Abstract
Toll-like receptors (TLRs) play a critical role in innate immunity against pathogens. Their stimulation induces the activation of NF-κB, an important inducer of HIV-1 replication. In recent years, an increasing number of studies using several cells types from HIV-infected patients indicate that TLRs play a key role in regulating the expression of proinflammatory cytokines and viral pathogenesis. In the present study, the effect of HIV-1 stimulation of monocyte-derived macrophage (MDM) and peripheral blood mononuclear cell (PBMC) subpopulations from healthy donors on the expression and functions of TLR2 and TLR4 was examined. In addition, and to complete the in vitro study, the expression pattern of TLR2 and TLR4 in 49 HIV-1-infected patients, classified according to viral load and the use of HAART, was determined and compared with 25 healthy subjects. An increase of TLR expression and production of proinflammatory cytokines were observed in MDMs and PBMCs infected with HIV-1 in vitro and in response to TLR stimulation, compared to the mock. In addition, an association between TLR expression and up-regulation of CD80 in plasmacytoid dendritic cells (pDCs) was observed. The ex vivo analysis indicated increased expression of TLR2 and TLR4 in myeloid dendritic cells (mDCs), but only of TLR2 in monocytes obtained from HIV-1-infected patients, compared to healthy subjects. Remarkably, the expression was higher in cells from patients who do not use HAART. In monocytes, there was a positive correlation between both TLRs and viral load, but not CD4+ T cell numbers. Together, our in vitro and ex vivo results suggest that TLR expression and function can be up-regulated in response to HIV-1 infection and could affect the inflammatory response. We propose that modulation of TLRs represents a mechanism to promote HIV-1 replication or AIDS progression in HIV-1-infected patients.
Introduction
Widespread dysregulations of the immune system have been described during HIV-1 infection. Among these, CD4+ T cell depletion together with increased levels of viremia and opportunistic infections are known to promote the progression to AIDS.1 HIV-infected individuals can also suffer chronic hyperactivation of the immune system and this persistent immune challenge with high levels of proinflammatory cytokines and increase in immune cell activation is thought to contribute to viral pathogenesis.1 Moreover, advanced HIV-1 infection is known to be associated with reduced innate response to new infectious challengers; indeed, HIV-1 infection can lead to markedly reduced numbers and altered functions of innate immune cells such as plasmacytoid dendritic cells (pDCs) and myeloid dendritic cells (mDCs).2,3 Given the central role of DCs in linking innate and adaptive immunity, abnormalities or dysregulation of their function could help to explain the altered responses observed in HIV-1-infected subjects. DCs are activated through innate signaling receptors such as the toll-like receptors (TLRs) and by other members of pattern-recognition receptors (PRRs).4,5 PRR activation by various stimulatory ligands induces inflammatory cytokine release, expression of costimulatory molecules, and migration of DCs into lymphoid tissues, culminating in the establishment of adaptive immunity.6,7
TLRs recognize several conserved microbial structures, including lipopolysaccharide (LPS), proteins, and nucleic acids, to activate a common signaling pathway responsible for inducing the expression of transcription factors, such as the activator protein 1 (AP-1) and the nuclear factor-κB (NF-κB),8,9 that are involved in regulating the expression of proinflammatory cytokines and interferon (IFN). Subsequently, NF-κB can activate the HIV-1 genome by binding to the viral long terminal repeats (LTRs) in the provirus, even in models of HIV-1 latency.10–15 TLRs are expressed in HIV-1 reservoir cells such as CD4+ T cells, macrophages, and DCs.16–19 Various reports indicated that HIV-derived RNA can activate pDCs via TLR7 and TLR8.4,20–22 Others studies have evaluated the ability of TLRs to modulate HIV-1 replication, as well as TLR modulation during HIV-1 infection. Indeed, in peripheral blood mononuclear cells (PBMCs) derived from chronic untreated HIV-1-infected patients, an increase in TLR6, TLR7, and TLR8 mRNA was reported; in contrast, in HIV-1-infected patients with advanced disease, an mRNA increase was observed for TLR2, TLR3, and TLR4.23 Using PBMCs from HIV-1-infected patients, Heggelund et al. (2004) also reported the up-regulation of several chemokines through TLR2 stimulation, compared to PBMCs derived from healthy donors.24 Additionally, it has also been reported that coculture of DCs and CD4+ T cells increases HIV-1 transmission through TLR2 stimulation.25
In HIV-1-infected patients, several alterations of TLR expression have been documented23,26–29 raising the possibility that during chronic infection proinflammatory signals can further augment HIV-1 genome expression. The expression and functions of TLRs have been extensively studied in cells from HIV-1-infected patients. Here, we report than MDM and PBMC subpopulations from healthy donors in vitro exposed to HIV-1 increase the levels of TLRs, the proinflammatory cytokines, and CD80 expression, in response to either HIV-1 infection or TLR2 and TLR4 stimulation. In addition, in a cohort of HIV-1-infected patients, increased expression of TLR2 and TLR4 was observed in mDCs, but only of TLR2 in monocytes. Together, our results indicate that in vitro and ex vivo HIV-1 infection can modify TLR expression, altering the in vitro expression of proinflammatory cytokines. These results suggest that HIV-1 could perpetuate the dysfunction and activation of the innate response, and might play a role in HIV-1 disease progression.
Materials and Methods
Patient cohort
Forty-nine HIV-1-infected patients were classified according to viral load (higher than or less than 400 copies of viral RNA/ml blood) and use of highly active antiretroviral treatment (HAART) (naive treatment or more than 6 continuous months of treatment, at the time of sample acquisition)30 (Table 1). Patients with cancer, patients with low hemoglobin values (less than 8 g/dl), and pregnant women were excluded. Twenty-five age-matched HIV-1-uninfected individuals without illness in the past 3 months were included in the study as controls; they were volunteers from the general population. According to ethical guidelines, signed informed consent was obtained from all patients and controls, before enrollment into the study. All biomedical investigations were approved by the bioethics committee of the Universidad de Antioquia and were conducted according to the principles of the Declaration of Helsinki.
Table 1.
Demographic Features of HIV-1-Infected Individuals and Controls
| |
HIV-1-infected individuals |
|
||
|---|---|---|---|---|
| Item | HAARTa VL<400 copies/ml n=26 | HAARTa VL>400 copies/ml n =12 | Without HAART Without HAART n =11 | Healthy controls n =25 |
| Age: median (range) | 36 (17–61) | 38 (23–58) | 28 (20–52) | 25 (19–47) |
| Male:Female | 13:13 | 8:4 | 6:5 | 10:15 |
| Viral load in RNA copies/ml | 41,500 | 12,850 | ||
| plasma: median (range) | Undetectable | (500–750,000) | (5,140–220,000) | N/A |
| CD4+ T cells/μl peripheral | ||||
| bloodb: median (range) | 387 (14–950) | 237 (31–950) | 481 (73–875) | 646 (460–1,143) |
Patients in HAART treatment were using combinations of nucleoside reverse transcriptase inhibitors (abacavir, lamivudine, didanosine, stavudine, and zidovudine), nonnucleoside reverse transcriptase inhibitors (efavirenz and nevirapine), and protease inhibitors (lopinavir, fosamprenavir, amprenavir, nelfinavir, and saquinavir) for more than 6 continuous months.
CD4+ T cell counts under 200 cells/μl, were receiving fluconazol, acliclovir, and TMS as prophylactic drugs.
HAART, highly active antiretroviral therapy; VL, viral load in copies of viral RNA/ml of plasma; N/A, not applicable.
HIV viral loads and CD4+ T cells counts
HIV-1 viral loads were assessed by certified commercial laboratories in total peripheral blood samples collected by venipuncture into vacutainers (BD Biosciences, San Jose, CA) containing ethylenediaminetetraacetic acid (EDTA). The CD4+ T cell counts were performed using the standard protocols and flow cytometry (FACS BD Biosciences, San Jose, CA).
Isolation and culture of PBMCs and primary human monocyte-derived macrophages (MDMs)
Fresh blood was collected from healthy donors by venipuncture in tubes containing preservative-free heparin. Fresh PBMCs were isolated by density gradient centrifugation using Ficoll/Histopaque (Sigma-Aldrich Chemical Co., St. Louis, MO). PBMCs were washed three times with phosphate-buffered saline (PBS) 1× and resuspended in RPMI 1640 (Invitrogen, San Diego, CA). Aliquots of fresh PBMCs containing approximately 3×106 cells were mixed directly in 1 ml Trizol reagent (Invitrogen, San Diego, CA) and frozen before RNA extraction. For ex vivo TLR expression assays, fresh PBMCs were used immediately after purification.
To obtain the MDMs, elutriated monocytes were cultured for 7 days in RPMI 1640, supplemented with 2 mM glutamine and 100 ng/ml human recombinant macrophage colony-stimulating factor (R&D Systems, Minneapolis, MN) at 37°C in the presence of 5% CO2 as previously described.31 After 7 days of culture, the resulting MDM monolayers were examined by microscopy: all the adherent cells had the morphology of MDMs. To assess TLR expression, the MDMs were washed and detached from the plastic plate using a cell dissociation buffer (an enzyme-free solution of EDTA plus PBS without Ca2+ and Mg2+) for staining. To increase the chance of detecting an at least 2-fold difference between virus- and mock-infected cultures, three independent experiments using MDMs from six healthy donors were used for HIV-1 infection in vitro and the samples treated with the TLR2 and TLR4 agonists. TLR2, TLR4, and cytokine expression were then determined.
