Abstract
Ischemic preconditioning (IPC) is a potent and effective means of protecting cells against ischemic injury. The protection has been demonstrated to involve release of paracrine factors that promote cell survival and angiogenesis, factors important for successful tissue engineering. The aim of the present study was to determine whether IPC of a vascular bed in vivo is an effective strategy to prepare it for tissue engineering with implanted cells. To test this hypothesis, an in vivo vascularized tissue engineering approach was employed, whereby polyacrylic chambers were placed around the femoral vessels of adult Sprague-Dawley rats. IPC was induced by 3 cycles of 5 min femoral artery occlusion interspersed with 5-min periods of reperfusion. Rats subjected to IPC generated bigger tissue constructs at 7 and 28 days postimplantation of empty chambers (∼50% increase in weight and volume, p<0.05). Morphometric counting of Masson trichrome stained tissue sections revealed significantly greater tissue construct volumes in ischemic preconditioned vascular beds at 7 and 28 days, increasing both fibrin matrix and vascularized tissue. Furthermore, morphometry of lectin-labeled blood vessels indicated an increase in vascular volume in IPC tissue constructs (∼100% increase vs. control, p<0.05). To investigate the cytoprotective effect of IPC, we implanted DiI-labeled neonatal rat cardiomyocytes in the chambers for 3 days, and IPC significantly reduced apoptosis of implanted cells as determined by the TUNEL assay and cleaved caspase-3 immunostaining. Furthermore, IPC significantly increased the cardiac muscle volume and vascular volume at 28 days after implantation of cardiomyocytes. In conclusion, in vivo IPC promotes survival of implanted cardiomyocytes and is associated with enhanced angiogenesis. IPC may represent a new approach to optimize tissue engineering with implanted cells.
Introduction
End-stage heart failure is a life threatening condition, and the current therapeutic options are limited to ventricular assist devices or heart transplantation.1,2 Ventricular assist devices are a short-term bridge to transplant option, while heart transplantation itself is limited by the shortage of the heart donors, life-long immune suppressive drug therapy and potential long-term graft failure.3 Engineering cardiac tissues with autologous cells can potentially provide immunocompatible cardiac tissues to regenerate the damaged myocardium. However, engineering viable cardiac grafts with sufficient thickness for clinical application in myocardial repair has been limited by the inability to generate three-dimensional cardiac grafts with adequate intrinsic vascular networks for oxygen and nutrient supply, leading to central necrosis of cardiac grafts.4 To overcome this shortcoming, various strategies have been developed to promote vascularization of engineered tissues by using proangiogenic growth factors such as fibroblast growth factor, transforming growth factor-β, and vascular endothelial growth factor,5,6 by employing cells that are capable of participating in neovascularization such as endothelial progenitor cells,5,6 and to prevascularize with biological microvascular grafts that readily inosculate with the host vasculature.7 We have developed an alternative strategy using an in vivo vascularized tissue engineering chamber based on femoral vessels placed within a protected space to generate a functional microcirculatory network over time.8 Using this method, a significant volume of vascularized and contractile cardiac tissue, with a maximum thickness of ∼2 mm, has been generated with neonatal rat cardiomyocytes.9 Despite successful formation of contractile cardiac tissue, the model is also characterized by rapid death of transplanted cells at early time points, most likely due to the hostile ischemic environment associated with delayed revascularization from the arteriovenous loop within the chamber until around 3 days postsurgery.8 Furthermore, the death of implanted cells triggers innate immune responses and accentuates the harshness of the microenvironment.8 Therefore, clinically adaptable cytoprotective strategies are needed to address this problem in tissue engineering.
Ischemic preconditioning (IPC) was first described in 1986 by Murry et al. in canine myocardium where brief episodes of sublethal myocardial ischemia can protect the heart from subsequent prolonged lethal ischemia-reperfusion injury.10 This phenomenon has been established as a powerful endogenous mechanism protecting against ischemic injury in almost all species, and in various organs and cell types. IPC activates endogenous repair mechanisms by inducing the release of several protective paracrine factors that activate multiple survival signaling pathways.11,12 The proangiogenic effect of IPC has also been reported by a number of studies in the infarcted myocardium as a result of increased vascular endothelial growth factor (VEGF) production.13–17 IPC is also known to potentiate mobilization and recruitment of endogenous stem and progenitor cells, such as endothelial progenitor cells,17 mesenchymal, and hematopoietic stem cells,18,19 into the infarcted myocardium for cardioprotection. The endothelial progenitor cells recruited by IPC have been shown to contribute to enhanced neovascularization in the ischemic myocardium through release of paracrine factors and direct incorporation into the microvasculature.17 These attractive beneficial effects attained by IPC lead us to propose that IPC might provide benefits for in vivo tissue engineering. In this proof-of-concept study, we developed a novel in vivo tissue engineering chamber and sought to determine whether IPC can promote tissue and vascular growth, and whether the survival of implanted cells was enhanced by IPC.
Materials and Methods
Animals
Experimental procedures were approved by the Animal Ethics Committee of St. Vincent Hospital (Melbourne, Australia) and were conducted in accordance with the Australian National Health and Medical Research Council guidelines for the care and maintenance of animals. Inbred Sprague-Dawley rats (Animal Resources Centre, Perth, Western Australia) were maintained in-house with a 12-h dark/light cycle and given water and chow ad libitum.
