured during basal and high-glucose 13C-labeling periods for Fresh (white bars), Frozen (gray bars), and DPS vitrified encapsulated βTC-tet cells (striped bars). *Statistical significance (p<0.05). Data are presented as mean±standard deviation (n=3).
Insulin secretion during small-scale GSIS experiment
To investigate whether the decreased ISR from the DPS group was due to the required scale-up of the vitrification process for the extraction procedure, insulin secretion was also tested on a smaller scale. Insulin secretion during the smaller scale GSIS experiment resulted in trends similar to those seen in the large-scale extraction experiments (Fig. 6A). Specifically, there was no difference in ISR per viable cell among the three groups during the basal period and between the Fresh and Frozen groups during the high-glucose period. At the 4- and 7-h time points during the high-glucose period, however, ISR was lower for DPS compared with Fresh and Frozen groups.
FIG. 6.
ISR from encapsulated βTC-tet cells during small-scale experiments. (A) Glucose-stimulated insulin secretion (GSIS) experiment, and (B) K+-induced depolarization experiment for Fresh (white bars), Frozen (gray bars), and DPS-vitrified groups (striped bars). (C) Stimulation indices from GSIS (white bars) and K+-induced depolarization (gray bars) for Fresh, Frozen, and DPS vitrified groups. *Statistical significance (p<0.05). Data are presented as mean±standard deviation (n=3).
Insulin secretion during K+-induced depolarization
To investigate whether the depolarization/exocytosis response was affected by vitrification with DPS, encapsulated cells were exposed to a high extracellular [KCl]. As indicated in Figure 6B, ISR measured during exposure to 47 mM KCl was much lower for DPS compared with the Fresh group at both the 1.5- and 2-h time points (p<0.05), and there was no difference between the Fresh and Frozen groups. In addition, there was no difference in ISR between the Fresh and DPS groups during the basal period. For the Fresh and Frozen groups, the stimulation index with high K+ was higher than that with glucose (Fig. 6C). However, there was no difference between the two indices for the DPS group. Also, there were no differences in intracellular insulin content measured at the end of the basal period among the three groups, with 23.79±3.53, 24.85±5.75, and 19.09±3.39 pmol/105 viable cells for Fresh, Frozen, and DPS vitrified groups, respectively.
Discussion
Cryopreservation is important for clinical translation of pancreatic substitutes, but recent studies have often shown impaired insulin secretion postcryopreservation in encapsulated islets or insulinoma cells.11,12,16 Although an overall reduction in viable cell number is likely a significant contributor to the impaired secretion, cryopreservation stresses may also affect the function of the remaining viable cells. In this study, we applied 13C NMR and isotopomer analysis to evaluate the effects of two cryopreservation methods on the intermediary metabolism of encapsulated βTC-tet cells and examined secretory function both after glucose and non-nutrient secretagogue stimulation. Using a modified, single pyruvate pool model of metabolism,24,25,28 we found that relative carbon flow through the TCA-cycle-associated pathways examined was not significantly affected by either conventional freezing or vitrification. Further, insulin secretion per viable cell was not affected by conventional freezing. However, the glucose-stimulated and depolarization-induced insulin secretion responses were significantly compromised by vitrification.
It is well established that intermediary metabolism is a crucial component in the GSIS response in beta cells.18–21 Understanding how metabolic pathways are affected by cryopreservation is important in better evaluating impaired insulin secretion and in designing optimal cryopreservation protocols. Specifically, the use of 13C NMR and isotopomer analysis allows for a comprehensive view of mitochondrial metabolism,29 by examining carbon flow through various metabolic pathways at once, which has not been done previously for cryopreserved encapsulated islets or beta cells. Although this method alone does not determine absolute metabolic fluxes, it does allow for the evaluation of relative metabolic fluxes and preferred metabolic pathways, which have shown significant correlations with insulin secretion.24,27
Reports in the literature are conflicting with respect to secretory function after cryopreservation in encapsulated islets11–15 or beta cells16 and also vary with species, type of cryopreservation method, and insulin secretion protocol used. Additionally, few studies have directly compared conventional freezing with vitrification.12,16 Studies on cryopreservation of encapsulated hamster islets have indicated impaired insulin secretion from Vitrified11,12 and Frozen12 constructs in vitro, with freezing leading to capsule breakage. On the other hand, Vitrified encapsulated βTC3 cells reportedly exhibited similar insulin secretion to nontreated controls, although Frozen encapsulated cells exhibited lower secretion.16 However, the particular cell line, freezing protocol, and vitrification solutions used were different than those used in the current study.