Preparation of virus stocks
Fully infectious HIV-1 particles were produced following the protocol of Swingler et al. (2007).32 Briefly, transient calcium phosphate cotransfection of human 293T cells (2×106 cells seeded in a 75-cm2 flask) with 25 μg of pNL4-3.GFP (X4-tropic virus, in which the GFP reporter gene replaced Nef), or with 20 μg of pNL4-3Δenv.GFP (in which the envelope gene was deleted and the GFP reporter gene replaced Nef), in the presence of 15 μg of a VSV-G expression vector, was used to obtain the pseudotyped viruses (HIV-1NL4-3.GFP VSV-G and HIV-1NL4-3Δenv.GFP VSV-G), which were used to obtain an efficient infection of the different cell models. Virus-containing supernatants were harvested 60 h posttransfection, filtered through a 0.22-μm pore membrane, and stored at −80°C until used. The virus content was measured by the extracellular reverse transcriptase (RT) activity radioassay as described previously.33 In addition, a high viral titer of HIV-1H9-HTLV-IIIcc was collected from H9-HTLV-IIIcc chronically HIV-1-infected cells (NIH AIDS Research and Reference Reagent Program) within 7 days of culture in RPMI. The supernatant of 293T cells empty vector-transfected or the supernatants of the H9 cell line were used as mock. Virus-containing supernatants were harvested, filtered through a 0.22-μm pore membrane, and normalized for virion content by a sandwich ELISA assay specific for the major viral core p24 protein, using the QuickTiter Lentivirus Titer Kit (Cell Biolabs, San Diego, CA) and following the manufacturer's instructions. Values of p24 (pg/ml) or reverse transcriptase units (RTU) were calculated on the basis of regression analysis of standards prepared from samples of known concentrations. The infectious capacity of the viral stocks was determined by Western blot, to detect Gag-derived proteins.
Monoclonal antibodies
The following monoclonal antibodies were obtained from BD Biosciences (BD Biosciences, San Jose, CA): Lin1 FITC (CD3, CD14, CD16, CD19, CD20, and CD56 cocktail), CD123 PE-Cy5, CD11c PE-Cy5, CD80 PE, and CD86 PE. TLR2 (clone TL2.1), and TLR4 (clone HTA125) were phycoerythrin conjugates (eBiosciences, San Diego, CA). Anti-BDCA-2 FITC and FcR blocking reagent were from Miltenyi Biotec, Auburn, CA. Conjugated isotype-control antibodies were used as controls.
Flow cytometry analysis
Flow cytometry was used to evaluate the effect of HIV-1 infection on TLR expression in monocytes, pDCs, mDCs, and MDMs. For this purpose, freshly isolated PBMCs were resuspended in PBS containing 0.5% bovine serum albumin (BSA) and 0.1% sodium azide for 1×107 cells/ml. After addition of the Fc-R blocking reagent, PBMCs were surface stained with the appropriate antibodies or controls for 25 min, fixed with 2% formaldehyde, and stored at 4°C until analysis. All samples were evaluated within 2–4 h of staining using a FACSCan flow cytometer (BD Biosciences, San Jose, CA). Logical gating was used to identify monocyte (CD14+), pDCs (BDCA-2+/CD123high), and mDCs (Lin1-/CD11chigh) populations.34 For DC evaluation, at least 200,000 gated events were acquired and at least 150,000 gated events for monocytes. The acquired events were analyzed using CellQuest software. TLR expression is expressed as the mean fluorescent intensity (MFI) of the overall cell subpopulation after subtraction of the isotype control.
Quantitative reverse-transcriptase real-time PCR for TLRs mRNAs
Interleukin (IL)-6 and tumor necrosis factor (TNF)-α mRNA in PBMCs infected with HIV-1 and treated with TLR agonists, or TLR2, TLR4, and TNF-α mRNAs in PBMCs from HIV-1-infected patients was determined by real time RT-PCR. For mRNA analysis, 1 μg of total RNA was treated with 2 units RNase-free DNase I (Fermentas, Glen Burnie, MD) for 1 h at 37°C to remove traces of genomic DNA. The first strand cDNA was synthesized using the Superscript III kit (Invitrogen, San Diego, CA) according to the manufacturer's instructions. Briefly, total RNA was incubated for 60 min at 42°C with RT (reverse transcriptase) and random hexamer primers, and then treated with RNase H for 20 min at 37°C. A none-RT control was used for each set of reactions. Specific primers were as previously reported by Kadowaki et al. (2001),35 with designed intron spanning, avoiding coamplification of genomic DNA in the real time RT-PCR step. The sequences were: TLR2 (F: GGCCAGCAAATTACCTGTGTG, R: CCAGGTAGGTCTTGGTGTTCA), TLR4 (F: CTGCAATGGATCAAGGACCA, R: TCCCACTCCAGGTAAGTGTT), TNF-α (F: GGCTCCAGGCGGTGCTTGTTC, R: AGACGGCGATGCGGCTGATG), IL-6 (F: ATTCGGTACATCCTCGAC, R: GGGGTGGTTATTGCATC), and β-Actin (F: ATCTGGCACCACACCTTCTACAATGAGCTGCG) R: CGTCATACTCCTGCTTGCTGATCCACATCTGC). β-actin was used as a housekeeping gene to account for variability in the amount of RNA transcribed and in the RT reaction itself. Real time PCR cycling was performed in duplicate (5 min at 95°C, followed by 38 cycles of 20 s at 95°C, 30 s at 55°C, and 30 s at 72°C) in 25 μl containing 1 μl of undiluted cDNA, 500 nmol of each primer, and 1×SYBR green PCR master mix (Qiagen, Valencia, CA). For comparative purposes, estimation of the relative amount of mRNA in the samples was determined according to the following formula: (1+X)–ΔCt, where X is the efficiency of the reaction and ΔCt is the difference between the Ct (the cycle at which the detected signal is significantly above the background signal) of the TLR mRNA of interest and the Ct of the endogenous control gene, β-actin. For all the experiments, the efficiency of the reactions was between 80% and 88%, and, thus, the formula was used with an average efficiency of 84% (1.84–ΔCt). The specificity of amplification was validated by observing a single peak at the expected Tm on the analysis of the melting curve.
In vitro HIV-1 stimulation study
PBMCs and MDMs were cultured at 2×106 cells/ml in 6-well polystyrene tissue culture plates at 37°C and 5% CO2, using complete RPMI 1640 medium (BioWhittaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal bovine serum, 50 U/ml penicillin/streptomycin (Sigma-Aldrich Chemical Co., St. Louis, MO), and 2 mM l-glutamine (Sigma-Aldrich Chemical Co., St. Louis, MO). The PBMCs and MDMs were then exposed to HIV-1 (50–6000 pg p24 HIV-1H9-HTLV-IIIcc, 300.000 RTU HIV-1NL4-3.GFP VSV-G, or 300.000 RTU HIV-1NL4-3Δenv.GFP VSV-G), or mock infected during 2 h or 18 h, for expression analysis, and during 2 h for cytokines response. The cells (PBMCs and MDM) were then washed and cultured a further 18 h with or without the TLR2 and TLR4 agonists: 10 nM palmitoyl-2-cysteine-serine-lysine-4-Pam2CSK4- (synthetic lipoprotein for TLR2/TLR6 heterodimer stimulation) or 10 nM palmitoyl-3-cysteine-serine-lysine-4-Pam3CSK4- (synthetic lipoprotein for TLR2/TLR1 heterodimer stimulation) and 10 ng/ml ultrapure LPS from Escherichia coli 0127:B8 (Invivogen, San Diego, CA), respectively. TLR2 and TLR4 expressions were evaluated by flow cytometry and RT-PCR. The supernatants were harvested after 18 h of culture and assayed for proinflammatory cytokines (TNF-α, IL-6, IL-8, and IL-1β) by ELISA and Cytometric Beads Array (CBA; BD Biosciences, San Jose, CA).
ELISA and CBA
To determine whether HIV-1 infection induces secretion of proinflammatory cytokines through TLR stimulation, an ELISA assay was used. Briefly, ELISA plates (Nunc, Rochester, NY) were coated overnight at 4°C with 50 μl/well of antihuman cytokine monoclonal antibodies at 1:250 dilution in bicarbonate coating buffer (0.1 M NaHCO3, pH 8.2). The wells were washed and blocked using 200 μl/well of PBS containing 10% heat-inactivated fetal bovine serum (PBS/10% FBS) for 2 h at room temperature. Serial dilutions of standard recombinant cytokines and culture supernatants were added in each well, and plates were incubated 2 h at room temperature. Biotinylated mouse antihuman cytokine antibody (BD Biosciences) diluted in PBS/10% FBS was added and incubation carried out at room temperature for 30 min. Avidin peroxidase in PBS/10% FBS was added to the wells and the mixtures incubated for 30 min at room temperature. The substrate [1:1 mixture of 3,3′,5,5′-tetramethylbenzidine (TMB) and H2O2] was added for 15 min and the plate was read at 450 nm. For CBA (BD Biosciences), culture supernatants were incubated for 3 h at room temperature with mixed capture beads and the human inflammation PE (phycoerythrin) detection reagent. After two washes, the acquisition on the flow cytometer was performed.
Additional assays were carried out to quantify the IL-6 and TNF-α mRNA levels by RT-PCR in stimulated cells, and to establish the expression of costimulatory molecules on cell membranes by flow cytometry. In these cases, the results are expressed as the fold induction calculated from the agonist mean value divided by the medium alone mean value. A 2-fold or greater increase was considered biologically significant.
Isolated pDCs and mDCs were not studied because HIV-1-infected individuals have much lower numbers of circulating pDCs and mDCs than uninfected individuals and too much blood would be required to obtain an adequate number of purified cells from these infected individuals.
Statistical analysis
Data were plotted and analyzed using the Prism 5.0 software (Graph Pad Software, CA). All the results of the in vitro assays shown represent at least three independent experiments. Unpaired two-tailed student t-tests (Mann–Whitney U-tests) and the ANOVA test were used to assess the statistical significance of TLR expression or function in the different groups, when more than three independent data were available. Spearman's test for matched correlations was performed. Values of *p<0.05 were considered significant, and values of **p<0.01 were considered highly significant.
