Abstract
BACKGROUND & AIMS
Liver disease is a significant cause of mortality among adults with α-1 antitrypsin (AAT) deficiency. Age and male sex are reported risk factors for liver disease. In the absence of adequate risk stratification, current recommendations are to intermittently test AAT-deficient adults for liver function. We evaluated this recommendation in a large group of adults with AAT deficiency, to determine the prevalence of increased levels of alanine aminotransferase (ALT) and identify risk factors for liver disease.
METHODS
We used the Alpha-1 Foundation DNA and Tissue Bank to identify a cross-section of AAT-deficient adults (n=647) with and without liver disease; individuals without AAT-deficiency were used as controls (n=152). Results from ALT tests were compared between groups.
RESULTS
The prevalence of liver disease among individuals with ATT-deficiency was 7.9%; an increased level of ALT was observed in 7.8% of ATT-deficient individuals, which did not differ significantly from controls. Mean levels of ALT fell within normal range for all groups. An increased level of ALT identified patients with liver disease with 11.9% sensitivity. The level of only γ- glutamyl transpeptidase was significantly higher in the AAT-deficient group than in controls (43 vs 30 IU/ml; P<.003). A childhood history of liver disease and male sex were risk factors for adult liver disease in the multivariate analysis.
CONCLUSIONS
An increased level of ALT does not identify adults with AAT deficiency who have liver disease. Male sex and liver disease during childhood might help identify those at risk.
Keywords: diagnostic factor, prognosis, protease inhibitor, cirrhosis
α-1 antitrypsin (AAT) is the most abundant endogenous serine protease inhibitor present in the circulation. It is a glycoprotein produced and secreted by the liver, and it primarily functions to inactivate neutrophil elastase and other serine proteases.1 The most common cause of AAT deficiency is a single point mutation resulting in a defective molecule that cannot be secreted from the liver effectively. In homozygous individuals (PI*ZZ), the low circulating levels of AAT causes the premature development of emphysema from the imbalance in protease/anti-protease activity in the lung. In contrast, liver injury is considered a “toxic gain of function” as the retained, abnormally folded soluble protein and insoluble inclusion bodies accumulate within the hepatocyte and cause damage.2
Although AAT deficiency is defined by a single gene defect, the phenotypic presentation of liver disease is highly variable. Children may present with jaundice and recover completely or progress to cirrhosis. In adults, liver disease is often undetected until cirrhosis or hepatocellular carcinoma are evident.3 The ATS/ERS published Standards for the Diagnosis and Management of Individuals with α-1 Antitrypsin deficiency in 2003.4 A recommendation was made to perform a regular assessment of simple liver function tests (LFTs) in those AAT deficient individuals who were asymptomatic or had lung disease alone “in the absence of firm evidence regarding optimal follow up.” This statement highlights the fact that the natural history of liver disease in PI*ZZ individuals is not well understood. The only prospective data available comes from the Swedish National neonatal screening program conducted from 1972–74. AAT deficient individuals were identified at birth and followed into young adulthood.5 Within the first 6 months of life, more than 50% of PI*ZZ children had either clinical signs of liver disease or abnormal LFTs.5 By adolescence, the majority of those followed had normal aminotransferase levels and no clinical signs of liver disease.6–8 Most recent reporting of liver enzyme testing in this cohort (n=89) included LFT testing at ages 22 to 30 years of age. Prevalence of an elevated alanine aminotransferase (ALT) was reported as 5–7% during this age range, and no clinical signs or complications of liver disease have developed.9
From these results, it may be easy to conclude that liver disease is not a significant issue for PI*ZZ adults. However, cirrhosis is frequently found on autopsy and causes significant mortality in older adults with AAT deficiency.10, 11 We hypothesize there is ongoing injury responsible for progression from asymptomatic young patients to older cirrhotic patients which may not be reflected by LFT abnormalities. Therefore, the aim of this study was to define the prevalence of elevated liver aminotransferases and liver disease in a large cross section of adults with AAT deficiency. The reported variation in onset and severity of hepatocyte damage also suggests other genetic and environmental factors modify an individuals' risk of developing liver disease. Consequently, we also sought to identify any risk factors associated with the development of liver disease.
