Abstract
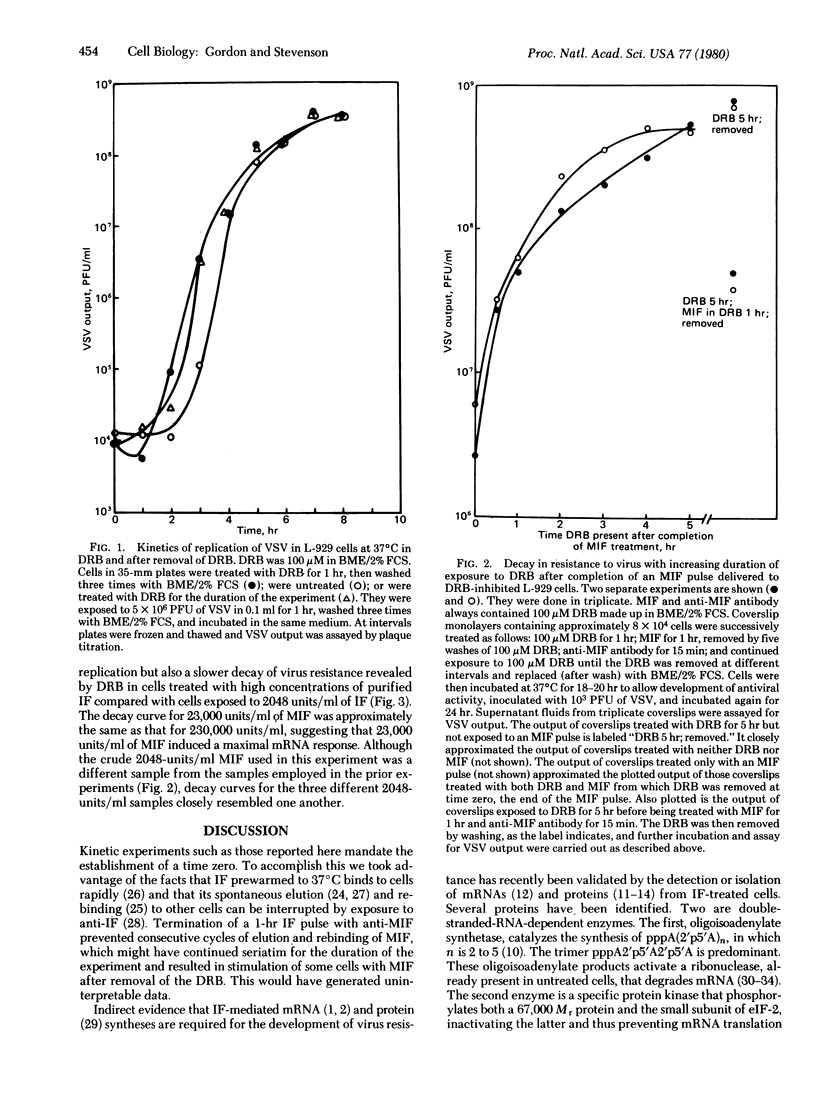
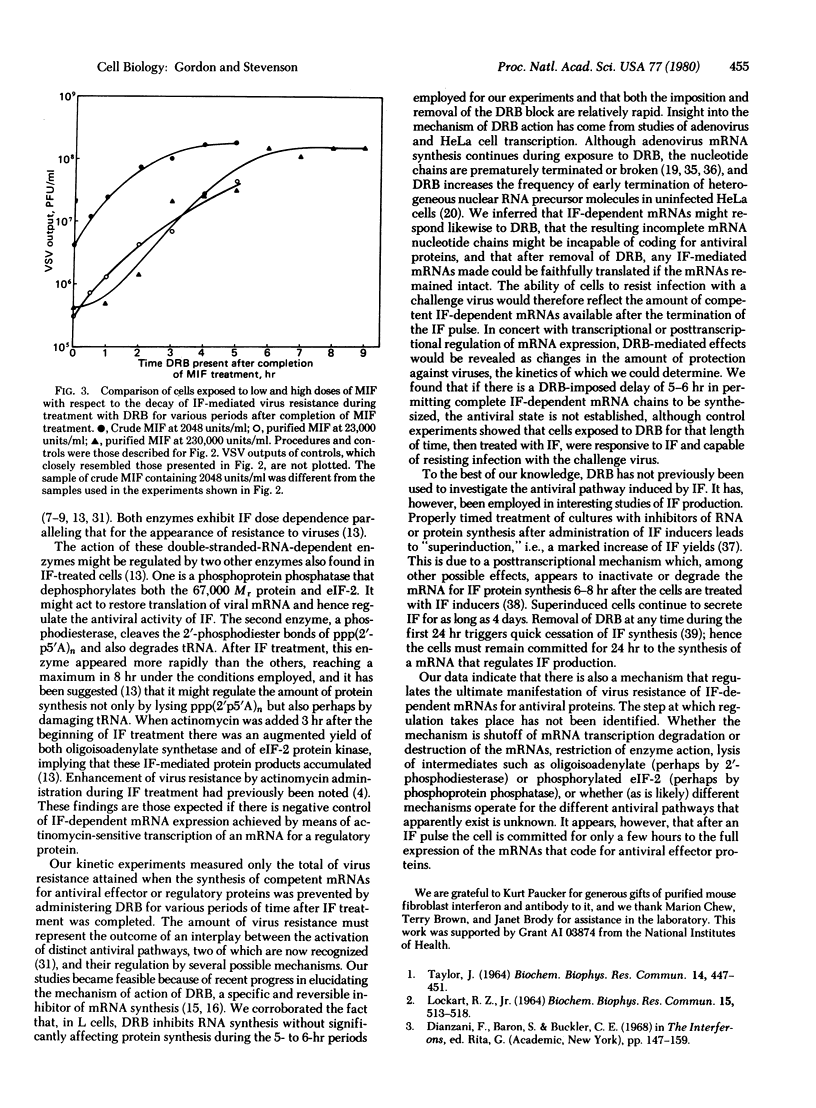
We used 5,6-dichloro-beta D-ribofuranosyl-benzimidazole (DRB), a selective and reversible inhibitor of mRNA production, to investigate the regulation of the pathway leading to resistance to viruses in cells treated with interferon (IF). DRB allows initiation of transcription but promotes premature termination of the nucleotide chains, so that it abolishes interferon-dependent protection against viruses. When the DRB is removed, synthesis of complete mRNAs can resume. Mouse L-929 cells were exposed to 100 microM DRB before and during a 1-hr pulse of IF followed by treatment with antibody to IF to prevent cell-to-cell spread of IF after that time. At different intervals thereafter the cells were washed and the DRB was replaced by medium; after further incubation, the cells were infected with vesicular stomatitis virus. Resistance to virus was inversely proportional to the duration of the block imposed by DRB. When the DRB was removed soon after the IF pulse, substantial protection from virus ensued, but none developed when removal of the DRB was deferred for 5-6 hr. Cells exposed to DRB for 5 hr, then pulsed with IF for 1 hr, still mounted a strong antiviral response. The data show that the ability of cells to resist viral infection decays within 5-6 hr after treatment with IF. Whether the decay is due to shutoff of transcription of mRNAs, or to their destruction or degradation, or whether regulation takes place at one or more subsequent steps in the antiviral pathway, remains to be determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Ball L. A. Induction of 2'5'-oligoadenylate synthetase activity and a new protein by chick interferon. Virology. 1979 Apr 30;94(2):282–296. doi: 10.1016/0042-6822(79)90462-8. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Nuclease activation by double-stranded RNA and by 2',5'-oligoadenylate in extracts of interferon-treated chick cells. Virology. 1979 Mar;93(2):348–356. doi: 10.1016/0042-6822(79)90239-3. [DOI] [PubMed] [Google Scholar]
- Berman B., Vilcek J. Cellular binding characteristics of human interferon. Virology. 1974 Feb;57(2):378–386. doi: 10.1016/0042-6822(74)90177-9. [DOI] [PubMed] [Google Scholar]
- Chany C., Fournier F., Rousset S. Potentiation of the antiviral activity of interferon by actinomycin D. Nat New Biol. 1971 Mar 24;230(12):113–114. doi: 10.1038/newbio230113a0. [DOI] [PubMed] [Google Scholar]