Results
TLR2 and TLR4 expression is differentially altered in PBMCs during early or late exposure to HIV-1 in vitro
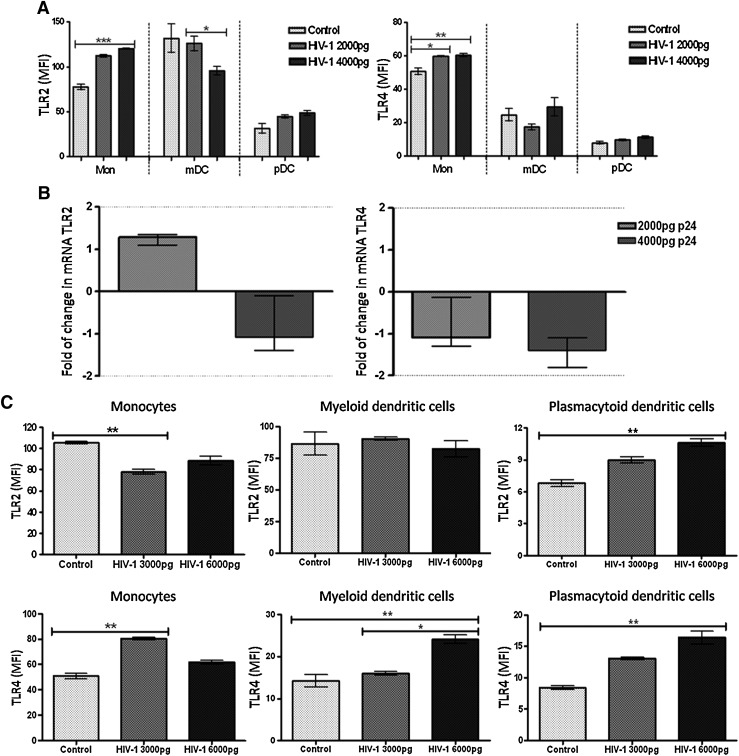
Assays were performed to evaluate the modulation of TLR2 and TLR4 expression in PBMCs exposed to HIV-1 in vitro for short or long times. First, 1×106 PBMCs from four healthy donors were incubated 2 h, with small doses (100 to 1000 pg p24) of HIV-1H9 HTLV-IIIcc, and then cultured 18 h more. The TLR2 and TLR4 expression level in monocytes, mDCs, and pDCs was measured by flow cytometry, and the mRNA level for both TLRs in PBMCs was measured by real time RT-PCR. Under these conditions, no significant differences were observed, compared to the mock (data no shown). When the viral doses were increased to 4000 pg p24 HIV-1H9 HTLV-IIIcc, up-regulation of TLR2 and TLR4 expression was observed in monocytes (Fig. 1A), but in mDCs there was a significant decrease in TLR2 expression as the quantity of virus was increased (Fig. 1A). However, when the PBMCs were incubated for 2 h with the virus, washed, and then incubated for 18 h, the expression of TLR2 and TLR4 in pDCs or of TLR4 in mDCs was not affected (Fig. 1A). In comparison to previous results at the protein levels, no changes were observed at the mRNA level for TLR2 and TLR4 (Fig. 1B).
FIG. 1.
Modulation of TLR2 and TLR4 expression in monocytes and in dendritic cells (DCs) exposed to HIV-1 in vitro. Peripheral blood mononuclear cells (PBMCs) (1×106) were exposed in vitro to HIV-1H9 HTLV-IIIcc (2000 and 4000 pg p24 HIV-1) for 2 h, and then incubated for 18 h at 37°C and 5% CO2 (A and B), or exposed in vitro to HIV-1H9 HTLV-IIIcc (3000 and 6000 pg p24 HIV-1) for 2 h, and then incubated 1 h at 37°C and 5% CO2 (C). TLR2 and TLR4 expression was measured at the protein level by flow cytometry (A and C) and at the mRNA level by real time RT-PCR (B). For flow cytometry analyses, mononuclear cells were gated according to physical characteristics, excluding dead cells. Monocytes were then gated as CD14+ cells, myeloid dendritic cells (mDCs) as Lin 1– CD11chigh, and plasmacytoid dendritic cells (pDCs) as Lin 1– CD123high. Each specific subpopulation was plotted as a histogram to show the expression of TLR2 and TLR4 by flow cytometry. The data are presented as overall mean fluorescence intensity (MFI) for toll-like receptors (TLRs) in each cell subpopulation, after subtraction of isotype staining background. Representative results of three independent experiments are shown as median and range. In the real time RT-PCR results, the dotted line represents 2-fold induction, compared to the control. Comparisons were performed using the Kruskal–Wallis ANOVA tests and Dunn's posttests. The level of significance was *p<0.05, **p<0.01, and ***p<0.001.
Our results suggest that late in the infection cycle (18 h after stimulus) the virus might down-regulate the TLR2 and TLR4 expression and the cell activation through these receptors. Based on these results, it seemed interesting to determine what happens with the expression of TLR2 and TLR4 during the first 3 h of the infection process. To do that, and based on previous reports on the kinetics of TLR expression,36–38 the experimental strategy was modified. The PBMCs (1×106) were stimulated for 2 h with HIV-1H9 HTLV-IIIcc (3000 and 6000 pg p24) and incubated for 1 h instead of 18 h. Interestingly, if the TLR expression analysis was performed in the first 3 h, a significant decrease in TLR2 expression in monocytes stimulated with 3000 pg p24 was observed, compared to mock-infected cells (Fig. 1C), but with higher doses, TLR2 expression slightly recovered. In contrast, a significant increase was observed for TLR2 in pDCs stimulated with 6000 pg p24, compared to mock-infected cells (Fig. 1C). In mDCs, stimulation with HIV-1 had no effect on TLR2 expression at 3 h. Regarding TLR4 expression, up-regulation was observed in monocytes when the cells were stimulated with 3000 pg p24 of HIV-1; interestingly, in mDCs and pDCs a dose-dependent up-regulation was observed compared to the mock (Fig. 1C). Remarkably, in mDCs increased expression of TLR4 was detected only at higher doses of stimulus (6000 pg p24).
In summary, these results show than PBMCs challenged with HIV-1H9 HTLV-IIIcc, in vitro, alter TLR2 and TLR4 expression in monocytes, pDCs, and mDCs. Together, our results suggest that during the first steps of HIV-1 infection (early infection), the antigen-presenting cells are activated mainly through TLR4, but TLR2 could be playing a very important role in monocytes and pDCs. In contrast, viral entry and subsequent production of viral proteins (late infection) could promote TLR2 activation in monocytes and mDCs, and expression of TLR4 in monocytes but not in DCs. To better understand this behavior, and because the cell line H9HTLV-IIIcc is a model of chronic HIV-1 infection, it seemed important to compare the level of expression of TLR2 and TLR4 in these cells and in H9 uninfected control cells. An increase in TLR2 and TLR4 expression in H9HTLV-IIIcc was observed compared to expression in control cells (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertonline.com/aid). In addition, an increase in TNF-α mRNA was observed in the H9-infected cells, suggesting that these cells are constantly producing proinflammatory cytokines (Supplementary Fig. S1B).
TLR2 and TLR4 activation in HIV-1-infected cells up-regulates proinflammatory cytokine release
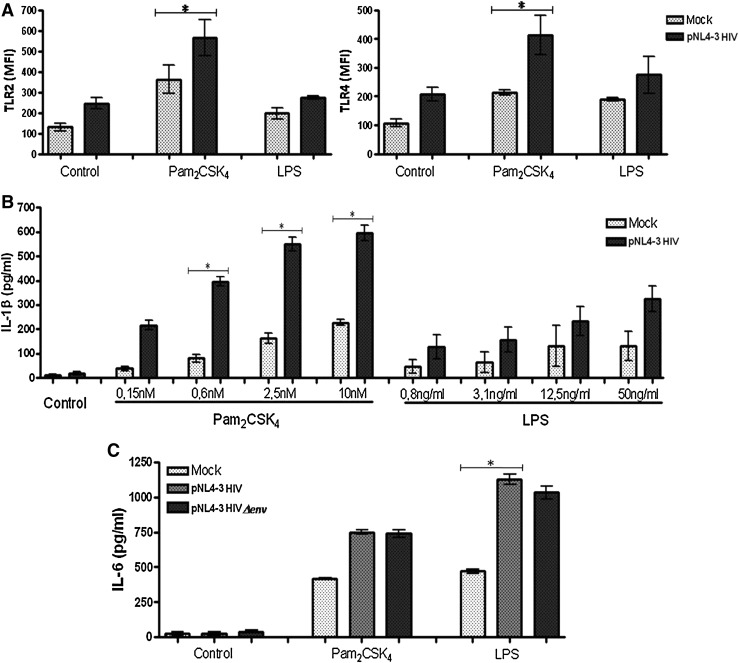
It has been reported that HIV-1 alters the pattern of expression and function of TLRs in cells from HIV-1-infected patients. To examine the expression and function of TLR2 and TLR4 in human macrophages from healthy donors and stimulated with HIV-1 in vitro, MDMs infected with HIV-1NL4-3.GFP VSV-G, prior TLR2 and TLR4 agonist exposures were obtained. Sixty percent of the MDMs were infected (data not shown) and HIV-1 replication (demonstrated by flow cytometry and fluorescent microscopy) was increased when the MDMs were treated with Pam2CSK4 and LPS (data not shown). Similar to viral replication, TLR expression in MDMs infected with HIV-1NL4-3.GFP VSV-G and stimulated with Pam2CSK4 or LPS was also increased. HIV-1 infection or TLR2 and TLR4 stimulation individually only slightly increased TLR2 and TLR4 expression on MDMs, but TLR stimulation on HIV-1-infected cells shows augmented expression level of TLR2 and TLR4, compared to the mock (Fig. 2A). Interestingly, when the MDMs were stimulated with the TLR2 agonist and infected with HIV-1NL4-3.GFP VSV-G, an increases in TLR4 expression was also observed, but not the inverse (TLR4 stimulation only slightly alters TLR2 expression) (Fig. 2A). In conclusion, our results show that the expression levels of TLR2 and TLR4 are increased in MDMs infected with HIV-1NL4-3.GFP VSV-G and in the presence of a TLR2 agonist.
FIG. 2.
Increased TLR2 and TLR4 expression and function in monocyte-derived macrophages (MDMs) HIV-1 infected in vitro. (A) MDMs were gated according to physical characteristics, excluding dead cells, and plotted as histograms to show the expression of TLR2 and TLR4 by flow cytometry. The data are presented as overall mean fluorescence intensity (MFI), after subtraction of isotype staining background. The MDMs were infected in vitro with HIV-1NL4-3.GFP VSV-G for 2 h before stimulation with TLR2 and TLR4 agonists (Pam2CSK4 and LPS, respectively) and incubated for 18 h at 37°C and 5% CO2. Comparisons were performed using unpaired two-tailed Student's t-tests (Mann–Whitney U-tests). The level of significance was *p<0.05. Interleukin (IL)-1β (B) release was quantified by ELISA in the supernatants of MDM cultures infected with HIV-1 for 2 h before stimulation with increasing doses of Pam2CSK4 (0.15 nM–10 nM) and lipopolysaccharide (LPS) (0.8–50 ng/ml) and, finally, incubated for 18 h at 37°C and 5% CO2. Comparisons were performed using unpaired two-tailed Student's t-tests (Mann–Whitney U-tests). The level of significance was *p<0.05. In (C) MDMs were infected with HIV-1NL4-3.GFP VSV-S or HIV-1NL4-3(env.GFP VSV-S for 2 h before stimulation with Pam2CSK4 or LPS and incubated for 20 h at 37°C and 5% CO2. IL-6 release was quantified by ELISA in the supernatants. Comparisons were performed using the Kruskal–Wallis ANOVA tests and Dunn's posttests. The level of significance was *p<0.05.