Although secretion from the Frozen group appeared slightly elevated compared with Fresh group in the large-scale 13C NMR experiments, the increase was not statistically significant. More importantly, insulin secretory function was significantly lower in the DPS vitrified group relative to the Fresh group (Fig. 5). This decreased secretion is likely not due to the large scale of the 13C experiments, as a similar trend was observed in the small-scale secretion study (Fig. 6A).
Although studies using 13C NMR and isotopomer analysis typically do not show correlations between all metabolic fluxes and insulin secretion, the significant decrease in insulin secretion in the DPS group without any corresponding changes in metabolic fluxes is noteworthy and unprecedented. This maintenance of carbon flow through TCA cycle pathways suggests that insulin secretion may be compromised downstream of the TCA cycle in the DPS-vitrified group.
Various steps downstream of the TCA cycle may be potentially affected by DPS treatment, with one possible area being depolarization/exocytosis. It has been previously shown that βTC-tet cells stimulated by high K+ had a higher fold induction in insulin secretion compared with cells stimulated by glucose.36 Similarly, in our study, significantly higher stimulation indices were found for high K+ stimulation compared with high-glucose stimulation for the Fresh and Frozen groups, but not the DPS group. The blunted insulin secretion from the DPS group after high K+ exposure and the similar stimulation indices between high K+ and high glucose exposure for DPS may indicate a defect in depolarization/exocytosis. This is corroborated by the similar intracellular insulin content among the three groups at the end of the basal period, which indicates that the lower induced secretion from the DPS group was not due to insufficient intracellular insulin. Although the reason for the reduced secretion is unclear, defects in the membrane depolarization mechanism itself or in subsequent steps, such as the opening of voltage-gated calcium channels and the rise in intracellular calcium, are possibilities.
Conclusions
Using 13C NMR and isotopomer analysis, we found that intermediary metabolism was maintained in encapsulated βTC-tet cells cryopreserved by either vitrification or conventional freezing. Additionally, insulin secretion was maintained postwarming in Conventionally Frozen constructs. Although vitrification may be advantageous due to better maintenance of biomaterial integrity in some systems, our study indicates compromised insulin secretion, possibly due to defects in late-stage secretion events. Overall, our results offer new insight into understanding insulin secretion pathways that are maintained after cryopreservation as well as those that should be investigated further to better understand compromised secretion from encapsulated beta cells postwarming.
Acknowledgments
This work was supported by the National Institutes of Health R01DK73991. The programs tcaCALC and tcaSIM were obtained from the University of Texas Southwestern and developed through H-47669-16 and RR-02584. Additionally, the authors thank L. Gelbaum for his NMR expertise and useful discussions.
Disclosure Statement
No competing financial interests exist.
