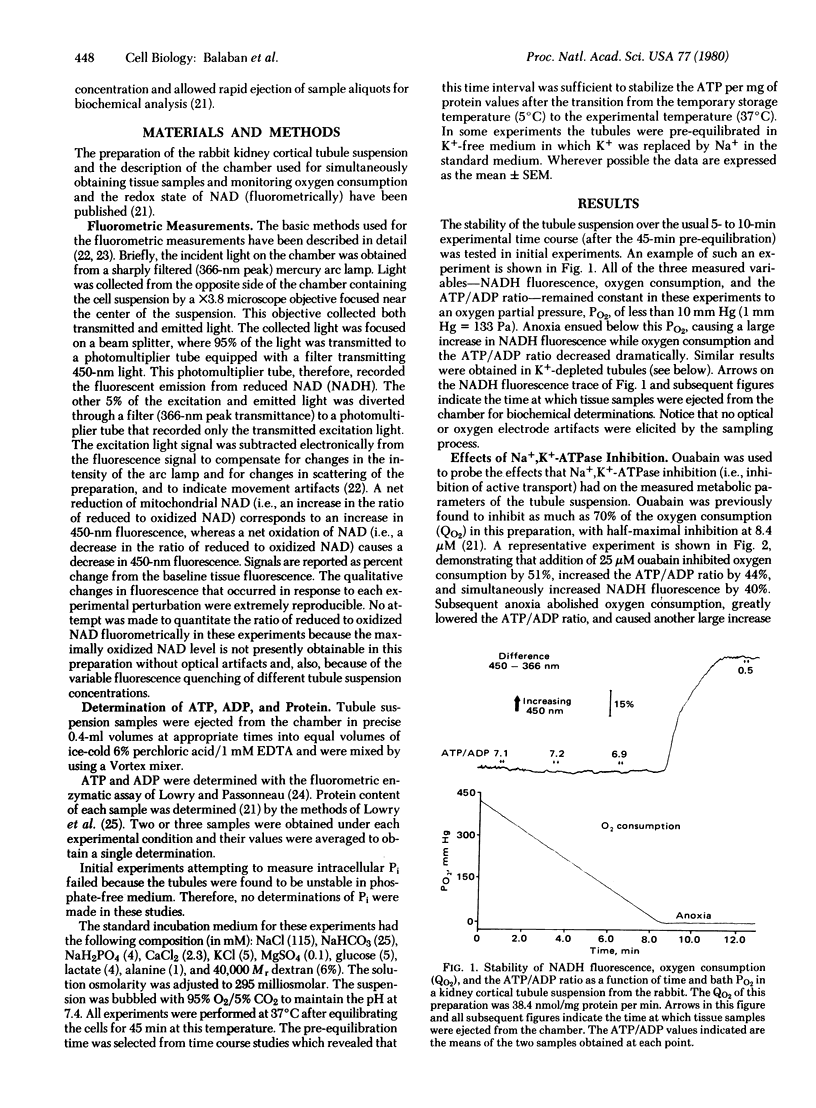
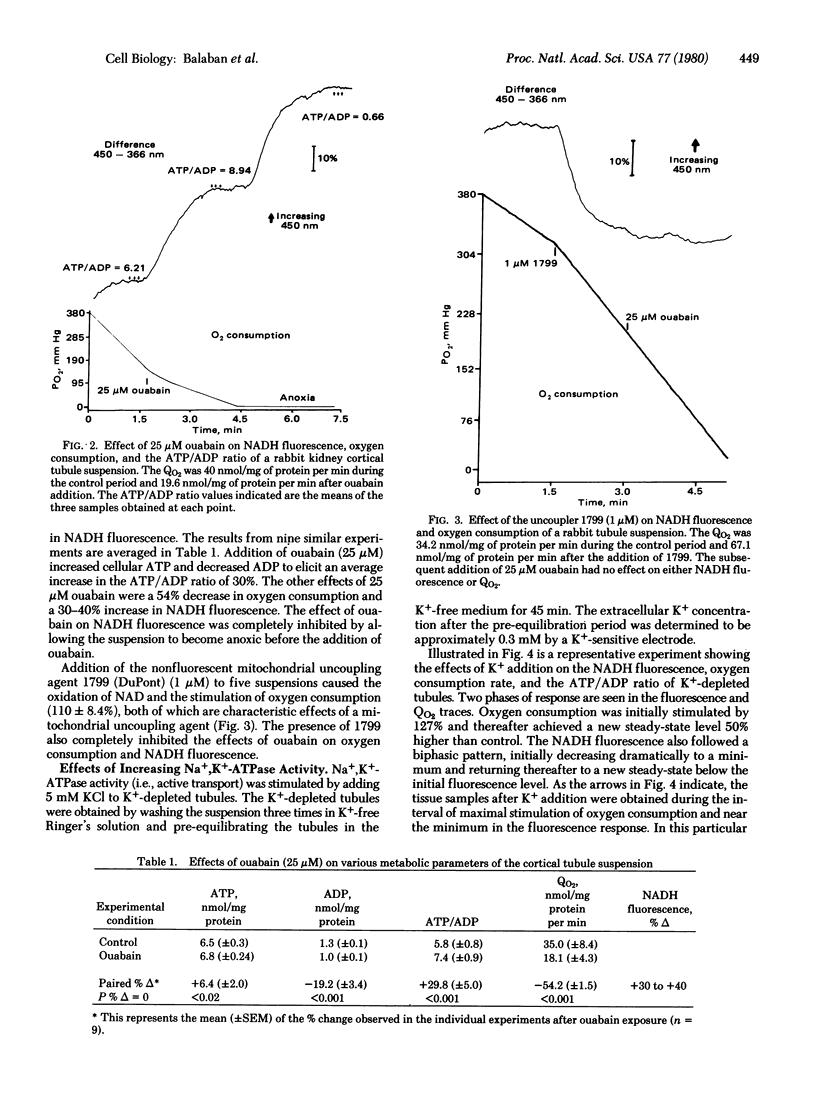
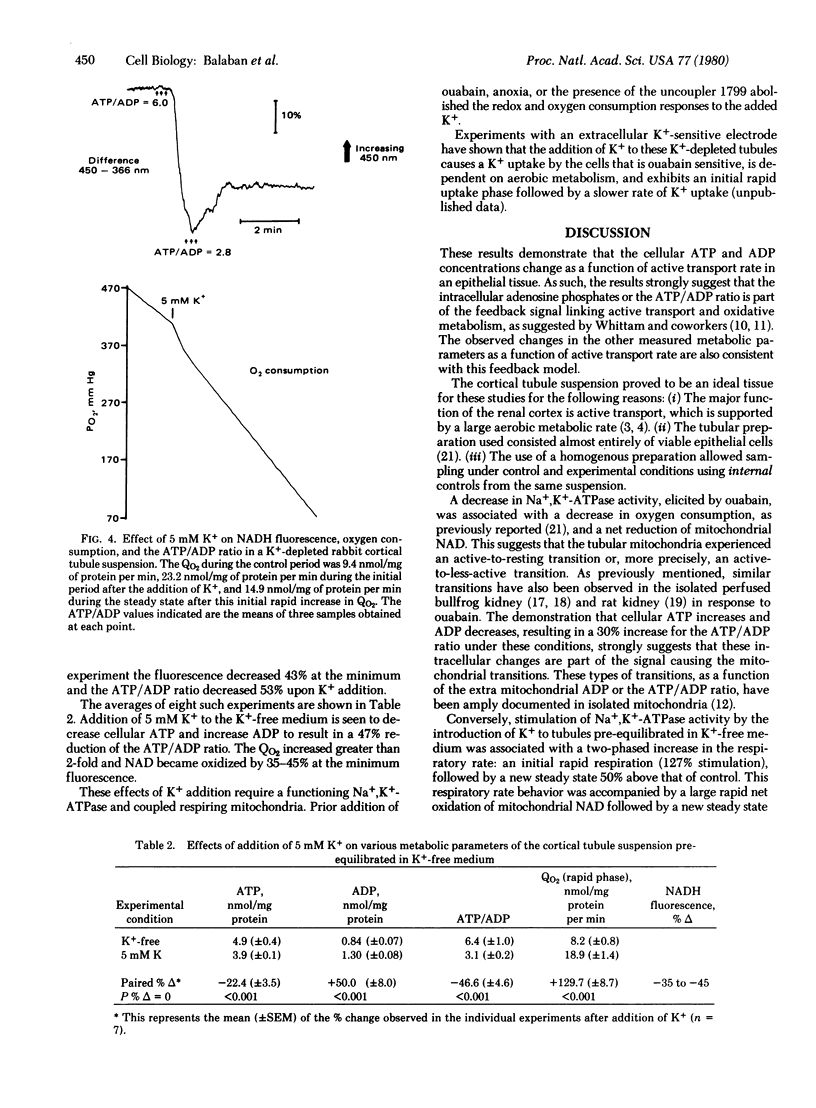
Abstract
We report the results of studies in which the cytoplasmic coupling between Na+,K+-ATPase activity (presumably a measure of active transport) and the mitochondrial respiratory rate was investigated in a tubule suspension from the rabbit kidney cortex. Simultaneous measurements of the redox state of mitochondrial nicotinamide adenine dinucleotide (NAD) (performed fluorometrically), the cellular ATP and ADP concentrations, and the oxygen consumption rate (QO2) were made under conditions known to alter the Na+,K+-ATPase turnover. Ouabain (25 microM) caused: (i) a 54% inhibition of QO2, (ii) a net reduction of NAD, and (iii) a 30% increase in the ATP/ADP ratio. The addition of K+ (5 ?M) to K+-depleted tubules caused: (i) an initial 127% stimulation of QO2 followed by a new steady-state QO2 50% above control, (ii) an initial large oxidation of NAD followed by a new steady state more oxidized than the control level, and (iii) a 47% decrease in the cellular ATP/ADP ratio. These data indicate that the cellular ATP and ADP concentrations or the ATP/ADP ratio may be part of the coupling mechanism linking Na+,K+-ATPase turnover and the aerobic metabolic rate in kidney.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Soltoff S. P., Storey J. M., Mandel L. J. Improved renal cortical tubule suspension: spectrophotometric study of O2 delivery. Am J Physiol. 1980 Jan;238(1):F50–F59. doi: 10.1152/ajprenal.1980.238.1.F50. [DOI] [PubMed] [Google Scholar]