- Dahl H., Degré M. A micro assay for mouse and human interferon. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):863–870. doi: 10.1111/j.0365-5563.1973.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Baron S. The continued presence of interferon is not required for activation of cells by interferon. Proc Soc Exp Biol Med. 1977 Sep;155(4):562–566. doi: 10.3181/00379727-155-39851. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Baron S. Unexpectedly rapid action of human interferon in physiological conditions. Nature. 1975 Oct 23;257(5528):682–684. doi: 10.1038/257682a0. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., Samuel C. E. Mechanism of interferon action. Properties of an interferon-mediated ribonucleolytic activity from mouse L929 cells. Virology. 1978 Aug;89(1):240–251. doi: 10.1016/0042-6822(78)90056-9. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN R. M., SONNABEND J. A. INHIBITION OF INTERFERON ACTION BY P-FLUOROPHENYLALANINE. Nature. 1964 Jul 25;203:366–367. doi: 10.1038/203366a0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Broeze R. J., Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979 Jun 7;279(5713):523–525. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Sen G. C., Dubois M. F., Ratner L., Slattery E., Lengyel P. Interferon action: two distinct pathways for inhibition of protein synthesis by double-stranded RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5893–5897. doi: 10.1073/pnas.75.12.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. W., Sehgal P. B., Darnell J. E. DRB-induced premature termination of late adenovirus transcription. Nature. 1978 Apr 13;272(5654):590–593. doi: 10.1038/272590a0. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Sehgal P. B., Darnell J. E., Jr Multiple discrete sites for premature RNA chain termination late in adenovirus-2 infection: enhancement by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2571–2575. doi: 10.1073/pnas.76.6.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Shulman L., Schmidt A., Chernajovsky Y., Fradin A., Revel M. Kinetics of the induction of three translation-regulatory enzymes by interferon. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3208–3212. doi: 10.1073/pnas.76.7.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E., Jr, Korant B. D. Fibroblast interferon induces synthesis of four proteins in human fibroblast cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1824–1827. doi: 10.1073/pnas.76.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUCKER K., CANTELL K. Neutralization of interferon by specific antibody. Virology. 1962 Sep;18:145–147. doi: 10.1016/0042-6822(62)90189-7. [DOI] [PubMed] [Google Scholar]