It now seemed interesting to determine whether the alteration in TLR2 and TLR4 expression by HIV-1 (Fig. 2A) has an effect on TLR function in MDMs. Experiments designed to measure the release of proinflammatory cytokines in supernatants and the expression of costimulatory molecules were undertaken in HIV-1-infected MDMs in vitro and stimulated with TLR2 and TLR4 agonists (Pam2CSK4 and LPS, respectively). A dose-dependent increase in IL-1β was observed (Fig. 2B); although the release of this cytokine was higher in response to the TLR2 agonist. IL-6 production was also increased after HIV-1 infection and in response to TLR2 and TLR4 agonists, compared to the mock (Fig. 2C). Higher amounts of IL-6 were observed when the MDMs were stimulated by TLR4. Interestingly, the expression level of IL-6 was independent of the HIV-1 source used, such as in the presence of HIV-1NL4-3.GFP VSV-G or without the HIV-1 envelope glycoproteins (HIV-1NL4-3Δenv.GFP VSV-G).
PBMCs and MDMs behave similarly toward HIV-1 infection
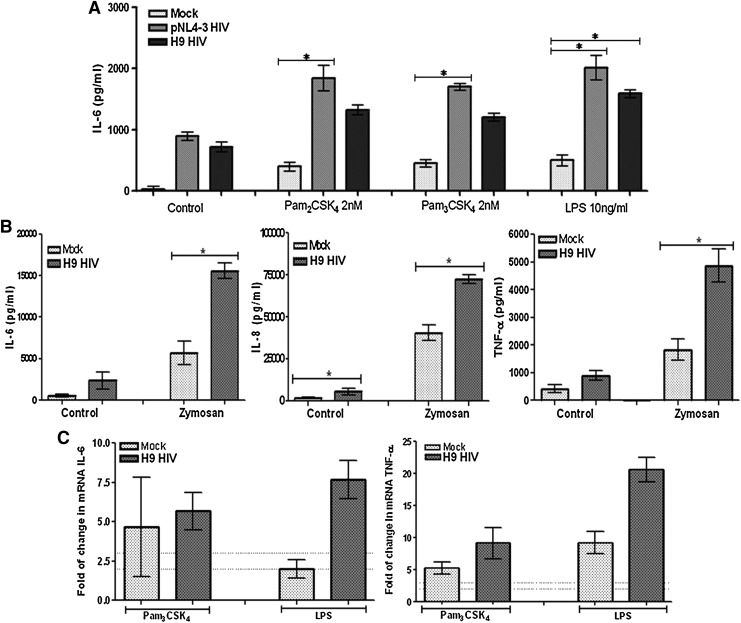
To establish whether PBMCs respond similarly to HIV-1 infection as MDMs do, PBMCs were in vitro exposed to HIV-1H9 HTLV-IIIcc or HIV-1NL4-3.GFP VSV-G and then stimulated with TLR2 and TLR4 agonists. As in previous results described for MDMs, increased IL-6 release was observed in HIV-1-exposed PBMCs, in response to TLR2 and TLR4 ligands (Fig. 3A). IL-6 release was similar whether TLR2 was activated with Pam2CSK4 or Pam3CSK4 (which stimulates the heterodimers TLR2/TLR6 or TLR2/TLR1, respectively). A high release of IL-6, IL-8, and TNF-α was also observed when the PBMCs were infected with HIV-1H9 HTLV-IIIcc and treated with zymosan (another TLR2 agonist) (Fig. 3B). Together, these results suggest that PBMCs and MDMs both are activated in response to TLR stimulation, leading to proinflammatory cytokine release, which is higher in the context of HIV-1 infection.
FIG. 3.
Increased cytokines expression in PBMCs exposed to HIV-1 in vitro and treated with TLR agonists. (A) IL-6 production was quantified by ELISA in the supernatants of PBMC cultures exposed in vitro to HIV-1NL4-3.GFP VSV-G or HIV-1H9 HTLV-IIIcc for 2 h before stimulation with TLR2 agonists (10 nM Pam2CSK4 or 10 nM Pam3CSK4) and the TLR4 agonist (10 ng/ml LPS), and then incubated for 18 h at 37°C and 5% CO2. Comparisons were performed using the Kruskal–Wallis ANOVA tests and Dunn's posttests. The level of significance was *p<0.05. (B) Tumor necrosis factor (TNF)-α, IL-8, and IL-6 production was quantified by Cytometric Beads Array (CBA; BD Biosciences) in the supernatants of PBMC cultures exposed in vitro to HIV-1H9 HTLV-IIIcc for 2 h before stimulation with the TLR2 agonist zymosan (10 μg/ml), for 18 h at 37°C and 5% CO2. Median and range are shown. Comparisons were performed using unpaired two-tailed Student's t-tests (Mann–Whitney U-tests). The level of significance was *p<0.05. (C) The mRNAs of IL-6 and TNF-α were quantified by real time RT-PCR in total RNA extracted from PBMC cultures exposed in vitro to HIV-1H9 HTLV-IIIcc for 2 h before stimulation with 10 nM Pam3CSK4 and 10 ng/ml LPS, for 2 h at 37°C and 5% CO2. Representative results of three independent experiments shown as median and range are presented, normalized to the control (mock-infected cells, not treated with PAMPs). Dotted line represents 2- and 3-fold induction.
To determine if a correlation exists between the production of IL-6 and TNF-α, and the rate of transcription, the mRNAs of both cytokines were quantified by real time RT-PCR. The level of the mRNAs of IL-6 and TNF-α was higher in PBMCs infected with HIV-1 and treated with LPS compared to the mock (Fig. 3C). When the cells were stimulated with the TLR2 agonists, just a slight increase in IL-6 and TNF-α mRNA levels was observed (Fig. 3C). These results are in agreement with the expression of TLR2 and TLR4 observed in PBMCs infected with increasing doses of HIV-1 (higher TLR expression and higher TLR responses) (Fig. 1).
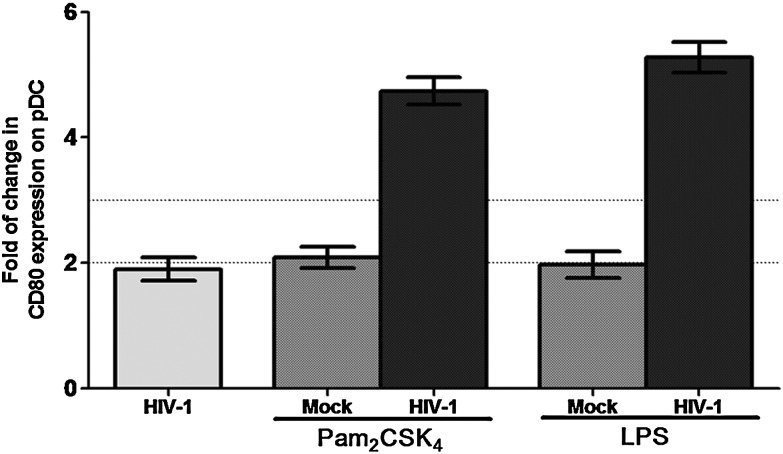
HIV-1 infection induces up-regulation of the maturation marker CD80 in pDC, especially in response to TLR2 and TLR4 ligands
Since MDMs and PBMCs produce a high secretion of proinflammatory cytokines (IL-6, IL-1β, and TNF-α) after infection with HIV-1 and through TLR2 and TLR4 activation with their agonist, we questioned whether this could be associated with DC maturation. For this purpose the expression of CD80 and CD86 was analyzed by flow cytometry in PBMCs exposed to HIV-1H9 HTLV-IIIcc in vitro before and after treatment with Pam2CSK4 and LPS. Twenty-four hours later, pDCs, mDCs, and monocytes were selected using the specific marker for each subcellular population and the expression of CD80 and CD86 was determined. An up-regulation of CD80 was observed in pDCs in vitro exposed to HIV-1 and stimulated by either TLR2 or TLR4 agonist, compared to the pDCs without TLR stimulation (Fig. 4). In contrast, the treatment of mDCs and monocytes with TLR2 and TLR4 agonists revealed no differences in CD80 expression between infected and mock (data no shown). When CD86 expression was analyzed, neither monocytes nor DCs showed a significant change in the expression pattern of CD86 (data not shown). Thus, collectively our results show that in response to TLR2 and TLR4 agonists, the PBMCs and MDMs secreted proinflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α), and in pDCs there was an up-regulation of CD80 expression, in a higher proportion in HIV-1-infected cells than in mock-infected cells. Our in vitro results were not associated with cell death, because cell viability (MTT assay) was close to 98% during the time course of the experiments, for dose of each TLR agonist and for the viral doses used (data not shown).
FIG. 4.
CD80 expression is increased in pDCs exposed in vitro to HIV-1. CD80 expression was measured by flow cytometry in total PBMCs exposed in vitro to HIV-1H9 HTLV-IIIcc for 2 h before stimulation with 10 nM Pam3CSK4 and 10 ng/ml LPS for 18 h at 37°C and 5% CO2. Mononuclear cells were gated according to physical characteristics excluding dead cells, and pDCs were then gated as Lin 1– CD123high cells. Representative results of three independent experiments are shown as median and range of the fold change in the expression of the marker [mean fluorescent intensity (MFI) in overall subpopulation, after isotype control subtraction] compared to the control without infection or TLR stimulation. Dotted line represents 2- and 3-fold induction, compared to the control.
CD4+ T cells are decreased in HIV-1-infected patients
The expression of TLR2 and TLR4 in monocytes, pDCs, and mDCs from HIV-1-infected patients was then evaluated to determine the behavior of TLR in vitro as described above, and ex vivo. The demographics of the patients examined are presented in Table 1. PBMCs from 49 HIV-1-infected patients were subdivided based on the viral load and the use or not of HAART (Table 1); 25 healthy donors were also examined as controls for TLR2 and TLR4 expression in monocytes, pDCs, and mDCs. The quantification of the cellular subpopulations was established to assess the possible differences between subject groups. In contrast to previous reports,3 there was no significant change in the percentage or the absolute count of monocytes, pDCs, and mDCs in peripheral blood obtained from HIV-1-infected patients compared with healthy donors (data not shown). However, as expected, the CD4+ T cell counts were decreased in the HIV-1-infected patients, especially in those whose viral load was higher than 400 RNA copies/ml, indicating the immune alterations that are prevalent during HIV infection (Table 1 and Supplementary Fig. S2).
