Abstract
The ability to trigger an innate immune response against opportunistic pathogens associated with HIV-1 infection is an important aspect of AIDS pathogenesis. Toll-like receptors (TLRs) play a critical role in innate immunity against pathogens, but in HIV-1 patients coinfected with opportunistic infections, the regulation of TLR expression has not been studied. In this context, we have evaluated the expression of TLR2 and TLR4 in monocytes, plasmacytoid dendritic cells, and myeloid dendritic cells of HIV-1 patients with or without opportunistic infections. Forty-nine HIV-1-infected individuals were classified according to viral load, highly active antiretroviral therapy (HAART), and the presence or absence of opportunistic infections, and 21 healthy subjects served as controls. Increased expression of TLR2 and TLR4 was observed in myeloid dendritic cells of HIV-1 patients coinfected with opportunistic infections (without HAART), while TLR4 increased in plasmacytoid dendritic cells, compared to both HIV-1 without opportunistic infections and healthy subjects. Moreover, TLR2 expression was higher in patients with opportunistic infections without HAART and up-regulation of TLR expression in HIV-1 patients coinfected with opportunistic infections was more pronounced in dendritic cells derived from individuals coinfected with Mycobacterium tuberculosis. The results indicate that TLR expression in innate immune cells is up-regulated in patients with a high HIV-1 load and coinfected with opportunistic pathogens. We suggest that modulation of TLRs expression represents a mechanism that promotes HIV-1 replication and AIDS pathogenesis in patients coinfected with opportunistic pathogens.
Introduction
Alterations in immune response are hallmarks of HIV-1 infection. Among these, CD4+ T cell depletion together with increased levels of viremia and opportunistic infections (OI) are considered major factors in promoting HIV/AIDS pathogenesis.1 HIV-1-infected individuals can also suffer from chronic hyperactivation of the immune system, and this persistent immune challenge together with high levels of proinflammatory cytokines and increases in immune cell activation is thought to contribute to viral pathogenesis.1 Moreover, advanced HIV-1 infection is known to be associated with reduced innate and adaptive immune responses to pathogens, which can promote unusual and severe manifestations of OI and AIDS progression. In addition to CD4+ T cell depletion, HIV infection can lead to markedly reduced numbers and altered functions of innate immune cells such as plasmacytoid dendritic cells (pDC) and myeloid dendritic cells (mDC).2,3 Given the central role of DCs in innate and adaptive immunity, it seemed possible that the functional abnormalities of these cells could trigger altered responses to opportunistic pathogens in HIV-1-coinfected subjects. DCs are activated through innate signaling receptors such as the Toll-like receptors (TLRs) and other members of pattern-recognition receptors (PRRs).4,5 PRR activation by various stimulatory substances culminates in the establishment of adaptive immunity, characterized by the release of inflammatory cytokines, expression of costimulatory molecules, and migration of DCs into lymphoid tissues.6,7 Other viruses such as hepatitis C virus, vaccinia virus, West Nile virus, and HTLV-1 can also alter immune effector mechanisms leading to dissemination of infection.8
TLRs recognize several conserved microbial structures, including lipids, proteins, and nucleic acids. For example, HIV-derived RNA can activate pDCs via TLR7/TLR8 and possibly TLR9.4,9–11 TLR engagement leads to activation of common signaling pathways, which results in the activation of the transcription factors, activator protein 1 (AP-1) and NF-κB.12,13 Importantly, TLR downstream signaling can also activate HIV-1 genome expression itself, through the interaction with long terminal repeats (LTR) in the HIV provirus.14–18 Previous studies have documented altered TLR expression in HIV-1-infected patients19–23 raising the possibility that proinflammatory signals during chronic infection and OI can further increase HIV expression. Thus, while TLR expression in HIV-1 patients coinfected with OI could be an important determinant of disease progression, the regulation of TLR expression in HIV-1 patients coinfected with OI has not been studied. As we are interested in this subject, we evaluated the expression of TLR2 and TLR4 in monocytes, pDC, and mDC of HIV-1 patients with or without OI. We report here an increased expression of TLR2 and TLR4 in DCs of HIV-1-infected patients with OI, especially in those coinfected with Mycobacterium tuberculosis (TB) and without HAART treatment, as well as high levels of TLR2 and TLR4 mRNAs in peripheral blood mononuclear cells (PBMCs) from coinfected patients.
Materials and Methods
Patient cohort
Forty-nine HIV-1-infected patients were classified in two groups according to the presence or absence of OI (+OI or −OI, respectively), viral load (higher than or less than 400 copies of viral RNA/ml blood), and use of HAART (naive treatment or more than 6 continuous months of treatment, at the time of sample acquisition). Twenty-one age-matched HIV-1 individuals uninfected by OI and without illness for the past 3 months were included in the study as controls, who were volunteers from the general population, unfamiliar with the study. According to ethical guidelines, signed informed consent was obtained before enrollment into the study. All biomedical investigations were conducted according to the principles of the Helsinki Declaration.
The diagnosis of the OI was made based on clinical manifestations (signs and symptoms) and using routine microbiology laboratory tests (serology, culture, and/or PCR; for TB x-rays were also used). HIV-1 patients with OI were treated with specific antibiotics/antivirals, according to international pharmaceutical guidelines. However, all the samples were taken within 1–2 days after specific diagnosis. According to the 1993 Centers for Disease Control (CDC) and Prevention classification system, all HIV-1 patients with OI were at the symptomatic AIDS stage (C2 and C3), including Pneumocystis jirovecii pneumonia, disseminated histoplasmosis, disseminated cryptococcosis, active pulmonary and extrapulmonary tuberculosis, esophageal candidiasis, and other opportunistic infections, common among Colombian patients (e.g., disseminated herpes simplex virus disease, syphilis, Pseudomonas spp., and Escherichia coli sepsis). Syphilis is not an opportunistic infection per se; however, Treponema pallidum is a very common coinfecting pathogen in Colombian HIV-1-infected patients, who have an increase risk for neurological complications and uveitis and have higher rates of treatment failure.24
Sample acquisition and preparation
Whole peripheral blood was collected by venipuncture into vacutainers (Becton Dickinson) containing heparin. HIV-1 viral loads were assessed by certified commercial laboratories. CD4+ T cells counts were performed by flow cytometry (FACS BD Biosciences, San Jose, CA). Fresh PBMCs were isolated by density gradient centrifugation using Ficoll–Hypaque (Histopaque 1077, Sigma Aldrich). Aliquots of fresh PBMCs, containing approximately 3×106 cells, were mixed directly in 1 ml Trizol reagent (Invitrogen) and frozen before RNA extraction.