Preparation of in vivo vascularized tissue engineering chamber
Adult male rats weighing between 320 and 430 g were anesthetized with isoflurane inhalation (4% induction and 2% maintenance). The groins were clipped of hair, skin swabbed with chlorhexidine/70% ethanol solution, and draped. The femoral vessels were exposed through a longitudinal incision made on the medial thigh. The femoral artery and vein were separated from their surrounding tissue attachments and small branches to the thigh musculature were cauterized to isolate the length of the vessels from the inguinal ligament to the branch point of the deep vein. Polyacrylic chambers (12×11×4-mm internal dimension; Department of Chemical and Biomolecular Engineering, University of Melbourne, Australia) were placed around the femoral vessels, by passing the intact vascular pedicle through slits on both ends of the chamber, creating a flow-through model with intact femoral circulation (Fig. 1). Lids were attached to the chamber base to create a protected volume for tissue growth. Skin wounds were then closed in two layers and the animals were allowed to recover. Polyacrylic chambers were sterilized with ultraviolet light in the laminar flow hood and enclosed in a sterile Petri dish until implantation.
FIG. 1.

(A) Macroscopic view of the polyacrylic tissue engineering chamber. (B) The tissue engineering chamber was placed around the dissected femoral artery (a) and vein (v) in the groin region of a rat. Plasma clot containing DiI-labeled neonatal rat cardiomyocytes (outlined by the dotted line) was implanted at the base of the chamber. Ischemic preconditioning (IPC) was induced by transient femoral artery occlusion at the proximal end of the artery just outside the chamber (arrowhead).
Induction of IPC
Animals randomized to receive IPC intervention had their common femoral arteries (between the inguinal ligament and the chamber) exposed and temporarily occluded for 5 min followed by 5 min of reperfusion, repeated three times immediately after inserting the chambers (Fig. 1B). Occlusion was achieved by using traumatic artery microclamps.
Preparation of neonatal rat cardiomyocytes
Neonatal rat ventricular cardiomyocytes (rCMs) were isolated from Sprague-Dawley rat ventricles using the Neonatal cardiomyocytes isolation system (Worthington Biochemical, Lakewood, NJ) as previously described.20 In brief, ventricles from neonatal rats (1–3 days old) were minced into 1–3 mm3 pieces and incubated in 50 μg/mL of trypsin solution overnight at 4°C. Trypsin inhibitor was then added and warmed to 37°C for 2 min and followed by collagenase digestion for 45–60 min. The tissue sample was then filtered through a 100 μm mesh and centrifuged at 1200 rpm for 5 min. rCMs were labeled with Cell Tracker CM-DiI (Invitrogen, Mulgrave, Victoria, Australia) at 3 μg per million cells for 5 min at 37°C and then for 15 min at 4°C. Fibrin scaffolds in the plasma clots are biocompatible and have low immunogenicity in vivo.21 Therefore, rCMs were suspended in syngeneic plasma clot isolated from adult inbred rats to minimize immunogenicity of implanted cells. Fresh rat blood was collected from inbred Sprague-Dawley rats into an ethylenediaminetetraacetic acid-Vacutainer tube and centrifuged at 1800 rpm for 15 min at 4°C to separate plasma from red blood cells. The plasma was collected in sterile Eppendorf tubes as 200 μL aliquots and stored at −20°C until use. Before implantation, 3×106 rCMs were suspended in 200 μL of plasma, and 30 μL of 2% calcium chloride was added to form plasma clot in the sterilized chamber before implantation into the rat (Fig. 1B).22
Experimental protocols
To characterize the effect of IPC on tissue growth in the in vivo tissue engineering chamber, rats were implanted with empty chambers and randomized to receive either IPC intervention or no intervention (control). Each rat was implanted with two chambers (left and right femoral vessels). The tissue constructs were harvested at 7 (n=4 from 3 rats in control, n=5 from 4 rats in IPC) or 28 days postimplantation (n=5 from 3 rats in control, n=5 from 3 rats in IPC) from anesthetized rats. Following construct harvest, the rats were sacrificed with Lethobarb (Virbac Animal Health, Milperra, NSW, Australia). In a second experiment to determine the cytoprotective effect of IPC, rats were implanted with chambers containing 3×106 DiI-labeled rCMs suspended in plasma clot and subjected to either IPC intervention or control. The tissue constructs containing the implanted rCMs were harvested at 3 (n=5 from 3 rats in control, n=5 from 3 rats in IPC) or 28 days postimplantation (n=7 from 4 rats in control, n=7 from 4 rats in IPC) from anesthetized rats. Following construct harvest, the animals were sacrificed with Lethobarb.
Harvest of chamber tissue and tissue processing
For tissue harvest, rats were anesthetized and the chambers were exposed and opened to examine the patency of the femoral vessels, which was verified by arterial pulsation and venous flow at both end of the chambers. The femoral vessels were cauterized outside the chamber, and the constructs were removed from the chamber. Tissues were blotted dry and weighed. The volume of tissue constructs was determined by a volume displacement measurement method in which the water displacement due to tissue volume is determined by its weight.23,24 A 50-mL container was filled with an isotonic sterile saline (0.9% NaCl) solution, placed on a balance and the initial weight (W1) was recorded. The tissue construct was submerged into the solution so that it was beneath the surface of the liquid, but not touching the bottom of the container. The resulting weight (W2) was recorded. The weight (in mg) of the fluid displaced by the tissue (W0) was then calculated as W0=W2–W1. The construct volume (μL) was then determined by dividing the weight of the fluid displaced (W0) of the construct by the specific gravity of the isotonic saline (g=1.0048). Tissue constructs were fixed in 4% paraformaldehyde for 24 h. For 3-day constructs, fragile tissues were embedded in 1.5% agarose containing 3.7% formaldehyde. Tissue constructs were then divided into three equal transverse sections (distal, middle, and proximal) and paraffin embedded for routine histology and immunohistochemistry. Tissue morphometry, angiogenesis, rCM apoptosis, and cardiac tissue were analyzed from all three sections of each sample by two observers (SYL and SH or PS, SH and PS were blinded to the identity of the tissues).