Patients and Methods
The Alpha-1 Foundation DNA and Tissue Bank was established by the Alpha-1 Foundation for the purpose of collecting blood and tissue samples and clinical information from individuals with AAT deficiency. The Alpha-1 DNA and Tissue Bank is located at the University of Florida College of Medicine in Gainesville, Florida. Enrollment started in 2001, and data was entered into a central database as it was collected. Blood samples were stored in a central repository. Samples are genotyped for AAT with end-point allelic discrimination on an ABI Prism 7500 Fast unit (Applied Biosystems). The genotyping process uses the Taqman® procedure to test for S and Z alleles. A probe is specifically designed for wild type S or Z regions, and it contains the reporter dye FAM™ and a quencher dye. A separate probe is specifically designed for the mutant S or Z regions with the reporter dye VIC™ and a quencher dye. The probes, along with corresponding S or Z forward and reverse primers, and the templates are placed in separate wells for S or Z. The polymerase chain reaction (PCR) exploits the 5' nuclease activity of the DNA polymerase to cleave a Taqman® probe during PCR. The Taqman® probe contains a reporter dye at the 5' end and a quencher dye at the 3' end. During the reaction, cleavage of the probe separates the reporter dye and quencher dye, which results in increased fluorescence of the reporter. Accumulation of the PCR products is detected directly by monitoring the increase in fluorescence of the reporter dye. Alleles that were not identified as Z or S were considered to be the common `wild type' M variant.
Case Identification
The study is a cross-sectional analysis to establish the prevalence of liver disease and LFT abnormalities in a group of adult AAT deficient individuals. The database was queried to identify all AAT deficient individuals with genotype PI*ZZ who were age ≥18. PI*ZZ individuals with and without liver disease were compared. Responses from the completed medical questionnaire were used to determine the presence of liver disease, which was defined as adult onset hepatitis, cirrhosis, liver tumor, or liver transplant. If patients reported viral hepatitis or other cause of liver disease, they were excluded. Only one patient with HCV was eliminated for this reason. An analysis was performed after defining an abnormal ALT as any value above the upper limit of normal established by the laboratory (0–55 IU/L for males and 0–40 IU/L for females). The same analysis was performed using a more conservative set of values for ALT that has been proposed in the literature.12 Nine patients who were post liver transplant were excluded from all laboratory analysis. A control group with the normal genotype (PI*MM) and no liver disease was also identified from the database.
Clinical, Demographic, and Laboratory Data
Clinical and demographic data were obtained from the medical questionnaire that was completed at time of enrollment. Information provided included age, age at diagnosis of AATD, gender, race, occupational exposure to dust and particles, tobacco and alcohol use, childhood history of acute or chronic hepatitis, jaundice within first month of life, pulmonary symptoms, hypertension, and diabetes. Results of spirometry testing, recorded as FEV1 measured in liters and percent predicted, were available in most patients. A single sample for each AAT deficient individual was tested for ALT, aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (GGT) by commercial laboratory testing (LabCorp). Patients were excluded if they were less than 18 years old at time of data and sample collection, available clinical data was inadequate, or the blood specimen was inadequate. Institutional IRB approval was obtained for the protocol.
Data analysis
Descriptive statistics were used for the baseline characteristics of the study group. For numerical data, the distribution, mean and standard deviation were noted. Comparisons were made using students t-test. For categorical data, the number and proportion of a given parameter were noted. Comparisons were made using chi square test. The factors predictive for liver disease were evaluated using multiple logistic regression analysis where the presence of liver disease was the dependent variable. All analysis was performed using SPSS version 17.0.
Results
A total of 800 AAT deficient individuals with genotype PI*ZZ were identified, but some met exclusion criteria: age <18 (n=13), incomplete clinical data reported in the medical questionnaire (n=43), or an inadequate blood specimen available for testing (n=97). As a result, 647 PI*ZZ individuals were included in the analysis.
Clinical features of liver and lung disease
The demographics of this cross section of AAT patients are shown in Table 1. The group was composed of 48.4% men and 51.6% women. As expected, only a few individuals reported a race other than Caucasian, and only 19 (2.9%) individuals did not respond to the question. The average age at inclusion was 54.7 (range 21–86), however, average age at time of diagnosis of AATD was significantly lower, particularly in those whom reported liver disease (p=0.012). The reported prevalence of any liver disease in the overall cohort was 7.9%. The prevalence of cirrhosis was 3.4% (22/647), and increased to 4.1% (18/437) in those greater than 50 years of age. Men were significantly more likely to have liver disease than women (p=0.003) and more likely to be cirrhotic (p=0.006). Hepatocellular carcinoma was present in 5.9% (n=3). Liver transplantation had occurred in 17.6% (n=9).