Monoclonal antibodies
The following monoclonal antibodies were obtained from BD Biosciences (BD, San Jose, CA): Lin1 FITC (CD3, CD14, CD16, CD19, CD20, and CD56 cocktail), CD123 PE-Cy5, and CD11c PE-Cy5. TLR2 (clone TL2.1) and TLR4 (clone HTA125) phycoerythrin conjugates were from eBiosciences. Anti-BDCA-2 FITC and the FcR blocking reagent were from Miltenyi (Auburn, CA). Conjugated isotype-control antibodies were used as controls.
Flow cytometry analysis
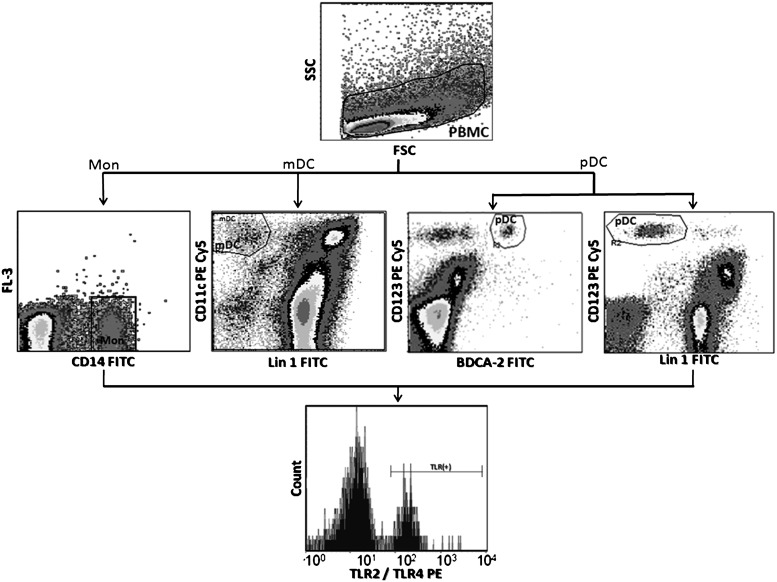
Freshly isolated PBMCs were resuspended in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 0.1% sodium azide. After addition of Fc-receptor blocking reagent (Miltenyi, Auburn, CA), PBMCs were surface stained with the appropriate antibodies or controls, fixed with 2% formaldehyde, and stored at 4°C until analysis. All samples were evaluated within 2–4 h of staining using a FACScan flow cytometer (BD Biosciences). Logical gating was used to identify monocyte (CD14+), mDC (Lin1−/CD11chigh), and pDC (BDCA-2+/CD123high) populations (Fig. 1). For DC evaluation, at least 200,000 gated events were acquired, and for monocytes at least 100,000 gated events. Acquired events were analyzed using the CellQuest software. TLR expression is presented as mean fluorescent intensity of the total cell subpopulation.
FIG. 1.
Gate strategies used to determine the expression of Toll-like receptor (TLR)2 and TLR4 on monocytes, myeloid dendritic receptors (mDCs), and plasmacytoid dendritic receptors (pDCs). Mononuclear cells were gated according to physical characteristics, excluding dead cells. Monocytes were then gated as CD14+ cells; mDC as Lin 1− CD11chigh; and pDC in two ways, BDCA-2+ CD123high or Lin 1− CD123high. Each specific subpopulation was plotted as histograms to show the expression of TLR2 and TLR4. Data are presented as overall mean fluorescence intensity (MFI), after subtraction of isotype staining background.
Quantitative reverse-transcriptase real-time PCR
One microgram of total RNA was treated with 2 units RNase-free DNase I (Fermentas) for 1 h at 37°C to remove traces of genomic DNA. The first strand cDNA was synthesized using the Superscript III kit (Invitrogen) according to the manufacturer's directions. Briefly, total RNA was incubated for 60 min at 42°C with reverse transcriptase and random hexamer primers, and then treated with RNase H for 20 min at 37°C. No RT control was used. Specific primers were designed intron spanning, to avoid coamplification of genomic DNA in the real time PCR step. The sequences were TLR2 (F: GGCCAGCAAATTACCTGTGTG, R: CCAGGTAGGTCTTGGTGTTCA), TLR4 (F: CTGCAATGGATCAAGGACCA, R: TCCCACTCCAGGTAAGTGTT), and β-actin (F: ATCTGGCACCACACCTTCTACAATGAGCTGCG, R: CGTCATACTCCTGCTTGCTGATCCACATCTGC). β-actin was used as a housekeeping gene to account for variability in the amount of RNA transcribed and in the RT reaction itself. Real-time PCR cycling was performed in duplicate (5 min at 95°C, followed by 38 cycles of 20 s at 95°C, 30 s at 55°C, and 30 s at 72°C) in 25 μl containing 2 μl of undiluted cDNA, 500 nmol of each primer, and 1X SYBR green PCR master mix (Qiagen). For comparative purposes, estimation of the relative amount of mRNA in the samples was determined according to the following formula: (1+X)−ΔCt, where X is the efficiency of the reaction, Ct is the cycle at which the detected signal is significantly above the background signal, and ΔCt is the difference between the Ct of the TLR mRNA of interest and the Ct of the endogenous control gene, β-actin. For all the experiments, the efficiency of the reactions was between 81% and 87%, and thus, the formula was used with an average efficiency of 85% (1.85−ΔCt). The specificity of amplification was validated by observing a single peak at the expected Tm on the analysis of the melting curve.
Statistical analysis
Data were plotted and analyzed using Prism 5.0 software (Graph Pad Software, CA). All the results of the in vitro assays shown represent at least three independent experiments. Unpaired two-tailed Student's t-tests (Mann–Whitney U-tests) and the ANOVA test were employed to assess the statistical significance of TLR expression in the different groups. Spearman's test for matched correlations was performed. Values of p<0.05 (*) were considered significant, whereas p values <0.01 (**) or <0.001 (***) were considered highly significant.
Results
Monocytes and mDC subpopulations decreased in HIV-1-coinfected patients
The demographics of the patients studied are shown in Table 1, and opportunistic pathogens present in HIV-1-coinfected patients are identified in Table 2. All the patients recruited for these studies were diagnosed with active disease at the time of sample collection, and were on antibiotic therapy. First, we quantified the cellular subpopulations to assess the possible differences between subject groups. We observed a significant decrease (p<0.01) in the percentage and the absolute count of monocytes/μl of blood from HIV patients coinfected with OI (OI+ median count: 284; range: 176–2066) compared with HIV-1-infected patients without OI (OI− median count: 650; range: 88–2002). In addition, a decrease in the relative number (percentage) of mDC (p<0.018) as well as in the absolute mDC count in total blood was observed in coinfected subjects (median: 0.17% range: 0.05–1.15%) when compared with OI subjects (median: 0.40% range: 0.22–1.15%). In contrast to previous reports, there was no change in the pDC subpopulation counts (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/aid). The results indicate alterations in cellular populations that are prevalent during HIV infection, primarily in patients with active coinfections.