Morphometric determination of tissue components
Paraffin-embedded 5 μm-thick sections were stained with Masson's trichrome for morphometric evaluation. Capillaries were identified by their anatomical structure characterized by a single layer of flat endothelial cells and with associated pericyte support. Small arteries were identified by their round lumen lined by endothelium and the internal elastic lamina (tunica intima), tunica media, and tunica adventitia were characterized by a layer of loose connective tissue. Small veins were identified by their irregular lumen lined with endothelium (tunica intima), tunica media comprised of one or several layers of vascular smooth muscle cells and a very thin tunica adventitia also comprised of loose connective tissue and fibroblasts. Compared to capillaries, both arterioles and venules have an inner diameter of 10 μm or greater.7 Sections were also stained with Mallory's phosphotungstic acid hematoxylin, ED-1 and hematoxylin, and eosin for fibrin, macrophages, and neutrophils, respectively (see Supplementary Materials for full protocol). Masson's trichrome stained transverse sections (distal, middle, and proximal section of each sample) were analyzed by video microscopy under ×20 magnification for tissue volume fractions with a computer-generated 9-point square grid (CAST system, Olympus Denmark, Albertslund, Denmark) applied systematically so that 10% of the total section area was assessed. The tissues were categorized into: (1) femoral vessels, (2) fibrin, and (3) vascularized tissues. Points falling over each tissue category were counted and these proportional counts of tissue components were expressed as a percentage of the total points counted (the percent volume of a tissue component). The absolute volume of each category in the tissue was calculated by multiplying the percent volume of each tissue by the total tissue construct volume at harvest.
Assessment of tissue angiogenesis
To identify newly formed vascular network sprouting from the femoral vein and artery, histochemistry was performed by incubating sections with Griffonia simplicifolia lectin (B1105; Vector Laboratories, Burlingame, CA) as previously described.8 After rehydration of sections, endogenous peroxidase was quenched with 3% hydrogen peroxide for 5 min. Tissues were treated with Proteinase K (Dako, Carpinteria, CA) for 4 min followed by incubation in biotinylated lectin at 1:300 for 30 min and HRP-streptavidin (Dako, Carpinteria, CA) at 1:400 for 30 min. The peroxidase activity was visualized with diaminobenzidine (DAB) (Thermo Scientific, Rockford, IL), and sections counterstained with hematoxylin. Complete transverse sections (distal, middle, and proximal section of each sample) were analyzed by video microscopy under ×20 magnification with a computer-generated 9-point square grid and 20% of the total area was sampled systemically.8 Points falling over lectin-positive vessels (except femoral artery and vein) in each tissue component (femoral vessels, fibrin, and vascularized tissues) were counted and expressed as a percentage of the total points counted in the respective tissue components. Absolute volume of blood vessels was calculated by multiplying the percentage of counted blood vessels in each tissue component by the tissue volume determined by volume displacement and in the morphometric analysis.
Cardiomyocyte apoptosis
To detect the apoptosis of rCM implanted in the tissue engineering chambers, paraffin-embedded 5 μm-thick sections of the 3-day constructs were stained with an antibody against cleaved caspase-3 (rabbit anti-human (Asp175) (5A1E) IgG, #9664; Cell Signaling, Danvers, MA) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (DeadEnd™ Colorimetric TUNEL system, G7130; Promega, Madison, WI). Sections underwent heat-mediated antigen retrieval in the citric acid buffer (pH 6.0, 30 min at 95°C), and endogenous peroxidase was quenched with 3% hydrogen peroxide for 5 min. Sections were then sequentially incubated in 10% normal goat serum for 30 min, cleaved caspase-3 antibody at 1:150 for 60 min, biotinylated goat anti-rabbit secondary antibody (BA1000; Vector, Burlingame, CA) at 1:200 for 30 min, avidin-biotinylated-peroxidase complex (Vectastain Elite ABC Standard, PK6100; Vector, Burlingame, CA) for 30 min, and DAB for 2 min. Nuclear counterstaining was performed with 6-diaminido-2-phenylindole (DAPI) (Molecular Probes, Invitrogen, Eugene, OR). The TUNEL assay was performed according to manufacturer's instructions, and cells with clear nuclear labeling were defined as TUNEL-positive cells. For each construct, nine random histological fields (three fields each from the femoral artery, the femoral vein, and the remote region) were captured with Olympus BX61/DP71 imaging system (Tokyo, Japan) at 400 times magnification. Bright field, DiI and DAPI images were merged and analyzed using NIH ImageJ software. The degree of apoptosis was calculated as (1) percentage of TUNEL+ DiI+ cells over total DiI+ cells and (2) percentage of cleaved caspase-3+ DiI+ cells over total DiI+ cells.
Assessment of cardiac muscle
To assess the cardiac tissue generated by implanted rCM, paraffin-embedded 5 μm-thick sections of the 28-day constructs were stained with an antibody against cardiac troponin T (mouse monoclonal IgG (1C11), ab8295; Abcam, Cambridge, MA). Dewaxed sections underwent heat-mediated antigen retrieval in the citric acid buffer (pH 6.0, 30 min at 95°C), and endogenous peroxidase was quenched with 3% hydrogen peroxide for 10 min. Sections were then sequentially incubated in 20% normal rabbit serum for 30 min, cardiac troponin T antibody at 1:1000 for 60 min, biotinylated rabbit anti-mouse secondary antibody (E0464; Dako, Carpinteria, CA) at 1:200 for 30 min, avidin-biotinylated-peroxidase complex (Vectastain Elite ABC Standard, PK6100; Vector, Burlingame, CA) for 30 min, DAB for 2 min, and counterstained with hematoxylin. Complete transverse sections (distal, middle, and proximal section of each sample) were analyzed by video microscopy under ×20 magnification with a computer-generated 9-point square grid and 20% of the total area was sampled systemically.8 Points falling over troponin T-positive cells were counted and expressed as a percentage of the total points counted. Absolute volume of cardiac tissue was calculated by multiplying the percentage of tissue stained positive with troponin T by the tissue volume determined by volume displacement at harvest.