Increased levels of expression of TLR2 and TLR4 in mDCs and monocytes from HIV-1-infected patients
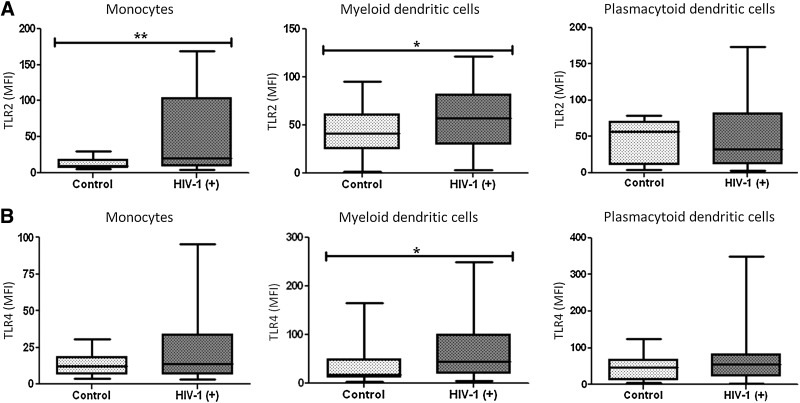
TLR2 and TLR4 expression was not affected by the age or gender of the healthy subjects included in the study (n=25, data not shown). However, the levels of expression of TLR2 and TLR4 varied greatly among the subjects included, mainly in the HIV-1-infected patients. Sanders et al. (2008) reported that TLR4 expression in lymphocytes was increased in HIV-1-infected individuals compared to healthy controls,39 suggesting a possible role of HIV-1 in modulating TLR expression. Recently, we have shown increased expression of TLR2 and TLR4 in mDCs, and TLR4 in pDCs from HIV-1-infected patients with opportunistic infections without HAART.34 In the present study, we determined the expression levels of the TLR2 and TLR4 proteins and mRNAs to assess whether HIV-1 infection alters TLR expression on antigen-presenting cells. TLR2 expression was increased in monocytes and mDCs (p<0.01 and p<0.05, respectively) from HIV-1-infected patients compared to healthy donors (Fig. 5A), but TLR4 increased only in mDCs (p<0.05) (Fig. 5B). No significant changes in TLR expression were observed in pDCs, consistent with the results described in Fig. 1A. Interestingly, the low MFI values of TLR expression (especially for TLR4 expression) in monocytes compared with DCs were due to high basal fluorescence in monocytes, which decreases the overall MFI, after subtraction of the isotype control. In conclusion, our finding demonstrates that in HIV-1-infected patients, there were changes in TLR2 and TLR4 levels in mDCs and TLR2 in monocytes.
FIG. 5.
Increased expression of TLR2 and TLR4 in monocytes and DCs from HIV-1-infected patients. Mononuclear cells were gated according to physical characteristics, excluding dead cells. TLR2 (A) and TLR4 (B) expression on monocytes (CD14+), mDCs (Lin1- CD11chigh), and pDCs (BDCA2+ CD123high) from total PBMCs were measured by flow cytometry. Data are presented as overall MFI, after subtraction of isotype staining background. The MFI of TLR2 and TLR4 in monocytes, mDCs, and pDCs were plotted for each group: controls (n=25) and HIV-1-infected patients (n=49). Comparisons were performed using unpaired two-tailed Student's t-tests (Mann–Whitney U-tests). The level of significance is *p<0.05 and **p<0.01.
HIV-1-infected patients HAART naive with high viral load showed the most notable up-regulation of TLR2 expression
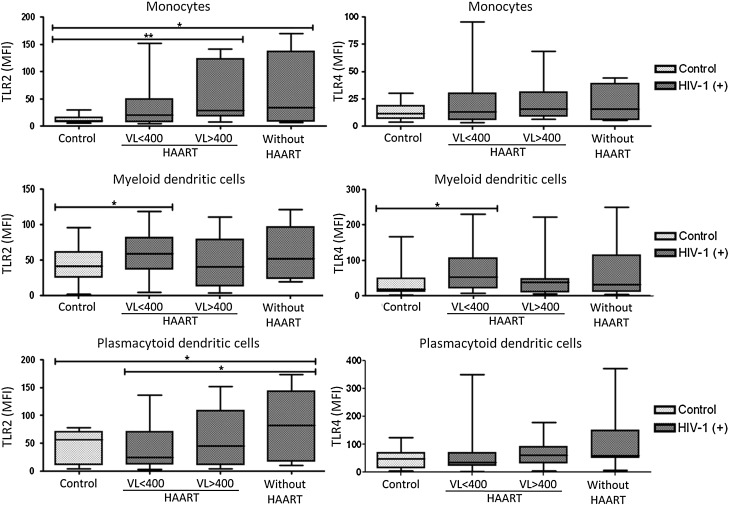
To determine whether viral load and/or HAART alter the expression pattern of TLR2 and TLR4, HIV-1-infected patients were divided into three groups: (1) HAART users with a viral load lower than 400 copies/ml, (2) HAART users with a viral load higher than 400 copies/ml, and (3) patients without HAART. The expression levels of both TLRs were evaluated. There was an up-regulation of TLR2 expression in monocytes and pDCs from HIV-1-infected patients without HAART, compared with the controls (Fig. 6). In monocytes an increase in TLR2 was also observed in patients with HAART and high viral loads (>400 viral RNA copies/ml). However, in mDCs a higher expression of TLR2 and TLR4 was observed in HIV-1-infected patients with HAART but with less than 400 viral RNA copies/ml (Fig. 6), suggesting that suppression of viral replication by HAART contributed to higher expression of TLRs. Interestingly, lower levels of TLR4 mRNA have been reported in HIV-1-infected patients whose HAART failed compared to healthy subjects.29 However, the presented results indicate that HIV-1 infection increases the expression levels of TLR2 and TLR4 depending on the viral load, the use of HAART, and possibly under precise circumstances, including the time of infection, or even a clinical unapparent superinfection. This is likely to be an important determinant of the immune pathogenesis of HIV-1 infection.
FIG. 6.
TLR2 and TLR4 expression in cells from HIV-1-infected patients based on HAART and viral load. TLR2 and TLR4 expression in monocytes (CD14+), mDCs (Lin1– CD11chigh), and pDCs (BDCA2+ CD123high) from total PBMCs was measured by flow cytometry. The data are presented as overall MFI after subtraction of the isotype staining background. The MFI of TLR2 and TLR4 in monocytes, mDCs, and pDCs was plotted for each group: controls (n=25), HIV-1-infected patients with HAART and viral loads less than 400 copies per ml of plasma (n=26), HIV-1-infected patients with HAART and viral loads higher than 400 copies per ml of plasma (n=12), and HIV-1-infected patients without HAART treatment (n=11). Comparisons were made by Kruskal–Wallis ANOVA tests and Dunn's posttests. The level of significance is *p<0.05 and **p<0.01.
Increased mRNA levels of TLR2, TLR4, and TNF-α in HIV-1-infected patients
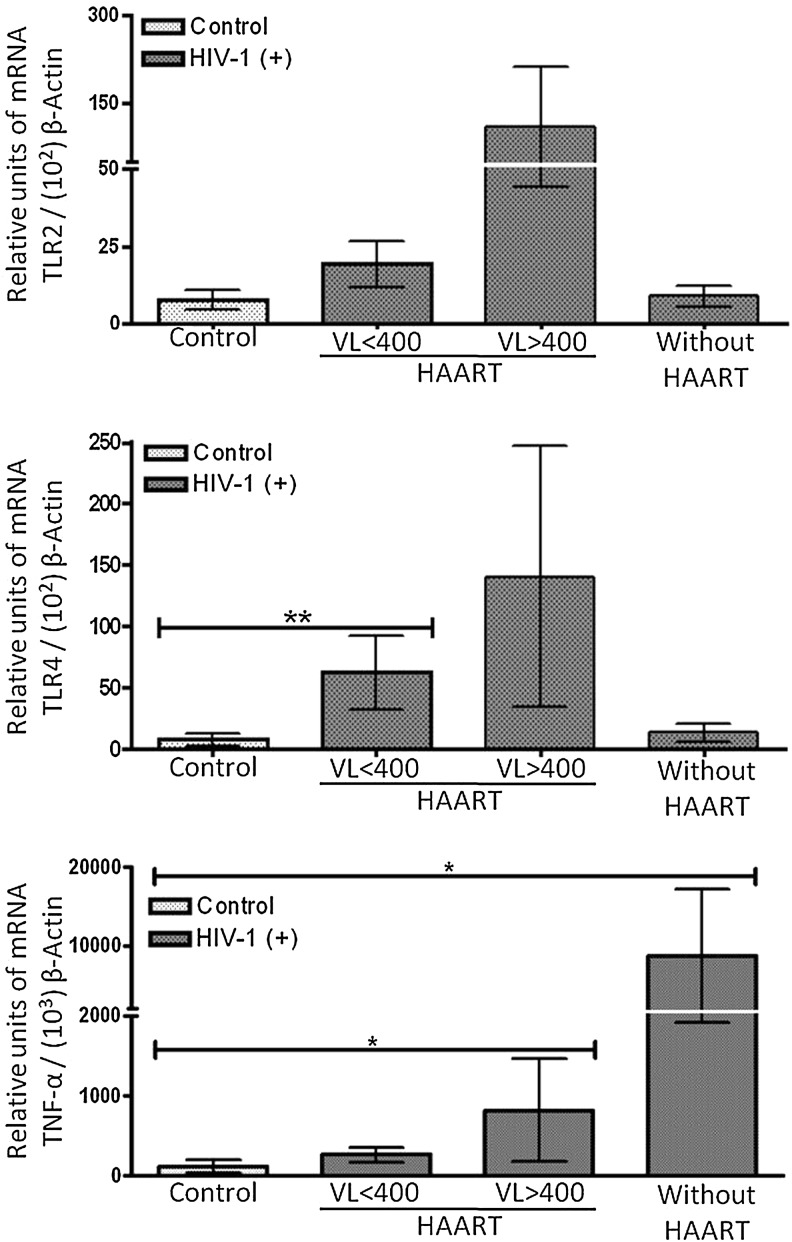
To assess the effect of viral load and HAART on TLR gene expression, we examined whether the levels of the TLR mRNAs were consistent with the increase in TLR2 and TLR4 protein expression observed by flow cytometry, but in total PBMCs. For TLR2 mRNA, there was a trend toward an increase in expression in PBMCs from HIV-1-infected patients with HAART, but it was higher in patients with high viral loads (Fig. 7). Interestingly, there was a significant increase in the transcript level of TLR4 on PBMCs of HIV-1-infected patients with HAART and low viral loads compared to healthy subjects (Fig. 7). No significant differences, but only slight increases were observed in patients with HAART and high viral loads; yet high variability was detected among patients tested for TLR4 expression. In patients without HAART, the level of TLR4 mRNA was very similar to that of the control subjects. Because flow cytometry analyses showed that CD14(–) cells (including NK, B, and T cells) presented no change in TLR2 or TLR4 expression when HIV-1-infected patients and healthy donors were compared (data not shown), we suggest that the increase in TLR transcripts observed occurs in the antigen-presenting cell subpopulations. Examining whether there were significant differences in TNF-α mRNA between the different groups of HIV-1-infected patients, we found that the TNF-α mRNA level was increased in HIV-1-infected patients with HAART (and high viral load) or without HAART (Fig. 7), indicating a functional link between the modulation of TLR expression (both protein and mRNA levels) and inflammatory status.