Table 1.
Demographic Features of HIV-1-Infected Individuals and Controls
| |
HIV-1-infected individuals |
|
|
|---|---|---|---|
| Item | With opportunistic infections (n=10) | Without opportunistic infections (n=39) | Healthy controls (n=21) |
| Age: median (range) | 25 (20–63) | 36 (20–61) | 24 (19–47) |
| Male:Female | 6:4 | 25:14 | 8:13 |
| Viral load in RNA copies/ml plasma: median (range) | 83,700 (734–231,000) | 17,750 (<50–750,000) | N/A |
| With HAARTa: without HAART | 4:6 | 30:9 | N/A |
| CD4+ T cells/μl peripheral bloodb: median (range) | 130 (4–406) | 330 (14–950) | 646 (460–1143) |
Patients in HAART treatment were using combinations of nucleotide and nucleoside reverse transcriptase inhibitors (abacavir, lamivudine, didanosine, stavudine, and zidovudine), nonnucleoside reverse transcriptase inhibitors (efavirenz and nevirapine), and protease inhibitors (lopinavir, fosamprenavir, amprenavir, nelfinavir, and saquinavir).
CD4+ T cell counts under 200 cells/μl; were receiving fluconazol, acyclovir, and TMS as prophylactic.
HAART, highly active antiretroviral therapy; NA, not applicable.
Table 2.
Opportunistic Infections in HIV-1-Infected Individuals
| Opportunistic infections | Number of patientsa |
|---|---|
| Mycobacterium tuberculosisb | 4 |
| Candidiasis (severe esophagitis) | 3 |
| Cryptococcosisc | 2 |
| Histoplasmosisc | 2 |
| HSV-1/2 infectionsc | 2 |
| Pneumocystis pneumonia | 2 |
| Otherd | 5 |
Some patients had infections by two or more pathogens.
Active pulmonary and extrapulmonary disease.
Disseminated infection.
Syphilis, pneumopathies, and bacterial infections, including Pseudomonas spp. and E. coli sepsis.
TLR2 and TLR4 are increased in DCs from HIV-1-coinfected patients with OI
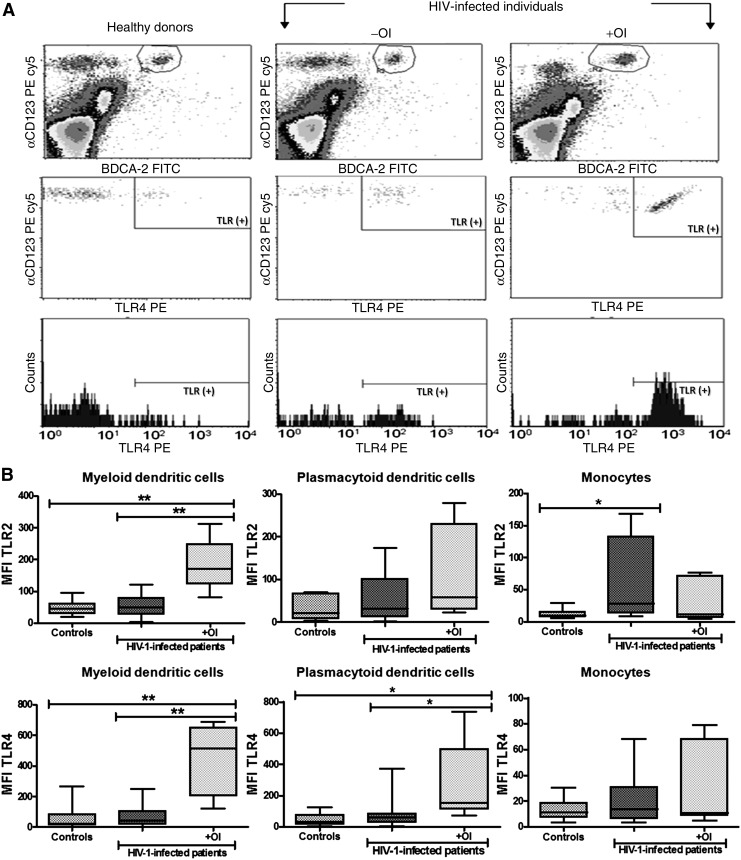
Sanders et al. reported that TLR4 expression in lymphocytes was increased in HIV-1-infected individuals compared to healthy controls,25 suggesting a possible role of HIV-1 in modulating TLR expression. We determined TLR2 and TLR4 protein and mRNA expression levels to assess whether coinfection with OI changes TLR expression in antigen-presenting cells of HIV-1-infected patients (Fig. 2). HIV-1-infected subjects with OI were subdivided based on the use of HAART for part of the analysis (Table 1 and Fig. 3). TLR2 and TLR4 expression was increased (p<0.01) in mDCs of HIV-1 patients coinfected with an OI compared with either HIV-1 patients without an OI or healthy donors (Fig. 2B). In addition, expression levels of both TLR2 and TLR4 in mDCs were different between HIV-1-infected patients with and without OI, but no significant difference in TLR2 or TLR4 expression was observed when HIV-1-infected patients without OI were compared to controls. Similar results were observed in pDCs from HIV-1 patients coinfected with OI, but only for TLR4 (p<0.05) (Fig. 2). In contrast, expression of TLR2 was increased in monocytes from HIV-1-infected patients without OI compared to healthy controls (p<0.05) (Fig. 2B). Interestingly, the low mean fluorescent intensity (MFI) values of TLR2 and TLR4 in monocytes compared with DCs is due to high basal fluorescence in monocytes, which decreases the overall MFI, after subtraction of the isotype control.
FIG. 2.
Increased expression of TLR2 and TLR4 in DCs from HIV-1 patients coinfected with opportunistic infection (OI). TLR2 and TLR4 expression on monocytes (CD14+), mDCs (Lin1− CD11chigh), and pDCs (BDCA2+ CD123high) from total peripheral blood mononuclear cells (PBMCs), and measured by flow cytometry. (A) Analysis of TLR4 expression in pDCs from the three groups of individuals, healthy donors, and HIV-1-infected patients with and without OIs. The PBMC gated events are shown to select the pDC subpopulation and TLR4 expression in these cells. For both TLRs and all cellular subpopulations evaluated, the strategy used for the analysis was the same. (B) The mean fluorescence intensity (MFI) of TLR2 and TLR4 in mDCs and monocytes was plotted for each group: controls (n=21), HIV-1-infected patients without OI (n=39), and HIV-1-infected patients with OI (n=10). Comparisons were performed using the Kruskal–Wallis ANOVA tests and Dunn's post-tests. The level of significance is p<0.05 (*) and p<0.01 (**).