Statistics
Values are shown as mean±standard error of the mean. Data were analyzed using the unpaired t-test or the two-way analysis of variance with the Bonferroni multiple comparison post hoc test where appropriate. p<0.05 was considered significant.
Results
Animal exclusions and body weight
During pilot studies, to establish this novel chamber model, it was found that rats with body weight less than 300 g were prone to femoral vessel occlusion at the edge of the chamber, so rats larger than 300 g were used for this study. Rat weight was similar between treatment groups (control, 382.2±9.3 g vs. IPC, 388.8±7.8 g, p>0.05). Eleven chambers were excluded from the study (five in the control group and six in the IPC group) due to complete occlusion of the femoral vein by thrombus with no histological evidence of revascularization or patency.
IPC increases tissue construct weight and volume
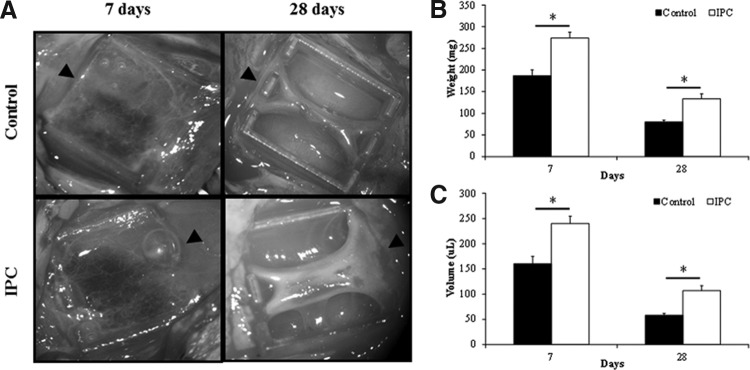
In rats implanted with empty chambers, the tissue constructs harvested at day 7 consisted mainly of a semi-solid fibrin scaffold filling the majority of the chamber (Fig. 2A). IPC significantly increased the construct weight (control, 187±14 mg vs. IPC, 274±13 mg, p<0.05) (Fig. 2B) and volume (control, 160.5±14.4 μL vs. IPC, 240.0±14.5 μL, p<0.05) (Fig. 2C) of tissue formed. The constructs harvested at day 28 consisted of a compact, vascularized connective tissue closely surrounding the femoral vessels and with fibrin scaffold at the periphery replaced by a smooth fibrous capsule. The constructs were narrowed toward the middle of the chambers (Fig. 2A). Similar to day 7 constructs, day 28 constructs harvested from rats subjected to IPC intervention weighed significantly more (control, 80±4 mg vs. IPC, 134±11 mg, p<0.05) (Fig. 2B) and had increased volume (control, 58.2±3.8 μL vs. IPC, 107.2±9.6 μL, p<0.05) (Fig. 2C).
FIG. 2.
(A) Representative photomacrograph of new tissues generated in the tissue engineering chamber at 7 and 28 days postimplantation, with or without IPC intervention. Arrowheads indicate the proximal end of the femoral vessels. The weight (B) and volume (C) of control and IPC tissue constructs harvested at 7 and 28 days. n=4–5. *indicates p<0.05.
Effect of IPC on tissue composition
In both the control and IPC-treated rats implanted with empty chambers, three distinct zones of tissue development were observed. Surrounding the femoral vessels was a layer of vascularized connective tissue comprised predominantly of collagen (Fig. 3B). This tissue was surrounded by less fibrous and more highly vascularized tissue, characterized by hyperpermeable capillary networks as evidenced by a significant presence of hemorrhage at the migrating front (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). This zone was previously termed the proliferative zone8 and comprised a significant proportion of red blood cells interspersed in fibrin matrix where individual cells of the granulation tissue, inflammatory cells, and angiogenic capillary tips were seen to invade the matrix to form new construct tissue (Fig. 3B). Arterioles, capillaries, and venules (Supplementary Fig. S1) were all evident with arterioles being more evident in the vicinity of the femoral artery. At the edge of the proliferative zone was a zone of fibrin matrix characterized by fine strands of interconnected fibers (Fig 3C) and was stained positive (blue, fibrin) with Mallory's phosphotungstic acid hematoxylin stain (supplementary Fig. S2). The fibrin matrix contained inflammatory cells such as neutrophils and macrophages (supplementary Fig. S1), occasional migrating myofibroblasts and endothelial cells, and areas of hemorrhage (Fig 3C). These three tissue zones were most prominent and clearly identifiable at 7 days, whereas at 28 days, these zones were predominantly replaced by vascularized connective tissue and only small remnants of fibrin matrix and proliferative zones were seen (Fig. 3A, D).
FIG. 3.
Masson's trichrome staining of tissue constructs for morphometric evaluation of tissue components. (A) Representative photomicrograph of tissue constructs harvested at 7 and 28 days. (B–E) Representative high-power photomicrographs of tissue morphology from different regions of the tissue construct as indicated in (A). (B) 7-day constructs with vascularized connective tissue (c) adjacent to the femoral vessels (v) and extended into hemorrhagic proliferative zone (p). (C) Fibrin matrix in 7-day constructs. (D) Collagen-rich vascularized connective tissue (c) in 28-day constructs containing mature arterioles (arrow heads) and venules (arrow). (E) Highly organized and compact capsular structure at the periphery of the 28-day construct. Scale bars=(A) 1 mm, (B–E) 50 μm. The absolute volume of fibrin matrix (F), vascularized tissue (G), and femoral vessels (H) of control and IPC tissue constructs harvested at 7 and 28 days. n=4–5. *indicates p<0.05.