FIG. 7.
HIV-1-infected individuals express higher levels of TLR2, TLR4, and TNF-α mRNAs than healthy donors. TLR2, TLR4, and TNF-α mRNA levels were measured using real-time RT-PCR, and normalized with the housekeeping gene β-actin. Relative units of transcripts versus β-actin transcripts are shown as median and range. Comparisons were by Kruskal–Wallis ANOVA tests and Dunn's posttests. The level of significance was *p<0.05 and **p<0.01. Controls (n=8), HIV-1-infected patients with HAART and viral loads less than 400 copies per ml (n=6), HIV-1-infected patients with HAART and viral loads more than 400 copies per ml (n=5), and HIV-1-infected patients without HAART (n=3).
Expression of TLR2 and TLR4 in monocytes and TLR2 in pDCs correlates positively with viremia in HIV-1-infected patients without HAART
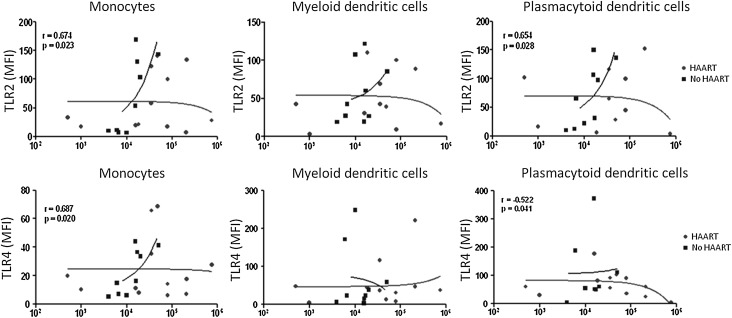
Finally, to determine the factors influencing TLR2 and TLR4 expression in monocytes, pDCs, and mDCs from HIV-1-infected patients, we examined whether there is a significant correlation between the expression of both TLRs and viral load. The analyses revealed a significant positive correlation between expression of TLR2 and viral load (patients without HAART) in monocytes and pDCs (r=0.674, p=0.023, and r=0.654, p=0.028, respectively) (Fig. 8). Similar results were observed for TLR4 in monocytes (r=0.687, p=0.020). There was no significant correlation between TLR4 and viral load in pDCs and mDCs or between TLR2 and viral load in mDCs (Fig. 8). No significant correlation was detected between TLR expression and CD4+ T cells count (data not shown).
FIG. 8.
Expression of TLR2 and TLR4 is positively correlated with viral load in HIV-1-infected patients without HAART. TLR2 expression was significantly correlated with viral load in monocytes (p=0.023, r=0.674) and pDCs (p=0.028, r=0.654) from HIV-1-infected patients without HAART. TLR4 expression was also significantly correlated with viral load in monocytes (p=0.020, r=0.687) from HIV-1-infected patients without HAART. In pDCs a negative correlation was observed between TLR4 expression and viral load in HIV-1-infected patients with HAART (p=0.041, r=–0.522). No significant correlations were observed in mDCs. HIV-1-infected patients with HAART (n=12) and HIV-1-infected patients without HAART (n=9). With the other patients it was not possible to obtain data on the viral load. The curve-fitting was a second degree polynomial curve by using the data linearization method. Spearman correlations were used with a significance level of p<0.05 two-tailed. In all cases the x-axis represents the viral load of the HIV-1-infected patients.
Discussion
Innate immune activation is critical to control infections and acts as bridge for adaptive immunity through TLR activation. However, in the case of HIV-1 infection, innate immune activation also drives HIV-1 replication via signaling pathways downstream of the TLRs.40 While some studies have explored how different types of cells from HIV-1-infected patients respond to TLR agonists (effect on expression and its functionality), the ultimate aim of this study was to determine the effect on TLR2 and TLR4 expression and functionality in PBMCs and MDMs derived from healthy subjects and in vitro infected with HIV-1 and then treated with agonist for the two TLRs. The altered expression of both TLRs in antigen-presenting cells from HIV-1-infected patients was also evaluated.
After in vitro HIV-1-infection of PBMCs, the modulation of TLRs expression in monocytes, pDCs, and mDCs was dose dependent (Fig. 1). As previously reported,34 flow cytometry and real time RT-PCR show that pDCs can express TLR2 and TLR4, suggesting an important role of these receptors in activating antiviral responses in the pDCs subsets as well as the induction of type I IFN production. Indeed, using functional assays, pDCs were shown to increase the expression of the costimulatory molecule CD80 in response to TLR2 and TLR4 stimulation (Fig. 4).
The results are in accordance with the previously reported by Martinson et al. (2007) about the higher expression of the activation and maturation markers CD40, CD83, and CD86 on pDC, in response to TLR7/8 stimulation; but they fail to show the overexpression of these molecules in HIV-1-infected patients, compared to healthy donors.41 In contrast, they found a decrease in IFN-α production in response to TLR7/TLR8 stimulation in HIV-1-infected patients, compared to healthy donors.41 Interestingly, TLR stimulation can also activate CD4+ and CD8+ T lymphocytes, determined by the expression of CD38, and even induce the central memory and effector CD4+ T cells to enter into cell cycle, and after activation can also induce apoptosis in CD4+ T cells.42 Remarkably, modulation of TLRs expression after HIV-1 infection behaved differently. When the cells were exposed in vitro to HIV-1 and cultured for 3 h only, TLR2 expression was up-regulated in pDCs, but down-regulated in monocytes; in contrast, TLR4 was increased in all three cellular subpopulations of antigen-presenting cells evaluated (Fig. 1C).
These results suggest that during early steps of infection both receptors could play an important role in initiating an immune response to control viral spread, which could include proinflammatory cytokines and type I IFN. However, when infection was extended to 18 h, the expression of TLR2 in Mon was increased, while TLR2 expression in pDCs and TLR4 expression in DCs were “normalized” (Fig. 1A). Normally, during the first 24 h of infection, HIV-1 has expressed all its proteins and a viral protein could be blocking the TLR2 and TLR4 pathway in DCs, as a mechanism to diminish host defense response. It has been reported that the HIV-1 Tat protein increases the half life of the TLR4 mRNA, but decreases TLR4 translation.43 Recently, it was reported that exposure to the HIV-1 proteins Tat and gp120 significantly alters TLR expression in astrocytes.44 Together, these results indicate that HIV-1 proteins alter the innate response, supporting our hypothesis. Other reports have shown that stimulation of TLR2, and even other TLRs such as TLR5, can increase cellular HIV-1 integration and trigger reactivation of the latent HIV-1 provirus in T cells, and can also activate virus gene expression in central memory CD4+ T cells.45 In addition, stimulation of TLR5 enhanced replication of HIV-1, while TLR9 stimulation suppressed viral replication.46
The differential effects of these TLR ligands on HIV-1 replication correlated with changes in production of chemokines, and the induction of cellular activation and proliferation.46 Therefore, the precise effect of TLR modulation on the HIV-1 life cycle must be investigated further. Once infection had been established (after 18 h), TLR2 and TLR4 were up-regulated in monocytes, probably to maintain the proinflammatory cytokine profile, which can also promote viral replication after provirus formation by activating NF-κB signaling in HIV-1-infected cells, similar to TLRs.10–14 At the same time, TLR2 expression was down-regulated in mDCs, the most important antigen-presenting cells, suggesting a viral mechanism to evade the immune response.19 Thus, TLR activation may promote HIV-1 infection due to the effect of downstream signaling effectors on viral replication.47
To evaluate the modulation of the TLRs in other cell subpopulation during HIV-1 infection, TLRs expression was assessed in MDMs. An increase in TLR2 and TLR4 expression either in MDMs infected by HIV-1 in vitro and stimulated with their specific agonist, or in monocytes, pDCs, and mDCs of HIV-1-infected patients, was detected. These observations agree with the findings that HIV-1 infection itself leads to up-regulation of TLRs.26 However, other authors have reported that TLRs signaling could be attenuated in HIV-1-infected cells,48,49 although this was observed in a chronically HIV-1-infected cell line, that may contain some metabolic alterations affecting intracellular pathways. Likewise, it has been reported that HIV-1 and its products can also modulate TLR expression and functions.27,50 For instance, ssRNA40 (HIV-1-derived RNA) increases TLR3 and TLR8 expression in activated T cells,39 as well as TLR4 mRNA expression,23,27 resulting in an increase in proinflammatory cytokine production in response to TLR stimulation by HIV-1. Increased TLR4 expression by T cell subsets has been reported in HIV-1-infected patients28; in chronic HIV-1-infected patients who failed to respond to HAART, reduced expression of TLR3, TLR4, and TLR9 together with increased expression of TLR7, which correlated with high HIV-1 RNA levels, was reported.29
Lately, we have also reported that TLR2 and TLR4 expression is increased in DCs from HIV-1-infected patients with opportunistic infections.34 All of these studies were obtained using cells derived from HIV-1-infected patients that have shown increased response in the presence of TLR ligands.23 Here, using MDMs or PBMCs from healthy subjects and infected in vitro with HIV-1 and stimulated with specific agonists for TLR2 and TLR4, an increase of IL-1β and IL-6, IL-8, and TNF-α was detected (Figs. 2B and C and 3A and B), or a trend to an increase of the mRNAs for IL-6 and TNF-α in PBMCs (Fig. 3C), similar to TNF-α, IL-12, and COX-2 in mDC from HIV-1-infected patients, previously reported.41 Based on these findings, we can speculate that HIV-1 has developed strategic mechanisms to alter the expression of TLRs and enhance proinflammatory cytokine production, which could play a key role in immune pathogenesis. However, it can also be argued that high expression of TLR2 and TLR4 in cells from HIV-1-infected patients, or in PBMCs from healthy subjects HIV-1 infected in vitro, results in increased HIV-1 replication.