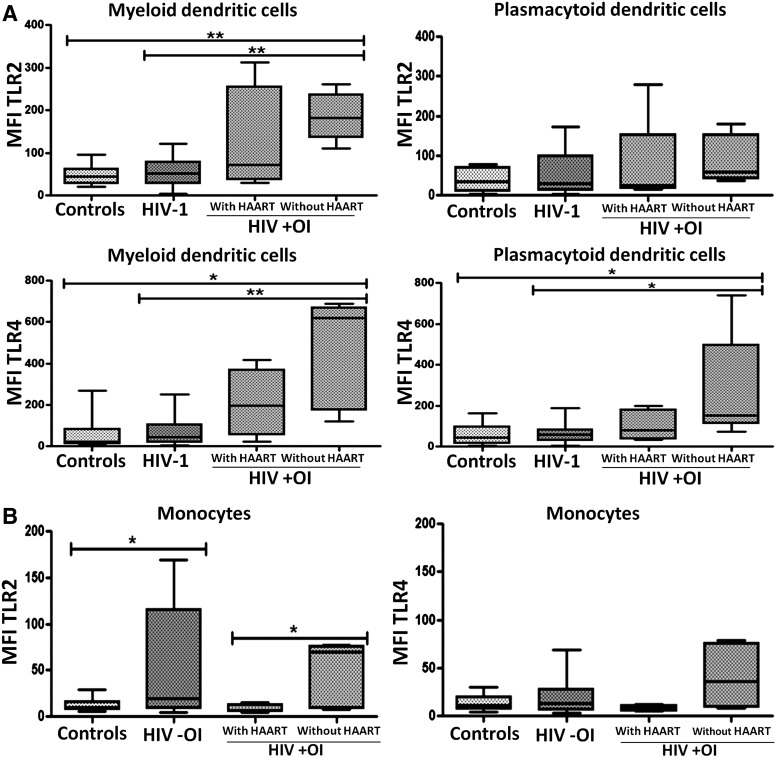
FIG. 3.
Higher expression of TLR2 and TLR4 in cells of HIV-1-coinfected patients with OI but without highly active antiretroviral therapy (HAART). OIs and HIV coinfection are associated with increased TLR expression in (A) dendritic cells and (B) monocytes from patients without HAART treatment. Mean fluorescence intensity (MFI) of TLR2 and TLR4 in mDCs and monocytes was plotted for each study group. Comparisons were by Kruskal–Wallis ANOVA tests and Dunn's post-tests. The level of significance is p<0.05 (*) and p<0.01 (**); controls (n=21), HIV −OI (HIV-1-infected patients without OIs, n=39), HIV +OI (HIV-1-infected patients with OIs) with HAART (n=4) and without HAART (n=6).
Surprisingly, we report TLR2 and TLR4 expression on pDC (defined as Lin1− CD123high or BDCA-2+ CD123high PBMCs) at the protein level in all the individuals in this study (Fig. 2 and Supplementary Fig. S2; Supplementary Data are available online at www.liebertonline.com/aid). These results were validated by the isotype control analysis (Supplementary Fig. S2A), as well as in purified pDCs derived from a healthy donor sample by using a magnetic bead-based methodology (Miltenyi, Germany), both at the protein level (Supplementary Fig. S2B) and the mRNA level (Supplementary Fig. S2C) (flow cytometry and real time RT-PCR, respectively).
HIV-1-infected patients coinfected with Mycobacterium tuberculosis showed the most notable up-regulation of TLR2 and TLR4
HIV-1-infected patients are frequently coinfected with TB, and active pulmonary or severe and disseminated forms of tuberculosis can be observed. To determine whether coinfection with M. tuberculosis specifically influenced the expression level of TLR2 and TLR4, HIV-1 patients coinfected with OI were subdivided by their type of coinfected microorganisms and evaluated for TLR expression levels. HIV-1 patients coinfected with TB presented the highest induction of TLR2 and TLR4 expression in monocytes and of TLR2 in mDCs (Table 3) when compared to HIV-1 patients coinfected with other opportunistic pathogens (Table 2) or when compared to healthy controls. These results demonstrate that HIV-1 infection can effectively synergize with certain types of OI to increase the expression levels of TLR2 and TLR4.
Table 3.
TLR2 and TLR4 Expression in Monocytes and Dendritic Cells from HIV-Infected Patients with Opportunistic-Infections
| |
Monocytes |
mDC |
pDC |
|||
|---|---|---|---|---|---|---|
| Clinical condition | TLR2 | TLR4 | TLR2 | TLR4 | TLR2 | TLR4 |
| HIV-1-infected patients | ||||||
| Syphilis | 8.13 | 9.89 | 70.59 | 71.46 | 65.57 | 151.60 |
| Fungia | 8.78 | 9.10 | 160.60 | 661.60 | 102.20 | 390.40 |
| M. tuberculosis | 72.36 | 68.16 | 197.30 | 617.90 | 50.19 | 153.40 |
| HSV-1/2a | 7.18 | 7.04 | 121.00 | 459.60 | 102.20 | 390.40 |
| HIV without OI | 19.92 | 13.31 | 52.08 | 44.37 | 29.86 | 59.13 |
| Healthy donors | 10.06 | 11.44 | 44.59 | 21.40 | 34.44 | 42.40 |
One of the HIV-1 coinfected patients with fungi (cryptococcosis and candidiasis) also had active and disseminated HSV-1/2 infection.
TLR2 and TLR4 were expressed as the mean fluourescent intensity (MFI) median of HIV-infected patients with each opportunist pathogen.
DC, dendritic cell.
DCs from HIV-1-infected patients with OI and without HAART present increased TLR2 and TLR4 expression
The pattern of TLR2 and TLR4 expression in individuals with or without HAART therapy was then evaluated. Notably, in the group of HIV-1 patients coinfected with OIs and without HAART treatment, there were increased TLR2 and TLR4 expression in mDCs, compared to HIV-1-infected patients without OI or compared to healthy controls (Fig. 3A). Similar results were observed in pDCs, but only in the case of TLR4 expression (Fig. 3A). In addition, monocytes of HIV-1 patients coinfected with OI and not undergoing HAART treatment expressed higher levels of TLR2 compared to monocytes of HIV-1-infected patients with OI and undergoing HAART therapy (Fig. 3B). Similar results were observed when the expression of TLR2 was determined in monocytes from HIV-1-infected patients without OI, as compared to controls (Fig. 3B). These results suggest that HAART treatment that modulates viral load can contribute to the effect of OI on the up-regulation of TLR2 and TLR4 in immune cells.