At 7 days, ∼80% of the tissue constructs consisted of fibrin matrix exuded from the femoral vessels (Fig. 3A), and IPC significantly increased the volume of fibrin matrix in the 7-day constructs (control, 125.5±17.8 μL vs. IPC, 189.0±11.8 μL, p<0.05) (Fig. 3F). In contrast, at 28 days, the tissue constructs consisted mainly of collagen-rich vascularized tissues with small areas of hemorrhagic tissue and granulation tissue at the periphery of the constructs (Fig. 3A, D, E). Compared to 7-day tissue constructs, the cellularity in the vascularized tissues was reduced and most of the blood vessels had matured into arterioles and venules (Figs. 3D and 4B). Cells at the periphery of the constructs were aligned closely in parallel to one another encapsulating the constructs forming a construct capsule (Fig. 3E). IPC significantly increased the volume of vascularized tissue in 28-day constructs (control, 46.5±3.4 μL vs. IPC, 89.8±9.5 μL, p<0.05) (Fig. 3G). The femoral artery and vein remained patent in all chambers with intact endothelial lining (Supplementary Fig. S1). The volume of femoral vessels remained similar at 7 and 28 days and was not affected by IPC intervention (Fig. 3H).
FIG. 4.
Vascularity of tissue constructs. Representative photomicrograph of lectin-stained sections from constructs harvested at 7 (A) and 28 (B) days. Micrographs on the right are higher power images of the respective constructs. Scale bars=0.1 mm. The percentage (C) and absolute volume (D) of blood vessels in vascularized tissue of control and IPC tissue constructs harvested at 7 and 28 days. n=4–5. *indicates p<0.05. Color images available online at www.liebertpub.com/tea
IPC increases vascular volume
Newly developed arterioles, capillaries, and venules were identified by lectin histology and were found mainly in granulation tissues and the proliferative zone (Fig. 4A, B). Compared to control, the percentage of blood vessels in the vascularized tissues was significantly higher in 7-day, but not in 28-day, constructs harvested from rats subjected to IPC intervention (Fig. 4C). IPC also significantly increased the vascular volume in the vascularized tissues formed in the tissue constructs isolated at 7 (control, 3.9±0.9 μL vs. IPC, 8.7±0.8 μL, p<0.05) and 28 days (control, 10.6±0.9 μL vs. IPC, 22.1±2.1 μL, p<0.05) (Fig. 4D). IPC also significantly increased the vascular volume in the fibrin matrix formed in the tissue constructs harvested at 7 days (Supplementary Fig. S3).
IPC enhances cell survival in vivo
Apoptosis of cardiomyocytes implanted in the chamber was identified by TUNEL-positive DiI-positive cells (Fig. 5A) or cleaved caspase-3-positive DiI-positive cells (Fig. 5B). IPC significantly reduced implanted cardiomyocyte apoptosis as determined by the TUNEL assay (control, 23.3%±2.8% vs. IPC, 11.8%±2.2%, p<0.05) (Fig. 5C) and cleaved caspase-3 staining (control, 41.6%±3.5% vs. IPC, 23.4%±3.7%, p<0.05) (Fig. 5D).
FIG. 5.
Apoptosis of implanted neonatal rat cardiomyocytes in day 3 tissue constructs. Representative figures of TUNEL (A) and cleaved caspase-3 (B) staining. Dil-label neonatal rat cardiomyocytes (red), DAPI nuclear staining (blue), and merged image are shown. Arrows indicate TUNEL/DiI-positive cells in (A) and cleaved caspase-3/DiI-positive cells in (B). Scale bars=50 μm. The percentage of TUNEL/DiI-positive (C) and cleaved caspase-3/DiI-positive (D) implanted cardiomyocytes in control and IPC tissue constructs harvested at 3 days. n=5. *indicates p<0.05.
IPC increases cardiac tissue volume
All constructs from the control and the IPC group, which had been implanted with rCM, showed spontaneous contraction at 4 weeks (Supplementary video V1). The constructs from the IPC group were significantly heavier (control, 129±7 mg vs. IPC, 179±17 mg, p<0.05) and larger (control, 116.0±8.1 μL vs. IPC, 161.7±16.2 μL, p<0.05) than those from the control group. Layers of compact troponin T-positive cardiac muscle were detected predominantly around the femoral vessels in both control and IPC groups (Fig. 6A). The majority of cardiomyocytes were elongated, showed striated sarcomeric staining for the contractile protein (troponin T, Fig. 6A), and tissues were well vascularized (Fig. 6B). The cardiac muscle volume in constructs harvested from the IPC group was nearly 3-fold larger than the control group (control, 3.3±0.7 μL vs. IPC, 9.0±2.0 μL, p<0.05) (Fig. 6B). The increased cardiac muscle volume was accompanied by an increase in vascular volume of the vascularized tissues in constructs harvested from rats subjected to IPC (control, 20.6±1.8 μL vs. IPC, 34.4±3.1 μL, p<0.05) (Fig. 6D). Furthermore, IPC also significantly increased the percentage of blood vessels in the vascularized tissues (control, 17.8%±0.9% vs. IPC, 21.8%±1.6%, p<0.05). Lectin-positive vessels were found to distribute among the cardiomyocytes (Fig. 6C).
FIG. 6.
Cardiac tissue volume and vascularity in day 28 tissue constructs implanted with neonatal rat cardiomyocytes. Serial tissue sections were immunostained for cardiac specific troponin T (A) and lectin (C). Boxed areas are magnified in right panels illustrating cardiomyocytes with striated sarcomeric staining for contractile proteins troponin T (A) and were closely associated with blood vessels (C). Scale bars=100 μm. The absolute volume of cardiac tissue (B) and blood vessels (D) in vascularized tissue of control and IPC tissue constructs harvested at 28 days. n=7. *indicates p<0.05. Color images available online at www.liebertpub.com/tea
Discussion
The major finding of the present study is that in vivo IPC benefits in vivo cardiac tissue engineering, leading to increase in both cardiac muscle formation and vascularity. These benefits are likely due to protection from cardiomyocytes high metabolic demand making them susceptible to the ischemic injury encountered at implantation.25 Using a three-dimensional in vivo vascularized tissue engineering chamber, we were able to show that IPC (1) increased tissue weight and volume, (2) increased vascular volume, (3) protected implanted neonatal rat cardiomyocytes from apoptotic cell death, and (4) increased cardiac muscle volume formed.