In support of this hypothesis, an association between progression of HIV disease and polymorphisms in TLR451,52 and TLR9,53,54 or in the levels of soluble TLR2,55 has been reported. In addition, a 3' UTR polymorphism in the NLRP3 gene, a member of the cytosolic NOD-like receptors, was also associated with increased susceptibility to HIV-1 infection.56 Overall, these results point to an important link between TLRs, or even other PRRs, and the inflammatory response in modulating AIDS progression.
It will be interesting to determine whether alteration of the TLR signaling pathway leads to an antiviral host response, or is detrimental to the host by enhancing viral replication and spread. Based on our results, and on previous reports,57 we propose that the role of TLRs during HIV-1 infection could be dual. During the initial phase of infection, TLR signaling could promote antiviral activity through IFN-α/β release,58 whereas during the chronic phase, TLR stimulation could induce a strong inflammatory response that would increase HIV-1 replication.
Some authors have reported that exposure of pDCs to viral gp120 leads to suppression of these cells in a TLR9-dependent response.59 However, our results show an association between up-regulation of TLR2, TLR4, and proinflammatory cytokines with pDC maturation, based on the expression of CD80 (Fig. 4) and TLR2 in pDCs (Fig. 6). An increase of TLR2, TLR4, and TNF-α in the H9 HTLV-IIIcc cells, a chronically infected cell line (data not shown), was observed, as also reported in U1 cells,49 arguing that during chronic HIV-1 infection, proinflammatory cytokine release is increased.
The main goal of HAART is to block HIV-1 replication and achieve immune reconstitution in HIV-1-infected patients. Here, we report that pDCs from HIV-1-infected subjects without HAART present higher expression levels of TLR2 than those from healthy donors. These results are in line with our previous findings in HIV-1-infected patients with opportunistic infections, who also have higher TLR2 and TLR4 levels in DCs.34 This could be associated with a higher viral load since TLRs can mediate the activation of HIV-1 LTR through the NF-κB pathway,10,11,14 suggesting than TLR expression levels could be influenced by viral factors and by the immunological state of the host.
Thus, the association between TLR expression and plasma HIV-1 viral load points to the regulation of expression by viral products rather than large shifts in innate cell populations. Interestingly, in monocytes expression of TLR2 and TLR4 was positively correlated with viral load, as well as TLR2 in pDCs, all of them in patients without HAART. This is similar to previous reports in chronically HIV-1-infected patients with advanced disease (CD4+ T cell count less than 200 cells/ml),23,27 as well as in coinfected patients with HIV-1 and opportunistic infections.34 Regarding other TLRs, previous studies have shown lower levels of TLR3, TLR7, and TLR9 in patients with high levels of HIV-1 RNA compared to those with lower levels of viremia.29 However, our data do not show a correlation between TLR expression and CD4+ T cell count (data not shown). Finally, the mechanisms by which HIV-1 infection increases TLR expression and function need to be further examined.
Based on these observations, we propose that increased TLR expression, together with a higher inflammatory response, could increase viral replication through NF-κB. In addition to increased TLR expression in monocytes/macrophages and DCs, up-regulation of TLR activity therefore also induces IFN-α/β secretion and increases loss of CD4+ T cells via TRAIL-mediated apoptosis.60 On the other hand, there is a second possibility, wherein patients with high viral loads, especially those without HAART treatment, maintain a proinflammatory state that also increases TLR expression. In both cases, TLR up-regulation in HIV-1-infected patients could represent an immunopathogenic event that would accelerate progression to AIDS, due to increased viral replication. The more interesting aspect of our study is that it was performed in three cellular subpopulations (monocytes, pDCs, and mDCs), the most important antigen-presenting cells, directly from HIV-1-infected patients or from healthy subjects (HIV-1-infected in vitro), and to our knowledge, this is the first study relating TLR2 and TLR4 expression in DCs and HIV-1 infection.
Up-regulation of TLR2 and/or TLR4 during viral infections has been reported. For example, overexpression of TLR2 and TLR4 in monocytes of hepatitis B virus (HBV)-infected patients61 and Dengue virus-infected patients was reported.62 However, down-modulation of TLR7 and TLR9 expression was reported in cells of hepatitis C virus- or HBV-infected patients, which negatively correlated with viral load.63,64 Together, these results suggest an important role of TLRs during viral infection and that viruses can modulate their expression to avoid antiviral immunity or to promote pathogenic events with altered TLR expression and inflammatory immune response.
Conclusions
In summary, we have described important changes in TLR expression as a consequence of HIV-1 infection. By increasing TLR expression in monocytes/macrophages and DCs, HIV-1 infection may progressively perturb the immune response (innate and adaptive) and disturb processes that normally protect individuals from pathogen-associated diseases. We propose that increased TLR expression during HIV-1 infection leads to increased innate sensing and responsiveness of the immune system that may also serve as a primary driver for immune activation and thus for HIV-1 progression. Therapies that could act on these processes may therefore provide additional opportunities to interrupt HIV-1-associated disease progression, in combination with the antiretroviral-specific therapy, HAART.
Finally, it is important to consider that therapeutic modulation of TLR signaling could be a double-edge sword, because inhibition of TLR function can help control the chronic inflammation state and decrease HIV-1 replication. On the other hand, it could also increase the susceptibility to infections by other microorganisms. For this reason, an integral understanding of the mechanisms modulating TLR expression and function is necessary to guide the potential use of TLR-based therapies for HIV-1 infection, and even other infectious diseases.
Supplementary Material
Acknowledgments
We acknowledge George Washington University for supplying numerous reagents used in this study. The authors also acknowledge the patients and control individuals who participated in this study and the collaboration of the personnel of the institutions where the patients were recruited.
J.C.H. and S.U.I. were responsible for the conception and design of the project as well as the acquisition, analysis, and interpretation of data. M.S. and E.L. critically reviewed the contents. All authors read and approved the final manuscript.
This study was supported by the COLCIENCIAS, Grant 111549326099. The founders had no role in the study design, data collection and analysis, decision to publish, or the preparation of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Levy JA. HIV pathogenesis: 25 years of progress and persistent challenges. AIDS. 2009;23:147–160. doi: 10.1097/QAD.0b013e3283217f9f. [DOI] [PubMed] [Google Scholar]
- 2.Almeida M. Cordero M. Almeida J. Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–271. [PubMed] [Google Scholar]
- 3.Finke JS. Shodell M. Shah K. Siegal FP. Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24:647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez JC. Montoya CJ. Urcuqui-Inchima S. [The role of toll-like receptors in viral infections: HIV-1 as a model] Biomedica. 2007;27:280–293. [PubMed] [Google Scholar]
- 5.Pulendran B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol. 2005;174:2457–2465. doi: 10.4049/jimmunol.174.5.2457. [DOI] [PubMed] [Google Scholar]
- 6.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 7.Palsson-McDermott EM. O'Neill LA. Building an immune system from nine domains. Biochem Soc Trans. 2007;35:1437–1444. doi: 10.1042/BST0351437. [DOI] [PubMed] [Google Scholar]
- 8.Kaisho T. Akira S. Regulation of dendritic cell function through toll-like receptors. Curr Mol Med. 2003;3:373–385. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- 9.Mogensen TH. Paludan SR. Reading the viral signature by toll-like receptors and other pattern recognition receptors. J Mol Med. 2005;83:180–192. doi: 10.1007/s00109-004-0620-6. [DOI] [PubMed] [Google Scholar]
- 10.Equils O. Faure E. Thomas L. Bulut Y. Trushin S. Arditi M. Bacterial lipopolysaccharide activates HIV long terminal repeat through toll-like receptor 4. J Immunol. 2001;166:2342–2347. doi: 10.4049/jimmunol.166.4.2342. [DOI] [PubMed] [Google Scholar]
- 11.Equils O. Schito ML. Karahashi H, et al. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: Implications of simultaneous activation of TLRs on HIV replication. J Immunol. 2003;170:5159–5164. doi: 10.4049/jimmunol.170.10.5159. [DOI] [PubMed] [Google Scholar]
- 12.Sundstrom JB. Little DM. Villinger F. Ellis JE. Ansari AA. Signaling through toll-like receptors triggers HIV-1 replication in latently infected mast cells. J Immunol. 2004;172:4391–4401. doi: 10.4049/jimmunol.172.7.4391. [DOI] [PubMed] [Google Scholar]
- 13.Mares D. Simoes JA. Novak RM. Spear GT. TLR2-mediated cell stimulation in bacterial vaginosis. J Reprod Immunol. 2008;77:91–99. doi: 10.1016/j.jri.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg RS. Aggerholm A. Bertelsen LS. Ostergaard L. Paludan SR. Role of mitogen-activated protein kinases, nuclear factor-kappaB, and interferon regulatory factor 3 in toll-like receptor 4-mediated activation of HIV long terminal repeat. Apmis. 2009;117:124–132. doi: 10.1111/j.1600-0463.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez OA. Li M. Ebersole JL. Huang CB. HIV-1 reactivation induced by the periodontal pathogens Fusobacterium nucleatum and Porphyromonas gingivalis involves toll-like receptor 2 [corrected] and 9 activation in monocytes/macrophages. Clin Vaccine Immunol. 2010;17:1417–1427. doi: 10.1128/CVI.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarember KA. Godowski PJ. Tissue expression of human toll-like receptors and differential regulation of toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 17.Pasare C. Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Caron G. Duluc D. Fremaux I, et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM. Hemmi H. Dendritic cells: Translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 20.Beutler B. Inferences, questions and possibilities in toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 21.Beignon AS. McKenna K. Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier A. Alter G. Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lester RT. Yao XD. Ball TB, et al. Toll-like receptor expression and responsiveness are increased in viraemic HIV-1 infection. AIDS. 2008;22:685–694. doi: 10.1097/QAD.0b013e3282f4de35. [DOI] [PubMed] [Google Scholar]