Increased mRNA levels of TLR2 and TLR4 in HIV-1 patients coinfected with opportunistic pathogens
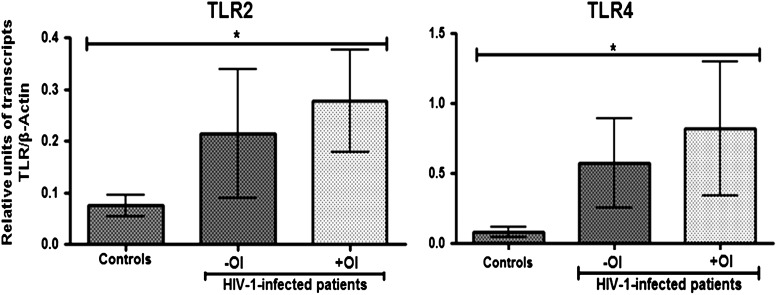
Consistent with the increase of TLR2 and TLR4 protein expression observed by flow cytometry, there was an increase in the mRNA level of both TLR2 and TLR4 in PBMCs of HIV-1 patients coinfected with OIs, when compared to healthy donors (p<0.05) (Fig. 4). Interestingly, HIV-1-infected patients also have increased levels of mRNA tumor necrosis factor (TNF)-α compared to the controls (data to be published separately).
FIG. 4.
HIV-1-infected individuals express higher levels of TLR2 and TLR4 mRNAs than healthy donors. TLR2 and TLR4 mRNA levels were measured using real-time PCR, and normalized with the housekeeping gene β-actin. Relative units of TLR2 and TLR4 transcripts versus β-actin transcripts are shown as a median and range. Comparisons were by Kruskal–Wallis ANOVA tests and Dunn's post-tests. The level of significance is p<0.05 (*); controls (n=21), −OI (HIV-1-infected patients without OIs, n=39), +OI (HIV-1-infected patients with OIs, n=10).
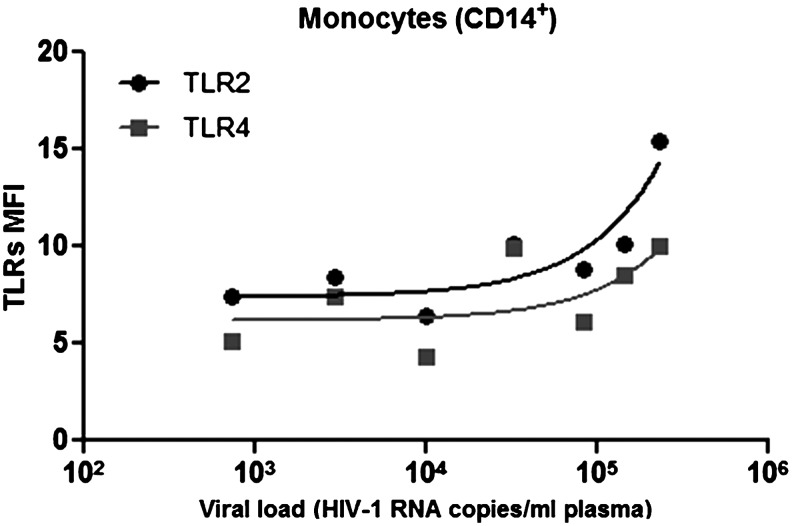
Expression of TLR2 in monocytes correlates positively with viremia in HIV-1 patients coinfected with opportunist pathogens
We next determined the correlation between plasma HIV-1 RNA levels with TLR2 and TLR4 expression in HIV-1-infected subjects with or without OI. Consistent with the hypothesis that HIV infection synergizes with OI to induce expression of TLR2 and TLR4, we observed that the expression levels of TLR2 in monocytes correlated positively with viral load (r=0.786, p=0.048). Additionally, there was a trend toward TLR4 correlation with viral load (r=0.679, p=0.109) (Fig. 5). A similar analysis for CD4+ T cell counts and TLR2 or TLR4 expression in HIV-1-infected patients with OI showed no significant correlations (data not shown).
FIG. 5.
TLR2 expression in monocytes is positively correlated with viral load in HIV-1-coinfected patients with OI. TLR2 expression was significantly correlated with viral load (p=0.048, r=0.786), and TLR4 tended toward correlating with viral load in HIV-1-coinfected subjects with OI (p=0.109, r=0.679). For the correlation, seven HIV-1-infected patients with OI were analyzed, but for the last three patients, it was not possible to obtain data on the viral load. Spearman correlations were used with a significance level of p<0.05 two-tailed.
Discussion
Innate immune activation is critical to control infections. In the case of HIV infection, however, innate immune activation also drives HIV replication via signaling pathways downstream of TLRs.26 Therefore, HIV is able to exploit the activation of innate immunity for its own advantage. This feature of HIV may have important implications for HIV-1-infected patients who are coinfected with opportunistic pathogens. Although it is known that OIs in HIV-1-infected individuals are frequently associated with increased viral loads,27 the mechanisms by which coinfecting pathogens facilitate AIDS progression remain poorly understood. Here, we evaluated the effect of coinfection on TLR2 and TLR4 expression in monocytes and DCs from in HIV-1-infected patients.
In vitro studies suggest that stimulation of TLR2, TLR4, and TLR9 causes up-regulation of viral replication14–18 by activating NF-κB in HIV-1-infected cells. However, activation of NF-κB through TLR4 alone is not sufficient to activate viral LTR and virus replication,28 while stimulation via TLR2 always induces LTR HIV activation. Presumably, this is because TLR4 signaling is also able to induce type I IFN release,29 which acts as an antiviral factor. On the other hand, the fungal zymosan, which stimulates TLR2 and dectin-1, was reported to inhibit HIV replication.30 Nevertheless, pure TLR2 and TLR5 ligands increase cellular HIV-1 integration, trigger reactivation of latent HIV-1 provirus in T cells, and activate virus gene expression in central memory CD4+ T cells.31 Thus, TLR activation may lead to modulation of HIV infection due to the effect of downstream signaling effectors on viral replication.