The initiation of tissue growth in the enclosed empty chamber containing only the femoral vessels was characterized by the deposition of fibrin matrix exuded from the femoral vessels. The fibrin matrix is known to be proangiogenic providing a biomaterial scaffold that encourages the infiltration of inflammatory cells, fibroblasts, pericytes, and vascular progenitors. Over time, these cells will undergo differentiation and maturation, and assemble into vascularized granulation tissues.8,26 In the present study, the higher volume of fibrin exudate induced by IPC in the 7-day tissue construct may contribute to a greater volume of intrinsic biological scaffold material to encourage a greater infiltration and deposition of inflammatory cells, fibroblasts, and vascular progenitors. This may result in the higher volume of granulation tissues we observed in the 28-day tissue constructs. This hypothesis is supported by our previous finding that the tissue growth and vessel development in in vivo chambers is directly proportional to the amount of available fibrin matrix.8,27 Interfering with the extravascular fibrin deposition with enoxaparin sodium has a negative impact on the size of the tissue construct and angiogenesis.27 The mechanism by which IPC increased fibrin volume is unclear. A recent study in retinal ischemia has suggested that IPC slightly increased vascular permeability28 and this may promote fibrin exudation into the tissue engineering chamber. Furthermore, IPC has also been reported to induce the release of VEGF,13,14,29 an angiogenic growth factor that is known to increase vascular permeability.30–32 Whether these mechanisms promote fibrin exudate from the femoral vessels in the IPC constructs warrants further investigation. The involvement of other endogenous vascular permeabilizing factors, such as histamine, serotonin, platelet-activating factor, and prostaglandins, certainly cannot be ruled out.32
Angiogenesis is fundamental to successful tissue engineering. Increase in vascularization and development of functional vascular networks improve nutrient and oxygen delivery and removal of metabolic products within the construct environment. In the present study, blood vessels were found mainly in the vascularized tissue outgrowth from the femoral vessels, and we observed increased vascular volume in IPC constructs, which was closely associated with an increase in vascularized tissue. Whether the increased vascular volume was simply the result of an increase in vascularized tissues remains undetermined. Interestingly, the vascular volume density in the vascularized tissue was higher in IPC constructs harvested at day-7 than later, suggesting that IPC may have a direct proangiogenic effect during early tissue formation. Previous studies have demonstrated that IPC promotes intrinsic angiogenesis in the infarcted myocardium through local release of signaling reactive oxygen species, upregulation of VEGF, nuclear translocation of PKCɛ, and upregulation of various proangiogenic transcription factors such as the specificity protein-1 and NFκB.13–15 Similarly, preconditioning with transient systemic hypoxia also stimulated myocardial angiogenesis in rats subjected to myocardial infarction.16,33 More recently, an in vitro study has demonstrated that exposing the tissue-engineered human oral mucosa to short periods of hypoxia, also increased secretion of soluble angiogenic factors, including VEGF and the placental growth factor.34 Exposure to hypoxic preconditioning also enhanced production of matrix metalloproteinase (MMP)-2, MMP-9, tissue inhibitors of matrix metalloproteinase (TIMP)-1 and TIMP-2, which are important regulators for angiogenesis.34 At 28 days, the vascular volume density was not different between control and IPC groups, suggesting that the hypervascularization mechanism was transient and overcome by subsequent tissue remodeling events. Our previous study using arteriovenous loop chambers, based on a similar in vivo vascularized tissue engineering strategy, had demonstrated that vascularization peaked between 7–10 days and tissues started to remodel from this time point.8,35 Collectively, IPC appears to accelerate the process of intrinsic angiogenesis during the early intense vascularization phase, and the vascular volume of the mature constructs harvested at later time points was likely to be determined by the volume of vascularized tissue.
In the present study, we suspended cardiomyocytes in plasma clots to avoid anoikis cell death and improve cell retention in the chamber. Anoikis is a programmed cell death induced by loss of adhesion-related survival signals as a result of detachment from matrix.36 In addition, the plasma clot is biodegradable, biocompatible in vivo, and simulates a naturally occurring microenvironment for implanted cells. Furthermore, autologous serum reduces the immunogenicity. Importantly, we showed that IPC enhanced the early survival of implanted cardiomyocytes. This is particularly important, because early chamber constructs were shown to become hypoxic and do not properly support the survival of implanted cells.8,22 The antiapoptotic effect of IPC has been shown to extend beyond cardiomyocytes and include almost all cell types in the body, for example, endothelial cells,14,33 retinal,28 neuronal,37 hepatic,38,39 and renal cells.40 In vivo IPC may also protect implanted stem cells from injury as suggested by a recent study that reported an improved survival of transplanted bone marrow mesenchymal stem cells in rat myocardium with ischemic postconditioning, where the stimulus of intermittent ischemia was applied during the early reperfusion period.41 Although, we did not investigate the mechanisms underlying the antiapoptotic effect of IPC, previous studies have implicated the involvement of various antiapoptotic proteins such as VEGF, Bcl-2, and survivin.14,42,43 Various protective signaling pathways have also been implicated, including PKC, PI3K-Akt, MAPKs, JAK-STAT, nitric oxide, GSK-3β, and others.11,12,42
IPC created a more benign environment in the recipient that protects implanted cells from apoptosis and we reason that, since most of the cell death occurred in the first few days postimplantation, the prosurvival advantage detected at early time points would be likely to persist long term. Indeed, the volume of cardiac muscle assessed at 4 weeks postimplantation of cardiomyocytes was significantly increased in the IPC group. The improvement of cell viability in the tissue engineering chamber may also serve to reduce the number of cells needed for implantation, and this is of particular importance when cells are limited in supply and are susceptible to cell death such as cardiomyocytes in cardiac tissue engineering,25 pancreatic islets for pancreas,44 hepatocytes for liver,45 renal cells for kidney,46 and pneumocytes for lung.47
Conclusions
We report here that IPC increased the size of tissue generated in an in vivo tissue engineering chamber and this was associated with increased microcirculatory vascular volume. We have also demonstrated that IPC enhanced survival of implanted cardiomyocytes and increased the cardiac muscle volume. Conceptually, in vivo IPC could be adapted as a clinically viable cytoprotection strategy to enrich vascular supply in such grafts and reduce loss of implanted cells, while avoiding extensive ex vivo manipulation of the cells to be implanted.