Table 1.
Clinical and Demographic Features of AAT Deficient Adults
| Variable | No Liver Disease n=596 | Liver Disease n=51 | p -value |
|---|---|---|---|
| Age in years, mean (range) | |||
| At time of Study | 54.90 (21–86) | 52.80 (22–82) | ns |
| At Diagnosis of AATD | 46.62 (6–85) | 43.94 (1–67) | 0.012 |
| Gender | |||
| Male, n(%) | 278 (46.6) | 35 (68.6) | 0.001 |
| Female, n(%) | 318 (53.4) | 16 (31.4) | |
| Race | |||
| Caucasian, n(%) | 569 (95.5) | 49 (96.1) | ns |
| Other, n(%) | 7 (1.3) | 3(3.9) | |
| Co-morbid Diseases | |||
| Diabetes, n(%) | 35 (5.9) | 2 (3.9) | ns |
| Hypertension, n(%) | 84 (14.1) | 8 (15.7) | |
| Tobacco Use | |||
| Never, n(%) | 175 (29.5) | 20 (39.2) | ns |
| Previous, n(%) | 402 (67.4) | 31 (60.8) | |
| Current, n(%) | 17 (2.9) | 0 (0.0) | |
| Alcohol use | |||
| Never, n(%) | 99 (16.6) | 12 (23.5) | 0.03 |
| Previous, n(%) | 128 (21.5) | 18 (35.3) | |
| Current, n(%) | 366 (61.4) | 21 (41.2) | |
| Occupational exposure | |||
| Yes, n(%) | 293 (49.2) | 30 (58.8) | ns |
| ≥Two more colds/year | |||
| Yes, n(%) | 260 (43.7) | 21 (41.2) | ns |
| Family History of Liver Disease | ns | ||
| Yes, n(%) | 100 (16.8) | 10 (19.7) | |
| Childhood History of Liver disease | |||
| Jaundice within first 30 days of life | 17 (2.9) | 9 (17.6) | <0.001 |
| Childhood Hepatitis | 2 (0.3) | 2 (3.9) | 0.033 |
| Laboratory Data, Mean (range) | |||
| ALT IU/ml (men) | 31.89(7–110) | 40.33(10–107)* | <0.001 |
| ALT IU/ml (women) | 24.36 (6–301) | 19.50 (11–39)* | ns |
| AST IU/ml | 43.27 (9–325) | 44.70 (7–183)* | ns |
| GGT IU/ml | 42.03 (2–456) | 45.93 (10–194)* | ns |
| CRP IU/ml | 0.40 (0.00–14.90) | 0.57 (0.00–3.04)* | ns |
ALT, alanine aminotransferase; AST, aspartate aminotransferase;
GGT, γ-glutamyl transpeptidase; CRP, C-reactive protein
Mean values are calculated after excluding post-liver transplant patients (n=9)
The majority of the PI*ZZ individuals reported respiratory symptoms as shown in Table 2. Shortness of breath was the most common symptom, with the mean duration of symptoms of 10.7 years (range, 1–70). The presence of pulmonary symptoms and medication use were not different between those with and without liver disease. Objectively, measurements of FEV1 and % predicted FEV1 were not different between the groups. Only a small percentage of individuals with and without liver disease reported no pulmonary symptoms or therapies directed at treatment.
Table 2.