- Blond D. M., Whittam R. The regulation of kidney respiration by sodium and potassium ions. Biochem J. 1964 Jul;92(1):158–167. doi: 10.1042/bj0920158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., COHEN P., JOBSIS F., SCHOENER B. Intracellular oxidation-reduction states in vivo. Science. 1962 Aug 17;137(3529):499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- Cala P. M., Cogswell N., Mandel L. J. Binding of [3H]ouabain to split frog skin: the role of the Na,K-ATPase in the generation of short circuit current. J Gen Physiol. 1978 Apr;71(4):347–367. doi: 10.1085/jgp.71.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Stubbs M., Miyata Y., Ditre C. M. Regulation of cellular metabolism by intracellular phosphate. Biochim Biophys Acta. 1977 Oct 12;462(1):20–35. doi: 10.1016/0005-2728(77)90186-4. [DOI] [PubMed] [Google Scholar]
- Franke H., Barlow C. H., Chance B. Oxygen delivery in perfused rat kidney: NADH fluorescence and renal functional state. Am J Physiol. 1976 Oct;231(4):1082–1089. doi: 10.1152/ajplegacy.1976.231.4.1082. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Preston A. S., Orloff J. Effect of ADH, aldosterone, ouabain, and amiloride on toad bladder epithelial cells. Am J Physiol. 1972 May;222(5):1071–1074. doi: 10.1152/ajplegacy.1972.222.5.1071. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F., Stainsby W. N. Oxidation of NADH during contractions of circulated mammalian skeletal muscle. Respir Physiol. 1968 May;4(3):292–300. doi: 10.1016/0034-5687(68)90035-2. [DOI] [PubMed] [Google Scholar]
- Kessler R. H., Landwehr D., Quintanilla A., Weseley S. A., Kaufmann W., Arcila H., Urbaitis B. K. Effects of certain inhibitors on renal sodium reabsorption and ATP specific activity. Nephron. 1968;5(6):474–488. doi: 10.1159/000179657. [DOI] [PubMed] [Google Scholar]