Supplementary Material
Acknowledgments
These studies were supported by grants from National Health and Medical Research Council of Australia (509271), the Heart Foundation, JO and JR Wicking Trust, and Principal Research Fellowship (to GJD). The O'Brien Institute and the Centre for Eye Research Australia acknowledge the Victorian State Government's Department of Innovation, Industry, and Regional Development's Operational Infrastructure Support Program.
Disclosure Statement
No competing financial interests exist.
References
- 1.Sarig U. Machluf M. Engineering cell platforms for myocardial regeneration. Expert Opin Biol Ther. 2011;11:1055. doi: 10.1517/14712598.2011.578574. [DOI] [PubMed] [Google Scholar]
- 2.Miyagawa S. Roth M. Saito A. Sawa Y. Kostin S. Tissue-engineered cardiac constructs for cardiac repair. Ann Thorac Surg. 2011;91:320. doi: 10.1016/j.athoracsur.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 3.Miniati D.N. Robbins R.C. Heart transplantation: a thirty-year perspective. Annu Rev Med. 2002;53:189. doi: 10.1146/annurev.med.53.082901.104050. [DOI] [PubMed] [Google Scholar]
- 4.Radisic M. Malda J. Epping E. Geng W. Langer R. Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 5.Nesselmann C. Li W. Ma N. Steinhoff G. Stem cell-mediated neovascularization in heart repair. Ther Adv Cardiovasc Dis. 2010;4:27. doi: 10.1177/1753944709353338. [DOI] [PubMed] [Google Scholar]
- 6.Naderi H. Matin M.M. Bahrami A.R. Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl. 2011;26:383. doi: 10.1177/0885328211408946. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd B.R. Hoying J.B. Williams S.K. Microvascular transplantation after acute myocardial infarction. Tissue Eng. 2007;13:2871. doi: 10.1089/ten.2007.0025. [DOI] [PubMed] [Google Scholar]
- 8.Lokmic Z. Stillaert F. Morrison W.A. Thompson E.W. Mitchell G.M. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J. 2007;21:511. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- 9.Morritt A.N. Bortolotto S.K. Dilley R.J. Han X. Kompa A.R. McCombe D. Wright C.E. Itescu S. Angus J.A. Morrison W.A. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- 10.Murry C.E. Jennings R.B. Reimer K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 11.Yellon D.M. Downey J.M. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 12.Das M. Das D.K. Molecular mechanism of preconditioning. IUBMB Life. 2008;60:199. doi: 10.1002/iub.31. [DOI] [PubMed] [Google Scholar]
- 13.Kawata H. Yoshida K. Kawamoto A. Kurioka H. Takase E. Sasaki Y. Hatanaka K. Kobayashi M. Ueyama T. Hashimoto T. Dohi K. Ischemic preconditioning upregulates vascular endothelial growth factor mRNA expression and neovascularization via nuclear translocation of protein kinase C epsilon in the rat ischemic myocardium. Circ Res. 2001;88:696. doi: 10.1161/hh0701.088842. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda S. Kaga S. Sasaki H. Zhan L. Zhu L. Otani H. Kalfin R. Das D.K. Maulik N. Angiogenic signal triggered by ischemic stress induces myocardial repair in rat during chronic infarction. J Mol Cell Cardiol. 2004;36:547. doi: 10.1016/j.yjmcc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Maulik N. Ischemic preconditioning mediated angiogenic response in the heart. Antioxid Redox Signal. 2004;6:413. doi: 10.1089/152308604322899486. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki H. Fukuda S. Otani H. Zhu L. Yamaura G. Engelman R.M. Das D.K. Maulik N. Hypoxic preconditioning triggers myocardial angiogenesis: a novel approach to enhance contractile functional reserve in rat with myocardial infarction. J Mol Cell Cardiol. 2002;34:335. doi: 10.1006/jmcc.2001.1516. [DOI] [PubMed] [Google Scholar]
- 17.Ii M. Nishimura H. Iwakura A. Wecker A. Eaton E. Asahara T. Losordo D.W. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 18.Gyongyosi M. Posa A. Pavo N. Hemetsberger R. Kvakan H. Steiner-Boker S. Petrasi Z. Manczur F. Pavo I.J. Edes I.F. Wojta J. Glogar D. Huber K. Differential effect of ischaemic preconditioning on mobilisation and recruitment of haematopoietic and mesenchymal stem cells in porcine myocardial ischaemia-reperfusion. Thromb Haemost. 2010;104:376. doi: 10.1160/TH09-08-0558. [DOI] [PubMed] [Google Scholar]
- 19.Kamota T. Li T.S. Morikage N. Murakami M. Ohshima M. Kubo M. Kobayashi T. Mikamo A. Ikeda Y. Matsuzaki M. Hamano K. Ischemic pre-conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 2009;53:1814. doi: 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Choi Y.S. Dusting G.J. Stubbs S. Arunothayaraj S. Han X.L. Collas P. Morrison W.A. Dilley R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J Cell Mol Med. 2010;14:878. doi: 10.1111/j.1582-4934.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed T.A. Dare E.V. Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 22.Tilkorn D.J. Bedogni A. Keramidaris E. Han X. Palmer J.A. Dingle A.M. Cowling B.S. Williams M.D. McKay S.M. Pepe L. Deftereos A. Morrison W.A. Penington A.J. Mitchell G.M. Implanted myoblast survival is dependent on the degree of vascularization in a novel delayed implantation/prevascularization tissue engineering model. Tissue Eng Part A. 2010;16:165. doi: 10.1089/ten.TEA.2009.0075. [DOI] [PubMed] [Google Scholar]