References
- 1.Papas K.K. Long R.C. Sambanis A. Constantinidis I. Development of a bioartificial pancreas: I. Long-term propagation and basal and induced secretion from entrapped beta TC3 cell cultures. Biotechnol Bioeng. 1999;66:4. [PubMed] [Google Scholar]
- 2.Black S.P. Constantinidis I. Cui H. Tucker-Burden C. Weber C.J. Safley S.A. Immune responses to an encapsulated allogeneic islet beta-cell line in diabetic NOD mice. Biochem Biophys Res Commun. 2006;340:1. doi: 10.1016/j.bbrc.2005.11.180. [DOI] [PubMed] [Google Scholar]
- 3.Zhou D. Sun A.M. Li X. Mamujee S.N. Vacek I. Georgiou J. Wheeler M.B. In vitro and in vivo evaluation of insulin-producing beta TC6-F7 cells in microcapsules. Am J Physiol Cell Physiol. 1998;274:5. doi: 10.1152/ajpcell.1998.274.5.C1356. [DOI] [PubMed] [Google Scholar]
- 4.Duvivier-Kali V.F. Omer A. Parent R.J. O'Neil J.J. Weir G.C. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes. 2001;50:8. doi: 10.2337/diabetes.50.8.1698. [DOI] [PubMed] [Google Scholar]
- 5.Kizilel S. Garfinkel M. Opara E. The bioartificial pancreas: progress and challenges. Diabetes Technol Ther. 2005;7:6. doi: 10.1089/dia.2005.7.968. [DOI] [PubMed] [Google Scholar]
- 6.Cui H. Tucker-Burden C. Cauffiel S.M. Barry A.K. Iwakoshi N.N. Weber C.J. Safley S.A. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation. 2009;88:2. doi: 10.1097/TP.0b013e3181abbfc1. [DOI] [PubMed] [Google Scholar]
- 7.Iwata H. Teramura Y. Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv Drug Deliv Rev. 2010;62:7. doi: 10.1016/j.addr.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Beck J. Angus R. Madsen B. Britt D. Vernon B. Nguyen K.T. Islet encapsulation: strategies to enhance islet cell functions. Tissue Eng. 2007;13:3. doi: 10.1089/ten.2006.0183. [DOI] [PubMed] [Google Scholar]
- 9.Mallett A.G. Korbutt G.S. Alginate modification improves long-term survival and function of transplanted encapsulated islets. Tissue Eng Part A. 2009;15:6. doi: 10.1089/ten.tea.2008.0118. [DOI] [PubMed] [Google Scholar]
- 10.Veriter S. Mergen J. Goebbels R.M. Aouassar N. Gregoire C. Jordan B. Leveque P. Gallez B. Gianello P. Dufrane D. In vivo selection of biocompatible alginates for islet encapsulation and subcutaneous transplantation. Tissue Eng Part A. 2010;16:5. doi: 10.1089/ten.TEA.2009.0286. [DOI] [PubMed] [Google Scholar]
- 11.Agudelo C.A. Teramura Y. Iwata H. Cryopreserved agarose-encapsulated islets as bioartificial pancreas: a feasibility study. Transplantation. 2009;87:1. doi: 10.1097/TP.0b013e318191b24b. [DOI] [PubMed] [Google Scholar]
- 12.Agudelo C.A. Iwata H. The development of alternative vitrification solutions for microencapsulated islets. Biomaterials. 2008;29:9. doi: 10.1016/j.biomaterials.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Zhou D.B. Vacek I. Sun A.M. Cryopreservation of microencapsulated porcine pancreatic islets—in vitro and in vivo studies. Transplantation. 1997;64:8. doi: 10.1097/00007890-199710270-00005. [DOI] [PubMed] [Google Scholar]
- 14.Inaba K. Zhou D. Yang B. Vacek I. Sun A.M. Normalization of diabetes by xenotransplantation of cryopreserved microencapsulated pancreatic islets—application of a new strategy in islet banking. Transplantation. 1996;61:2. doi: 10.1097/00007890-199601270-00001. [DOI] [PubMed] [Google Scholar]
- 15.Schneider S. Klein H.H. Preserved insulin secretion capacity and graft function of cryostored encapsulated rat islets. Regul Pept. 2011;166:1. doi: 10.1016/j.regpep.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee N. Chen Z.Z. Sambanis A. Song Y. Effects of cryopreservation on cell viability and insulin secretion in a model tissue-engineered pancreatic substitute (TEPS) Cell Transplant. 2005;14:7. doi: 10.3727/000000005783982882. [DOI] [PubMed] [Google Scholar]
- 17.Stiegler P.B. Stadlbauer V. Schaffellner S. Halwachs G. Lackner C. Hauser O. Iberer F. Tscheliessnigg K. Cryopreservation of insulin-producing cells microencapsulated in sodium cellulose sulfate. Transplant Proc. 2006;38:9. doi: 10.1016/j.transproceed.2006.08.188. [DOI] [PubMed] [Google Scholar]