We observed a marked increase of TLR2 and TLR4 expression in mDCs of HIV-1 patients coinfected with different pathogens in vivo. Similar results were observed in monocyte-derived macrophages and PBMCs from healthy donors and in vitro infected with HIV-1, treated with TLR2 and TLR4 agonist. Consequently, with this increase in TLR expression, an effect on the functionality of TLRs was observed based in the high expression of proinflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α, and the induction of pDC maturation, compared to mock infection (data to be published separately). These results extend previous findings that HIV-1 infection itself leads to up-regulation of TLR2.19 Likewise, HIV-1 and its products can also modulate TLR expression and functions. For instance, the ssRNA40 (HIV-1-derived RNA) increased TLR3 and TLR8 expression in activated T cells,25 as well as TLR4 mRNA expression,20,21 together with an increased proinflammatory response to TLR ligands. Increased TLR4 expression by T cell subsets was reported in HIV-1-infected patients.22 Chronic HIV-1-infected patients who failed to respond to HAART showed reduced expression of TLR3, TLR4, and TLR9 together with increased expression of TLR7, which correlated with high HIV-1 RNA levels.23 The results suggest that the virus-mediated immune-modulator pathway involved in regulating TLR expression could represent an important pathogenic event in chronic HIV-1 infection.
Consistent with this hypothesis, an association between HIV disease progression and polymorphisms in TLR432 and TLR9,33,34 or the levels of soluble TLR2,35 has been reported. Interestingly, a 3′-UTR polymorphism in the NLRP3 gene, a member of the cytosolic NOD-like receptors, was also associated with increased susceptibility to HIV-1 infection.36 Overall, these results point to an important role of the TLRs, or even other PRRs, in modulating AIDS progression.
Whether TLR signaling is beneficial for the host, or enhances virus replication and spread, depends on different factors: the kind of TLRs activated, the doses of TLR ligands involved, the duration of the stimulation, the cell types stimulated, and the stage of HIV-1 infection (acute vs. chronic). Based on our results, we propose a dual role of TLR during HIV-1 infection. During the initial phase of infection, TLR signaling could promote IFN-α/β release, which could have antiviral effects. However during chronic infection, TLR stimulation could induce a strong inflammatory response that would increase HIV-1 replication. This can explain the different results obtained by several research groups, showing an increase or decrease in HIV-1 replication,14,37,38 or even transmission from DCs to CD4+ T cells,39 after TLR stimulation when using different TLR ligands, virus strains, and cellular models (mastocytes,16 DCs,40 macrophages, lymphoid tissue,41 and microglia42), or animal models (transgenic mice that incorporate the HIV-1 genome).15,43
The main goal of HAART is to block HIV-1 replication and achieve immune reconstitution in HIV-1-infected patients. In this study we report that mDCs of HIV-1-infected subjects with OI and without HAART present higher expression levels of TLR2 and TLR4 than those from coinfected patients on HAART therapy. This could be associated with a higher viral load since TLR2 and TLR4 are able to mediate the activation of HIV-1 LTR through the NF-κB pathway.14,15,18 Together, these results suggest that TLR expression levels could be influenced by viral factors, opportunistic pathogens, and the immunological state of the host.
We have also observed that the percentage of monocytes and mDCs decreased significantly in HIV-1 patients coinfected with OI and without HAART. In contrast, the percentage of pDCs was similar in all the groups of HIV-1-infected individuals studied (Supplementary Fig. S1). Interestingly, despite a lower percentage of these cell types, the mDCs of HIV-1-infected subjects with OI and without HAART expressed higher levels of TLR2 and TLR4, the pDCs expressed higher levels of TLR4, and the monocytes expressed higher levels of TLR2. Previous reports have shown restoration in cellular subsets following HAART.44,45 HIV-1 patients coinfected with OIs in our study exhibited restoration of mDCs, as well as monocytes, after HAART.
Based on these observations, we propose that increased TLR expression, together with a higher inflammatory response, increases viral replication through NF-κB. In addition to increased TLR4 expression in pDCs, its activity might also, thereby, induce IFN-α secretion and increased loss of CD4+ T cells via TRAIL-mediated apoptosis.46 On the other hand, there is a second possibility, whereby patients with higher viral load, especially those without HAART, maintain a proinflammatory state that also increases TLR expression. In both cases, TLR up-regulation in HIV-1-infected patients could represent an immune-pathogenic event that would accelerate the progression to AIDS in patients not on HAART, primarily in the context of an opportunist infection.
TB is one of the most important OIs in HIV-1-infected patients, especially among Colombian subjects. Patients with TB and without HIV-1 infection showed no difference in TLR2 expression in monocytes compared to healthy donors,47 while TLR8 expression was up-regulated in patients with acute TB, as well as in differentiated macrophages upon infection with the M. bovis bacille Calmette-Guérin.48 Our results demonstrate that TLR2 and TLR4 are most strongly up-regulated in mDCs of patients coinfected with TB and HIV-1, which could lead to enhanced activation of cells by TB-derived products.
After HAART use, the responder patients (subjects with HAART in whom the viral load was significantly reduced) show an apparent “normalization” of TLR expression, despite low CD4 cell counts. Thus, the association between TLR expression and plasma HIV-1 viral load points toward the regulation of expression by viral products rather than large shifts in innate cell populations. Interestingly, in monocytes, expression of TLR2 was also positively correlated with the viral load, and as one might expect, it was lower in coinfected patients with HAART compared to patients without HAART. This is similar to previous reports in chronic HIV-1-infected patients with advanced disease (CD4+ T cell count less than 200 cells/ml).20,22 However, our data do not show a correlation between TLR expression and CD4+ T cell count (data not shown). Finally, the mechanisms by which HIV-1 infection and opportunistic pathogens increase TLR expression and possibly TLR functions need to be further examined.
Similarly, up-regulation of TLR2 and/or TLR4 during viral infections has been reported. For example, overexpression of TLR2 and TLR4 in monocytes of hepatitis B virus (HBV)-infected patients49 and dengue virus-infected patients was reported.50 However, down-modulation of TLR7 and TLR9 expression was reported in cells of HCV- or HBV-infected patients, which negatively correlated with viral load.51,52 Together, these results suggest an important role of TLRs during viral infection. On the one hand, they act to increase antiviral immunity, but they can also promote pathogenic events with altered TLR expression.
Remarkably, we have also observed increased expression of TLR4 in pDC (defined as Lin1− CD123high or BDCA-2+ CD123high PBMCs) at the protein level in HIV-1-infected patients. This is the first report of TLR2 and TLR4 expression (at the protein level) on pDCs (Supplementary Fig. S2), although previous reports showed very low amounts of TLR2 transcripts in BDCA-4-purified pDCs, as well as minimal CD80 induction after 48 h of lipopolysaccharide (LPS) stimulation.53,54 Moreover, we also observed this phenomenon of TLR2 and TLR4 expression on pDCs derived from patients with other viral infections (unpublished data).