- Kiil F. Renal energy metabolism and regulation of sodium reabsorption. Kidney Int. 1977 Mar;11(3):153–160. doi: 10.1038/ki.1977.23. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maxild J. Energy requirements for active transport of p-aminohippurate in renal cortical slices. Arch Int Physiol Biochim. 1973 Sep;81(3):501–521. doi: 10.3109/13813457309073400. [DOI] [PubMed] [Google Scholar]
- Nellans H. N., Finn A. L. Oxygen consumption and sodium transport in the toad urinary bladder. Am J Physiol. 1974 Sep;227(3):670–675. doi: 10.1152/ajplegacy.1974.227.3.670. [DOI] [PubMed] [Google Scholar]
- Rea C., Segal S. ATP content of rat kidney cortex slices: relation to alpha-aminoisobutyric acid uptake. Kidney Int. 1972 Aug;2(2):101–106. doi: 10.1038/ki.1972.77. [DOI] [PubMed] [Google Scholar]
- Sachs G. Ion pumps in the renal tubule. Am J Physiol. 1977 Nov;233(5):F359–F365. doi: 10.1152/ajprenal.1977.233.5.F359. [DOI] [PubMed] [Google Scholar]
- Van Rossum G. D. Relation of the oxidoreduction level of electron carriers to ion transport in slices of avian salt gland. Biochim Biophys Acta. 1968 Jan 15;153(1):124–131. doi: 10.1016/0005-2728(68)90152-7. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. Active cation transport as a pace-maker of respiration. Nature. 1961 Aug 5;191:603–604. doi: 10.1038/191603a0. [DOI] [PubMed] [Google Scholar]
- Whittembury G. Sodium and water transport in kidney proximal tubular cells. J Gen Physiol. 1968 May;51(5 Suppl):303S+–303S+. [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M., Dutton P. L. Thermodynamic relationships in mitochondrial oxidative phosphorylation. Annu Rev Biophys Bioeng. 1974;3(0):203–230. doi: 10.1146/annurev.bb.03.060174.001223. [DOI] [PubMed] [Google Scholar]
- ZERAHN K. Oxygen consumption and active sodium transport in the isolated and short-circuited frog skin. Acta Physiol Scand. 1956 May 31;36(4):300–318. doi: 10.1111/j.1748-1716.1956.tb01327.x. [DOI] [PubMed] [Google Scholar]



