Abstract
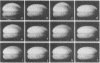
In most species the cell cycle is arrested in the unfertilized egg. After fertilization the cell cycle is reestablished and a rapid series of cleavages ensues. Preceding the first cleavage in Xenopus the egg undergoes a contraction of its cortex, called the "surface contraction wave," which can be visualized by time-lapse cinematography. This wave of contraction is propagated in a circular manner from the animal pole to the equator. We have found that eggs prevented from cleaving by treatment with antimitotic drugs undergo a sequence of periodic surface contraction waves timed with the cleavage cycle in untreated eggs. In addition, artificially activated eggs, which fail to cleave presumably for lack of a functioning centriole, undergo the same periodic contractions. No nuclear material is required for the periodic waves because a separated egg fragment, produced by constricting a fertilized egg, still undergoes contraction waves with the same period as the cleaving nucleated fragment. These results demonstrate that some expression of the cell cycle persists in the absence of any nuclear material or centrioles, suggesting to us that a biological clock exists in the cytoplasm or cortex of vertebrate eggs, which may be involved in timing the cell cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL L. G. Some mechanisms involved in cell division. Nature. 1962 Jan 13;193:190–191. doi: 10.1038/193190a0. [DOI] [PubMed] [Google Scholar]
- BROWN D. D., LITTNA E. RNA SYNTHESIS DURING THE DEVELOPMENT OF XENOPUS LAEVIS, THE SOUTH AFRICAN CLAWED TOAD. J Mol Biol. 1964 May;8:669–687. doi: 10.1016/s0022-2836(64)80116-9. [DOI] [PubMed] [Google Scholar]
- Flickinger R. A., Lauth M. R., Stambrook P. J. An inverse relation between the rate of cell division and RNA synthesis per cell in developing frog embryos. J Embryol Exp Morphol. 1970 Jun;23(3):571–582. [PubMed] [Google Scholar]
- Fraser L. R. Physico-chemical properties of an agent that induces parthenogenesis in Rana pipiens eggs. J Exp Zool. 1971 Jun;177(2):153–172. doi: 10.1002/jez.1401770204. [DOI] [PubMed] [Google Scholar]
- GROSS P. R., COUSINEAU G. H. MACROMOLECULE SYNTHESIS AND THE INFLUENCE OF ACTINOMYCIN ON EARLY DEVELOPMENT. Exp Cell Res. 1964 Feb;33:368–395. doi: 10.1016/0014-4827(64)90002-3. [DOI] [PubMed] [Google Scholar]
- Gilkey J. C., Jaffe L. F., Ridgway E. B., Reynolds G. T. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol. 1978 Feb;76(2):448–466. doi: 10.1083/jcb.76.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey R. D., Wolf D. P., Hedrick J. L. Formation and structure of the fertilization envelope in Xenopus laevis. Dev Biol. 1974 Jan;36(1):44–61. doi: 10.1016/0012-1606(74)90189-4. [DOI] [PubMed] [Google Scholar]
- Hara K. "Double camera" time-lapse micro-cinematography. Simultaneous filming of both poles of the amphibian egg. Mikroskopie. 1970 Sep;26(5):181–184. [PubMed] [Google Scholar]
- Heidemann S. R., Kirschner M. W. Aster formation in eggs of Xenopus laevis. Induction by isolated basal bodies. J Cell Biol. 1975 Oct;67(1):105–117. doi: 10.1083/jcb.67.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst R. T., Reitsma H. R. A device for localized micro-extraction and injection of cytoplasm in the amphibian egg with simultaneous marking of the wound site. Mikroskopie. 1977 Jan;32(11-12):345–350. [PubMed] [Google Scholar]
- Maller J., Poccia D., Nishioka D., Kidd P., Gerhart J., Hartman H. Spindle formation and cleavage in Xenopus eggs injected with centriole-containing fractions from sperm. Exp Cell Res. 1976 May;99(2):285–294. doi: 10.1016/0014-4827(76)90585-1. [DOI] [PubMed] [Google Scholar]
- Mano Y. Cytoplasmic regulation and cyclic variation in protein synthesis in the early cleavage stage of the sea urchin embryo. Dev Biol. 1970 Jul;22(3):433–460. doi: 10.1016/0012-1606(70)90162-4. [DOI] [PubMed] [Google Scholar]
- Petzelt C. NH3-treatment of unfertilized sea urchin eggs turns on the Ca2+-ATPase cycle. Exp Cell Res. 1976 Oct 1;102(1):200–204. doi: 10.1016/0014-4827(76)90315-3. [DOI] [PubMed] [Google Scholar]
- SAKAI H. Studies on sulfhydryl groups during cell division of sea urchin egg. III. SH groups of KC1-soluble proteins and their change during cleavage. J Biophys Biochem Cytol. 1960 Dec;8:609–615. doi: 10.1083/jcb.8.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T. Cyclic changes in the cortical layer of non-nucleated fragments of the newt's egg. J Embryol Exp Morphol. 1979 Jun;51:183–193. [PubMed] [Google Scholar]