Conclusions
In summary, we have identified marked changes in TLR expression as a consequence of OIs in HIV-1 coinfections. By increasing TLR expression in DCs, HIV-1 infection may progressively perturb the immune response and control processes that normally protect individuals from coinfection-associated diseases. We propose that increased TLR expression during HIV infection leads to increased innate sensing and responsiveness of the immune system that may also serve as a primary driver for immune activation and thus HIV-1 progression. Therapies that could act on these processes may therefore provide additional opportunities to interrupt HIV-1-associated disease progression, in combination with antiretroviral specific therapy, HAART.
Finally, it is important to consider that therapeutic modulation of TLR signaling could be a double-edged sword, because the inhibition of TLR function can help control the chronic inflammation state and decrease HIV-1 replication. On the other hand, it could also decrease the susceptibility to infections by other microorganisms. For this reason, an integral understanding of the mechanisms modulating TLR expression and function is necessary to guide the potential use of TLR-based therapies for HIV-1 infection, and even other infectious diseases.
Supplementary Material
Acknowledgments
We thank Silvia Torres for technical assistance. We also acknowledge the Université Jean Monnet and the George Washington University for supplying numerous reagents used in this study. The authors also acknowledge the patients and control individuals who participated in this study, the collaboration of the personnel of the institutions in which the patients were recruited, the infectious diseases physicians, and the flow cytometry staff. This study was supported by COLCIENCIAS (Colombia), Grant 111549326099. J.C.H. and S.U.I. elaborated the design of the project as well as the acquisition, analysis, and interpretation of the data. S.P. and E.L. critically reviewed the contents. All authors read and approved the final manuscript.
Author Disclosure Statement
No conflict of interest exist.
References
- 1.Levy JA. HIV pathogenesis: 25 years of progress and persistent challenges. AIDS. 2009;23:147–160. doi: 10.1097/QAD.0b013e3283217f9f. [DOI] [PubMed] [Google Scholar]
- 2.Almeida M. Cordero M. Almeida J. Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–271. [PubMed] [Google Scholar]
- 3.Finke JS. Shodell M. Shah K. Siegal FP. Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24:647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez JC. Montoya CJ. Urcuqui-Inchima S. [The role of toll-like receptors in viral infections: HIV-1 as a model.] Biomedica. 2007;27:280–293. [PubMed] [Google Scholar]
- 5.Pulendran B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol. 2005;174:2457–2465. doi: 10.4049/jimmunol.174.5.2457. [DOI] [PubMed] [Google Scholar]
- 6.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 7.Palsson-McDermott EM. O'Neill LA. Building an immune system from nine domains. Biochem Soc Trans. 2007;35:1437–1444. doi: 10.1042/BST0351437. [DOI] [PubMed] [Google Scholar]
- 8.Bowie AG. Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 10.Beignon AS. McKenna K. Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier A. Alter G. Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaisho T. Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med. 2003;3:373–385. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- 13.Mogensen TH. Paludan SR. Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J Mol Med. 2005;83:180–192. doi: 10.1007/s00109-004-0620-6. [DOI] [PubMed] [Google Scholar]
- 14.Equils O. Faure E. Thomas L. Bulut Y. Trushin S. Arditi M. Bacterial lipopolysaccharide activates HIV long terminal repeat through Toll-like receptor 4. J Immunol. 2001;166:2342–2347. doi: 10.4049/jimmunol.166.4.2342. [DOI] [PubMed] [Google Scholar]
- 15.Equils O. Schito ML. Karahashi H, et al. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: Implications of simultaneous activation of TLRs on HIV replication. J Immunol. 2003;170:5159–5164. doi: 10.4049/jimmunol.170.10.5159. [DOI] [PubMed] [Google Scholar]
- 16.Sundstrom JB. Little DM. Villinger F. Ellis JE. Ansari AA. Signaling through Toll-like receptors triggers HIV-1 replication in latently infected mast cells. J Immunol. 2004;172:4391–4401. doi: 10.4049/jimmunol.172.7.4391. [DOI] [PubMed] [Google Scholar]
- 17.Mares D. Simoes JA. Novak RM. Spear GT. TLR2-mediated cell stimulation in bacterial vaginosis. J Reprod Immunol. 2008;77:91–99. doi: 10.1016/j.jri.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg RS. Aggerholm A. Bertelsen LS. Ostergaard L. Paludan SR. Role of mitogen-activated protein kinases, nuclear factor-kappaB, and interferon regulatory factor 3 in Toll-like receptor 4-mediated activation of HIV long terminal repeat. Apmis. 2009;117:124–132. doi: 10.1111/j.1600-0463.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- 19.Heggelund L. Muller F. Lien E, et al. Increased expression of toll-like receptor 2 on monocytes in HIV infection: Possible roles in inflammation and viral replication. Clin Infect Dis. 2004;39:264–269. doi: 10.1086/421780. [DOI] [PubMed] [Google Scholar]
- 20.Lester RT. Yao XD. Ball TB, et al. Toll-like receptor expression and responsiveness are increased in viraemic HIV-1 infection. AIDS. 2008;22:685–694. doi: 10.1097/QAD.0b013e3282f4de35. [DOI] [PubMed] [Google Scholar]
- 21.Lester RT. Yao XD. Ball TB, et al. HIV-1 RNA dysregulates the natural TLR response to subclinical endotoxemia in Kenyan female sex-workers. PLoS One. 2009;4:e5644. doi: 10.1371/journal.pone.0005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller Sanders C. Cruse JM. Lewis RE. Toll-like receptor and chemokine receptor expression in HIV-infected T lymphocyte subsets. Exp Mol Pathol. 2010;88:26–31. doi: 10.1016/j.yexmp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Scagnolari C. Selvaggi C. Chiavuzzo L, et al. Expression levels of TLRs involved in viral recognition in PBMCs from HIV-1-infected patients failing antiretroviral therapy. Intervirology. 2009;52:107–114. doi: 10.1159/000218082. [DOI] [PubMed] [Google Scholar]