- 24.Heggelund L. Damas JK. Yndestad A, et al. Stimulation of toll-like receptor 2 in mononuclear cells from HIV-infected patients induces chemokine responses: Possible pathogenic consequences. Clin Exp Immunol. 2004;138:116–121. doi: 10.1111/j.1365-2249.2004.02595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thibault S. Fromentin R. Tardif MR. Tremblay MJ. TLR2 and TLR4 triggering exerts contrasting effects with regard to HIV-1 infection of human dendritic cells and subsequent virus transfer to CD4+ T cells. Retrovirology. 2009;6:42. doi: 10.1186/1742-4690-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heggelund L. Muller F. Lien E, et al. Increased expression of toll-like receptor 2 on monocytes in HIV infection: Possible roles in inflammation and viral replication. Clin Infect Dis. 2004;39:264–269. doi: 10.1086/421780. [DOI] [PubMed] [Google Scholar]
- 27.Lester RT. Yao XD. Ball TB, et al. HIV-1 RNA dysregulates the natural TLR response to subclinical endotoxemia in Kenyan female sex-workers. PLoS One. 2009;4:e5644. doi: 10.1371/journal.pone.0005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller Sanders C. Cruse JM. Lewis RE. Toll-like receptor and chemokine receptor expression in HIV-infected T lymphocyte subsets. Exp Mol Pathol. 2009;88:26–31. doi: 10.1016/j.yexmp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Scagnolari C. Selvaggi C. Chiavuzzo L, et al. Expression levels of TLRs involved in viral recognition in PBMCs from HIV-1-infected patients failing antiretroviral therapy. Intervirology. 2009;52:107–114. doi: 10.1159/000218082. [DOI] [PubMed] [Google Scholar]
- 30.Bartlett JA. Chen SS. Quinn JB. Comparative efficacy of nucleoside/nucleotide reverse transcriptase inhibitors in combination with efavirenz: Results of a systematic overview. HIV Clin Trials. 2007;8:221–226. doi: 10.1310/hct0804-221. [DOI] [PubMed] [Google Scholar]
- 31.el Dirani-Diab R. Andreola ML. Nevinsky G, et al. Biochemical characterization of the p51 sub-unit of human immunodeficiency virus reverse transcriptase in homo- and heterodimeric recombinant forms of the enzyme. FEBS Lett. 1992;301:23–28. doi: 10.1016/0014-5793(92)80202-r. [DOI] [PubMed] [Google Scholar]
- 32.Swingler S. Mann AM. Zhou J. Swingler C. Stevenson M. Apoptotic killing of HIV-1-infected macrophages is subverted by the viral envelope glycoprotein. PLoS Pathog. 2007;3:1281–1290. doi: 10.1371/journal.ppat.0030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swingler S. Brichacek B. Jacque JM. Ulich C. Zhou J. Stevenson M. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature. 2003;424:213–219. doi: 10.1038/nature01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez JC. Arteaga J. Paul S. Kumar A. Latz E. Urcuqui Inchima S. Up-regulation of TLR2 and TLR4 in dendritic cells, in response to HIV-1 and co-infection with opportunistic pathogens. AIDS Res Hum Retroviruses. 2011;27:1099–1109. doi: 10.1089/aid.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadowaki N. Ho S. Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan X. Xiu F. An H. Wang X. Wang J. Cao X. Fever range temperature promotes TLR4 expression and signaling in dendritic cells. Life Sci. 2007;80:307–313. doi: 10.1016/j.lfs.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Yang H. Wei J. Zhang H. Lin L. Zhang W. He S. Upregulation of toll-like receptor (TLR) expression and release of cytokines from P815 mast cells by GM-CSF. BMC Cell Biol. 2009;10:37. doi: 10.1186/1471-2121-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghanim H. Mohanty P. Deopurkar R, et al. Acute modulation of toll-like receptors by insulin. Diabetes Care. 2008;31:1827–1831. doi: 10.2337/dc08-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders CM. Cruse JM. Lewis RE. Toll-like receptors, cytokines and HIV-1. Exp Mol Pathol. 2008;84:31–36. doi: 10.1016/j.yexmp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Aukrust P. Muller F. Lien E, et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179:74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 41.Martinson JA. Roman-Gonzalez A. Tenorio AR, et al. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol. 2007;250:75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funderburg N. Luciano AA. Jiang W. Rodriguez B. Sieg SF. Lederman MM. toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One. 2008;3:e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Hara SP. Small AJ. Gajdos GB. Badley AD. Chen XM. Larusso NF. HIV-1 Tat protein suppresses cholangiocyte toll-like receptor 4 expression and defense against Cryptosporidium parvum. J Infect Dis. 2009;199:1195–1204. doi: 10.1086/597387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Hage N. Podhaizer EM. Sturgill J. Hauser KF. Toll-like receptor expression and activation in astroglia: Differential regulation by HIV-1 Tat, gp120, and morphine. Immunol Invest. 2011;40(5):498–522. doi: 10.3109/08820139.2011.561904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thibault S. Imbeault M. Tardif MR. Tremblay MJ. TLR5 stimulation is sufficient to trigger reactivation of latent HIV-1 provirus in T lymphoid cells and activate virus gene expression in central memory CD4+ T cells. Virology. 2009;389:20–25. doi: 10.1016/j.virol.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Brichacek B. Vanpouille C. Kiselyeva Y, et al. Contrasting roles for TLR ligands in HIV-1 pathogenesis. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding J. Rapista A. Teleshova N, et al. Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation. J Immunol. 2010;184:2814–2824. doi: 10.4049/jimmunol.0902125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noursadeghi M. Tsang J. Miller RF, et al. Genome-wide innate immune responses in HIV-1-infected macrophages are preserved despite attenuation of the NF-kappa B activation pathway. J Immunol. 2009;182:319–328. doi: 10.4049/jimmunol.182.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordone SK. Ignacio GA. Su L, et al. Failure of TLR4-driven NF-kappa B activation to stimulate virus replication in models of HIV type 1 activation. AIDS Res Hum Retroviruses. 2007;23:1387–1395. doi: 10.1089/aid.2007.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoshino S. Konishi M. Mori M, et al. HIV-1 Vpr induces TLR4/MyD88-mediated IL-6 production and reactivates viral production from latency. J Leukoc Biol. 2010;87:1133–1143. doi: 10.1189/jlb.0809547. [DOI] [PubMed] [Google Scholar]
- 51.Pine SO. McElrath MJ. Bochud PY. Polymorphisms in toll-like receptor 4 and toll-like receptor 9 influence viral load in a seroincident cohort of HIV-1-infected individuals. AIDS. 2009;23:2387–2395. doi: 10.1097/QAD.0b013e328330b489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papadopoulos AI. Ferwerda B. Antoniadou A, et al. Association of toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms with increased infection risk in patients with advanced HIV-1 infection. Clin Infect Dis. 2010;51:242–247. doi: 10.1086/653607. [DOI] [PubMed] [Google Scholar]
- 53.Bochud PY. Hersberger M. Taffe P, et al. Polymorphisms in toll-like receptor 9 influence the clinical course of HIV-1 infection. AIDS. 2007;21:441–446. doi: 10.1097/QAD.0b013e328012b8ac. [DOI] [PubMed] [Google Scholar]
- 54.Soriano-Sarabia N. Vallejo A. Ramirez-Lorca R, et al. Influence of the toll-like receptor 9 1635A/G polymorphism on the CD4 count, HIV viral load, and clinical progression. J Acquir Immune Defic Syndr. 2008;49:128–135. doi: 10.1097/QAI.0b013e318184fb41. [DOI] [PubMed] [Google Scholar]
- 55.Heggelund L. Flo T. Berg K, et al. Soluble toll-like receptor 2 in HIV infection: Association with disease progression. AIDS. 2004;18:2437–2439. [PubMed] [Google Scholar]
- 56.Pontillo A. Brandao LA. Guimaraes RL. Segat L. Athanasakis E. Crovella S. A 3'UTR SNP in NLRP3 gene is associated with susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr. 2010;54:236–240. doi: 10.1097/QAI.0b013e3181dd17d4. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed N. Hayashi T. Hasegawa A, et al. Suppression of human immunodeficiency virus type 1 replication in macrophages by commensal bacteria preferentially stimulating toll-like receptor 4. J Gen Virol. 2010;91:2804–2813. doi: 10.1099/vir.0.022442-0. [DOI] [PubMed] [Google Scholar]
- 58.Mosoian A. Teixeira A. Burns CS, et al. Prothymosin-alpha inhibits HIV-1 via toll-like receptor 4-mediated type I interferon induction. Proc Natl Acad Sci USA. 2010;107:10178–10183. doi: 10.1073/pnas.0914870107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinelli E. Cicala C. Van Ryk D, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stary G. Klein I. Kohlhofer S, et al. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–3863. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y. Lian JQ. Huang CX, et al. Overexpression of toll-like receptor 2/4 on monocytes modulates the activities of CD4(+)CD25(+) regulatory T cells in chronic hepatitis B virus infection. Virology. 2010;397:34–42. doi: 10.1016/j.virol.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Azeredo EL. Neves-Souza PC. Alvarenga AR, et al. Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leucocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever. Immunology. 2010;130:202–216. doi: 10.1111/j.1365-2567.2009.03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou J. Huang Y. Tian D. Xu D. Chen M. Wu H. Expression of toll-like receptor 9 in peripheral blood mononuclear cells from patients with different hepatitis B and C viral loads. J Huazhong Univ Sci Technolog Med Sci. 2009;29:313–317. doi: 10.1007/s11596-009-0310-2. [DOI] [PubMed] [Google Scholar]
- 64.Chang S. Kodys K. Szabo G. Impaired expression and function of toll-like receptor 7 in hepatitis C virus infection in human hepatoma cells. Hepatology. 2010;51:35–42. doi: 10.1002/hep.23256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.