- 18.Matschinsky F.M. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:2. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 19.Newsholme P. Gaudel C. McClenaghan N.H. Nutrient regulation of insulin secretion and beta-cell functional integrity. Adv Exp Med Biol. 2010;654 doi: 10.1007/978-90-481-3271-3_6. [DOI] [PubMed] [Google Scholar]
- 20.Jensen M.V. Joseph J.W. Ronnebaum S.M. Burgess S.C. Sherry A.D. Newgard C.B. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2008;295:6. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jitrapakdee S. Wutthisathapornchai A. Wallace J.C. MacDonald M.J. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia. 2010;53:6. doi: 10.1007/s00125-010-1685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabos K.J. Parkinson J.A. Hewage C. Nelson L.J. Sadler I.H. Hayes P.C. Plevris J.N. 1H NMR spectroscopy as a tool to evaluate key metabolic functions of primary porcine hepatocytes after cryopreservation. NMR Biomed. 2002;15:3. doi: 10.1002/nbm.765. [DOI] [PubMed] [Google Scholar]
- 23.Loven A.D. Olsen A.K. Friis C. Andersen B. Phase I and II metabolism and carbohydrate metabolism in cultured cryopreserved porcine hepatocytes. Chem Biol Interact. 2005;155:1. doi: 10.1016/j.cbi.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Simpson N.E. Khokhlova N. Oca-Cossio J.A. Constantinidis I. Insights into the role of anaplerosis in insulin secretion: a C-13 NMR study. Diabetologia. 2006;49:6. doi: 10.1007/s00125-006-0216-5. [DOI] [PubMed] [Google Scholar]
- 25.Simpson N.E. Constantinidis I. 13C NMR isotopomeric analysis and its application in the study of endocrine cell metabolism and function. Acta Biomed. 2007;78(Suppl 1) [PubMed] [Google Scholar]
- 26.Cline G.W. Lepine R.L. Papas K.K. Kibbey R.G. Shulman G.I. 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem. 2004;279:43. doi: 10.1074/jbc.M311842200. [DOI] [PubMed] [Google Scholar]
- 27.Lu D. Mulder H. Zhao P. Burgess S.C. Jensen M.V. Kamzolova S. Newgard C.B. Sherry A.D. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proc Natl Acad Sci U S A. 2002;99:5. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson N.E. Grant S.C. Gustavsson L. Peltonen V.M. Blackband S.J. Constantinidis I. Biochemical consequences of alginate encapsulation: a NMR study of insulin-secreting cells. Biomaterials. 2006;27:12. doi: 10.1016/j.biomaterials.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey F.M.H. Rajagopal A. Malloy C.R. Sherry A.D. C-13-NMR—a simple yet comprehensive method for analysis of intermediary metabolism. Trends Biochem Sci. 1991;16:1. doi: 10.1016/0968-0004(91)90004-f. [DOI] [PubMed] [Google Scholar]
- 30.Sherry A.D. Jeffrey F.M.H. Malloy C.R. Analytical solutions for C-13 isotopomer analysis of complex metabolic conditions: substrate oxidation, multiple pyruvate cycles, and gluconeogenesis. Metab Eng. 2004;6:1. doi: 10.1016/j.ymben.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Efrat S. Fusco-DeMane D. Lemberg H. al Emran O. Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci U S A. 1995;92:8. doi: 10.1073/pnas.92.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambanis A. Mukherjee I.N. Song Y.C. Cryoprotectant delivery and removal from murine insulinomas at vitrification-relevant concentrations. Cryobiology. 2007;55:1. doi: 10.1016/j.cryobiol.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss A.D. Forbes J.F. Scheuerman A. Law G.K. Elliott J.A. McGann L.E. Jomha N.M. Statistical prediction of the vitrifiability and glass stability of multi-component cryoprotective agent solutions. Cryobiology. 2010;61:1. doi: 10.1016/j.cryobiol.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Malloy C.R. Sherry A.D. Jeffrey F.M. Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers. Am J Physiol. 1990;259(3 Pt 2) doi: 10.1152/ajpheart.1990.259.3.H987. [DOI] [PubMed] [Google Scholar]
- 35.Malloy C.R. Sherry A.D. Jeffrey F.M.H. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric-acid cycle of the heart by C-13 NMR-spectroscopy. J Biol Chem. 1988;263:15. [PubMed] [Google Scholar]
- 36.Fleischer N. Chen C. Surana M. Leiser M. Rossetti L. Pralong W. Efrat S. Functional analysis of a conditionally transformed pancreatic beta-cell line. Diabetes. 1998;47:9. doi: 10.2337/diabetes.47.9.1419. [DOI] [PubMed] [Google Scholar]