- 24.Mofenson LM. Brady MT. Danner SP, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: Recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58:1–166. [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders CM. Cruse JM. Lewis RE. Toll-like receptors, cytokines and HIV-1. Exp Mol Pathol. 2008;84:31–36. doi: 10.1016/j.yexmp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Aukrust P. Muller F. Lien E, et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: Persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179:74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 27.Chaisson RE. Gallant JE. Keruly JC. Moore RD. Impact of opportunistic disease on survival in patients with HIV infection. AIDS. 1998;12:29–33. doi: 10.1097/00002030-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Nordone SK. Ignacio GA. Su L, et al. Failure of TLR4-driven NF-kappa B activation to stimulate virus replication in models of HIV type 1 activation. AIDS Res Hum Retroviruses. 2007;23:1387–1395. doi: 10.1089/aid.2007.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawai T. Takeuchi O. Fujita T, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 30.Pimenta-Inada HK. Jucá LG. Cirne-Santos CC. Bou-Habib DC. The toll-like receptor 2 ligand zymosan inhibits HIV-1 replication in human primary cells. In the XVII International AIDS Conference; Mexico. 2008. [Google Scholar]
- 31.Thibault S. Imbeault M. Tardif MR. Tremblay MJ. TLR5 stimulation is sufficient to trigger reactivation of latent HIV-1 provirus in T lymphoid cells and activate virus gene expression in central memory CD4+ T cells. Virology. 2009;389:20–25. doi: 10.1016/j.virol.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Pine SO. McElrath MJ. Bochud PY. Polymorphisms in toll-like receptor 4 and toll-like receptor 9 influence viral load in a seroincident cohort of HIV-1-infected individuals. AIDS. 2009;23:2387–2395. doi: 10.1097/QAD.0b013e328330b489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bochud PY. Hersberger M. Taffe P, et al. Polymorphisms in Toll-like receptor 9 influence the clinical course of HIV-1 infection. AIDS. 2007;21:441–446. doi: 10.1097/QAD.0b013e328012b8ac. [DOI] [PubMed] [Google Scholar]
- 34.Soriano-Sarabia N. Vallejo A. Ramirez-Lorca R, et al. Influence of the Toll-like receptor 9 1635A/G polymorphism on the CD4 count, HIV viral load, and clinical progression. J Acquir Immune Defic Syndr. 2008;49:128–135. doi: 10.1097/QAI.0b013e318184fb41. [DOI] [PubMed] [Google Scholar]
- 35.Heggelund L. Flo T. Berg K, et al. Soluble toll-like receptor 2 in HIV infection: Association with disease progression. AIDS. 2004;18:2437–2439. [PubMed] [Google Scholar]
- 36.Pontillo A. Brandao LA. Guimaraes RL. Segat L. Athanasakis E. Crovella S. A 3'UTR SNP in NLRP3 gene is associated with susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr. 2010;54:236–240. doi: 10.1097/QAI.0b013e3181dd17d4. [DOI] [PubMed] [Google Scholar]
- 37.Bafica A. Scanga CA. Schito M. Chaussabel D. Sher A. Influence of coinfecting pathogens on HIV expression: Evidence for a role of Toll-like receptors. J Immunol. 2004;172:7229–7234. doi: 10.4049/jimmunol.172.12.7229. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu T. Kida Y. Kuwano K. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through Toll-like receptors. Immunology. 2004;113:121–129. doi: 10.1111/j.1365-2567.2004.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thibault S. Fromentin R. Tardif MR. Tremblay MJ. TLR2 and TLR4 triggering exerts contrasting effects with regard to HIV-1 infection of human dendritic cells and subsequent virus transfer to CD4+ T cells. Retrovirology. 2009;6:42. doi: 10.1186/1742-4690-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J. Li G. Bafica A, et al. Neisseria gonorrhoeae enhances infection of dendritic cells by HIV type 1. J Immunol. 2005;174:7995–8002. doi: 10.4049/jimmunol.174.12.7995. [DOI] [PubMed] [Google Scholar]
- 41.Brichacek B. Vanpouille C. Kiselyeva Y, et al. Contrasting roles for TLR ligands in HIV-1 pathogenesis. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suh HS. Zhao ML. Choi N. Belbin TJ. Brosnan CF. Lee SC. TLR3 and TLR4 are innate antiviral immune receptors in human microglia: Role of IRF3 in modulating antiviral and inflammatory response in the CNS. Virology. 2009;392:246–259. doi: 10.1016/j.virol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Equils O. Salehi KK. Cornataeanu R, et al. Repeated lipopolysaccharide (LPS) exposure inhibits HIV replication in primary human macrophages. Microbes Infect. 2006;8:2469–2476. doi: 10.1016/j.micinf.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z. Fu J. Zhao Q, et al. Differential restoration of myeloid and plasmacytoid dendritic cells in HIV-1-infected children after treatment with highly active antiretroviral therapy. J Immunol. 2006;176:5644–5651. doi: 10.4049/jimmunol.176.9.5644. [DOI] [PubMed] [Google Scholar]
- 45.Groot F. van Capel TM. Kapsenberg ML. Berkhout B. de Jong EC. Opposing roles of blood myeloid and plasmacytoid dendritic cells in HIV-1 infection of T cells: Transmission facilitation versus replication inhibition. Blood. 2006;108:1957–1964. doi: 10.1182/blood-2006-03-010918. [DOI] [PubMed] [Google Scholar]
- 46.Stary G. Klein I. Kohlhofer S, et al. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3723–3724. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez MD. Garcia Y. Montes C, et al. Functional and phenotypic changes in monocytes from patients with tuberculosis are reversed with treatment. Microbes Infect. 2006;8:2492–2500. doi: 10.1016/j.micinf.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Davila S. Hibberd ML. Hari Dass R, et al. Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS Genet. 2008;4:e1000218. doi: 10.1371/journal.pgen.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y. Lian JQ. Huang CX, et al. Overexpression of Toll-like receptor 2/4 on monocytes modulates the activities of CD4(+)CD25(+) regulatory T cells in chronic hepatitis B virus infection. Virology. 2010;397:34–42. doi: 10.1016/j.virol.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Azeredo EL. Neves-Souza PC. Alvarenga AR, et al. Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leucocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever. Immunology. 2010;130:202–216. doi: 10.1111/j.1365-2567.2009.03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang S. Kodys K. Szabo G. Impaired expression and function of toll-like receptor 7 in hepatitis C virus infection in human hepatoma cells. Hepatology. 2010;51:35–42. doi: 10.1002/hep.23256. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J. Huang Y. Tian D. Xu D. Chen M. Wu H. Expression of toll-like receptor 9 in peripheral blood mononuclear cells from patients with different hepatitis B and C viral loads. J Huazhong Univ Sci Technolog Med Sci. 2009;29:313–317. doi: 10.1007/s11596-009-0310-2. [DOI] [PubMed] [Google Scholar]
- 53.Krug A. Towarowski A. Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 54.Hornung V. Rothenfusser S. Britsch S, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.