- Radke K. L., Colby C., Kates J. R., Krider H. M., Prescott D. M. Establishment and maintenance of the interferon-induced antiviral state: studies in enucleated cells. J Virol. 1974 Mar;13(3):623–630. doi: 10.1128/jvi.13.3.623-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Zilberstein A., Shulman L., Federman P., Berissi H., Revel M. Interferon action: isolation of nuclease F, a translation inhibitor activated by interferon-induced (2'-5') oligo-isoadenylate. FEBS Lett. 1978 Nov 15;95(2):257–264. doi: 10.1016/0014-5793(78)81006-0. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Fraser N. W., Darnell J. E., Jr Early Ad-2 transcription units: only promoter-proximal RNA continues to be made in the presence of DRB. Virology. 1979 Apr 15;94(1):185–191. doi: 10.1016/0042-6822(79)90448-3. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I. Halogenated benzimidazole ribosides, Novel inhibitors of RNA synthesis. Biochem Pharmacol. 1978;27(21):2475–2485. doi: 10.1016/0006-2952(78)90313-1. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I. Two mechanisms contribute to the superinduction of poly(I).poly(C)-induced human fibroblast interferon production. Virology. 1979 Jan 15;92(1):240–244. doi: 10.1016/0042-6822(79)90230-7. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Human interferon production: superinduction by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Science. 1975 Oct 17;190(4211):282–284. doi: 10.1126/science.1179208. [DOI] [PubMed] [Google Scholar]
- Tamm I., Hand R., Caliguiri L. A. Action of dichlorobenzimidazole riboside on RNA synthesis in L-929 and HeLa cells. J Cell Biol. 1976 May;69(2):229–240. doi: 10.1083/jcb.69.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Kikuchi T. Early termination of heterogeneous nuclear RNA transcripts in mammalian cells: accentuation by 5,6-dichloro 1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5750–5754. doi: 10.1073/pnas.76.11.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Sehgal P. B. Halobenzimidazole ribosides and RNA synthesis of cells and viruses. Adv Virus Res. 1978;22:187–258. doi: 10.1016/s0065-3527(08)60775-7. [DOI] [PubMed] [Google Scholar]
- Taylor J. Inhibition of interferon action by actinomycin. Biochem Biophys Res Commun. 1964;14:447–451. doi: 10.1016/0006-291x(64)90084-1. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Begon-Lours J., Gresser I. A method for the large scale production of potent interferon preparations. Proc Soc Exp Biol Med. 1974 Jul;146(3):809–815. doi: 10.3181/00379727-146-38196. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A., Kohase M. Superinduction of interferon with metabolic inhibitors: possible mechanisms and practical applications. J Infect Dis. 1976 Jun;133 (Suppl):A22–A29. doi: 10.1093/infdis/133.supplement_2.a22. [DOI] [PubMed] [Google Scholar]
- Young C. S., Pringle C. R., Follett E. A. Action of interferon in enucleated cells. J Virol. 1975 Feb;15(2):428–429. doi: 10.1128/jvi.15.2.428-429.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]