- 23.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57. [PubMed] [Google Scholar]
- 24.Hughes S. Archimedes revisited: a faster, better, cheaper method of accurately measuring the volume of small objects. Phys Educ. 2005;40:468. [Google Scholar]
- 25.Vunjak-Novakovic G. Tandon N. Godier A. Maidhof R. Marsano A. Martens T.P. Radisic M. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010;16:169. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Hinsbergh V.W. Collen A. Koolwijk P. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 27.Lokmic Z. Thomas J.L. Morrison W.A. Thompson E.W. Mitchell G.M. An endogenously deposited fibrin scaffold determines construct size in the surgically created arteriovenous loop chamber model of tissue engineering. J Vasc Surg. 2008;48:974. doi: 10.1016/j.jvs.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Abcouwer S.F. Lin C.M. Wolpert E.B. Shanmugam S. Schaefer E.W. Freeman W.M. Barber A.J. Antonetti D.A. Effects of ischemic preconditioning and bevacizumab on apoptosis and vascular permeability following retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2010;51:5920. doi: 10.1167/iovs.10-5264. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto E. Ogita T. Nakaoka T. Matsuoka R. Takao A. Kira Y. Rapid induction of vascular endothelial growth factor expression by transient ischemia in rat heart. Am J Physiol. 1994;267:H1948. doi: 10.1152/ajpheart.1994.267.5.H1948. [DOI] [PubMed] [Google Scholar]
- 30.Dvorak H.F. Nagy J.A. Feng D. Brown L.F. Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 31.van Bruggen N. Thibodeaux H. Palmer J.T. Lee W.P. Fu L. Cairns B. Tumas D. Gerlai R. Williams S.P. van Lookeren Campagne M. Ferrara N. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy J.A. Benjamin L. Zeng H. Dvorak A.M. Dvorak H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki H. Ray P.S. Zhu L. Otani H. Asahara T. Maulik N. Hypoxia/reoxygenation promotes myocardial angiogenesis via an NF kappa B-dependent mechanism in a rat model of chronic myocardial infarction. J Mol Cell Cardiol. 2001;33:283. doi: 10.1006/jmcc.2000.1299. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Amodio S. Tra W.M. Rakhorst H.A. Hovius S.E. van Neck J.W. Hypoxia preconditioning of tissue-engineered mucosa enhances its angiogenic capacity in vitro. Tissue Eng Part A. 2011;17:1583. doi: 10.1089/ten.TEA.2010.0429. [DOI] [PubMed] [Google Scholar]
- 35.Jiang F. Zhang G. Hashimoto I. Kumar B.S. Bortolotto S. Morrison W.A. Dusting G.J. Neovascularization in an arterio-venous loop-containing tissue engineering chamber: role of NADPH oxidase. J Cell Mol Med. 2008;12:2062. doi: 10.1111/j.1582-4934.2008.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zvibel I. Smets F. Soriano H. Anoikis: roadblock to cell transplantation? Cell Transplant. 2002;11:621. doi: 10.3727/000000002783985404. [DOI] [PubMed] [Google Scholar]
- 37.Pong K. Ischaemic preconditioning: therapeutic implications for stroke? Expert Opin Ther Targets. 2004;8:125. doi: 10.1517/14728222.8.2.125. [DOI] [PubMed] [Google Scholar]
- 38.Peralta C. Serafin A. Fernandez-Zabalegui L. Wu Z.Y. Rosello-Catafau J. Liver ischemic preconditioning: a new strategy for the prevention of ischemia-reperfusion injury. Transplant Proc. 2003;35:1800. doi: 10.1016/s0041-1345(03)00571-2. [DOI] [PubMed] [Google Scholar]
- 39.Yamada F. Saito T. Abe T. Tsuchiya T. Sato Y. Kenjo A. Kimura T. Gotoh M. Ischemic preconditioning enhances regenerative capacity of hepatocytes in long-term ischemically damaged rat livers. J Gastroenterol Hepatol. 2007;22:1971. doi: 10.1111/j.1440-1746.2006.04711.x. [DOI] [PubMed] [Google Scholar]
- 40.Bonventre J.V. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens. 2002;11:43. doi: 10.1097/00041552-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Fang J. Chen L. Fan L. Wu L. Chen X. Li W. Lin Y. Wang W. Enhanced therapeutic effects of mesenchymal stem cells on myocardial infarction by ischemic postconditioning through paracrine mechanisms in rats. J Mol Cell Cardiol. 2011;51:839. doi: 10.1016/j.yjmcc.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Z.Q. Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res. 2002;55:438. doi: 10.1016/s0008-6363(02)00442-x. [DOI] [PubMed] [Google Scholar]
- 43.Kaga S. Zhan L. Altaf E. Maulik N. Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. J Mol Cell Cardiol. 2006;40:138. doi: 10.1016/j.yjmcc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Merani S. Shapiro A.M. Current status of pancreatic islet transplantation. Clin Sci (Lond) 2006;110:611. doi: 10.1042/CS20050342. [DOI] [PubMed] [Google Scholar]
- 45.Fox I.J. Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40:878. doi: 10.1016/j.jhep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Shokeir A.A. Harraz A.M. El-Din A.B. Tissue engineering and stem cells: basic principles and applications in urology. Int J Urol. 2010;17:964. doi: 10.1111/j.1442-2042.2010.02643.x. [DOI] [PubMed] [Google Scholar]
- 47.Song J.J. Ott H.C. Bioartificial lung engineering. Am J Transplant. 2012;12:283. doi: 10.1111/j.1600-6143.2011.03808.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.