Lung Disease in AAT Deficient Adults With and Without Liver Disease
| Variable | No Liver Disease n=596 | Liver Disease n=51 | p -value |
|---|---|---|---|
| Symptoms, n(%) | |||
| Cough | 239 (40.1) | 24 (47.1) | ns |
| Wheeze | 463 (77.7) | 38 (74.5) | ns |
| Shortness of breath | 511 (85.7) | 39 (76.5) | ns |
| No symptoms | 38 (6.4) | 7 (13.7) | ns |
| Medications, n(%) | |||
| Inhaled bronchodilator | 411 (69.0) | 33 (64.7) | ns |
| Inhaled corticosteroid | 297 (49.8) | 18 (35.3) | ns |
| Supplemental oxygen | 235 (39.4) | 18 (35.3) | ns |
| Augmentation therapy | 423 (71.0) | 34 (66.7) | ns |
| No therapies | 78 (13.1) | 7 (13.7) | ns |
| Spirometry | |||
| FEV1/Liters (range) | 1.76 (0.33–5.57) | 1.93 (0.43–4.35) | ns |
| % Predicted FEV1 (range) | 54.74 (12–133) | 56.87 (13–118) | ns |
FEV1, Forced expiratory volume
Laboratory Testing
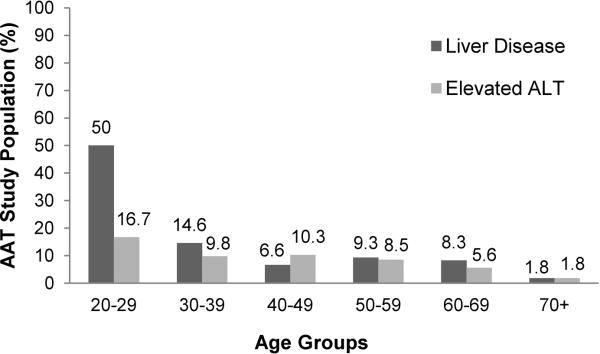
The laboratory data in the PI*ZZ individuals with and without liver disease was comparable (Table 1). The only statistically significant difference noted between groups was for the ALT in men, which was higher in the group with liver disease (p<0.001). When the ALT analysis was limited to individuals with cirrhosis, the results were similar. No significant differences for women (ALT, 24.4 vs. 22.2 IU/ml, p=0.82) and a significantly higher mean ALT for men (31.9 vs. 44.5 IU/ml, p=0.02). Despite the statistical significance of the higher ALT in men, the values were in the defined range for normal. When using the lab specified upper limit of normal for ALT, only 7.8% of this adult PI*ZZ population had an abnormal ALT. When the patients were stratified by age, the prevalence of reported liver disease and ALT abnormalities did not increase with age (Figure 1). The outlier was the age group of 20–29, which also had the smallest number of patients.
Figure 1. Prevalence of liver disease and elevated ALT by age group.
The prevalence of ALT abnormalities was approximately 10% or less in all age groups other than the 20–29 year olds. This age group had the fewest members (n=6) which may explain the differences noted. The prevalence of reported liver disease did not increase with age.
Defining a “normal” range for ALT has been problematic because reference populations have unknowingly included individuals with liver disease. Using a narrow range for normal ALT (0–19 IU/ml for women and 0–30 IU/ml for men) has been reported to improve sensitivity, especially in those with subclinical liver disease.12 We repeated our analysis using this stricter definition of normal, and the overall prevalence of abnormal ALT increased to 49.2%.
The diagnostic characteristics of an elevated ALT using the commercial lab definition and set of strict values are compared in Table 3. Overall, the diagnostic accuracy of an elevated ALT is poor, regardless of the cutoffs for normal. The sensitivity is low at 11.9% and does not improve to an acceptable level with a lower cutoff. Moreover, the positive and negative likelihood ratios (LR) approximate 1, suggesting that an abnormal ALT will fail to change the post-test probability of liver disease. With this observation in mind, we compared lab values from individuals with AAT deficiency to age and gender matched controls with a normal AAT genotype, MM, and no liver disease. No statistical difference was noted in the prevalence of an abnormal ALT, mean ALT, or AST (Table 4). The GGT was significantly higher in those with AAT deficiency. The same results were noted when the analysis was limited to liver disease (n=51) vs. controls (data not shown).
Table 3.
Diagnostic Accuracy of Elevated ALT for AAT Liver Disease
| Lab Defined Normal ALT | Strict Normal ALT | |
|---|---|---|
| Sensitivity | 11.9% | 52.4% |
| Specificity | 92.4% | 51.0% |
| PPV | 10% | 7.0% |
| NPV | 92.3% | 93.8% |
| LR+ | 1.57 | 1.07 |
| LR− | 0.95 | 0.93 |
PPV, positive predictive value; NPV, negative predictive value; LR+, likehood ratio positive, LR −, Likehood ratio negative
Table 4.
Comparison of Lab Data for AAT Deficiency and Normal Controls
| ZZ n=638^ | MM n=152 | p -value | |
|---|---|---|---|
| Age * | 54.7 (21–86) | 53.0 (26–82) | ns |
| Gender (M:F) | 304:334 | 64:88 | ns |
| Abnormal ALT (%) | 7.8 | 4.5 | ns |
| ALT* IU/ml (men) | 32.68 (7–110) | 33.48 (7–110) | ns |
| ALT* IU/ml (women) | 24.13 (6–301) | 22.01 (7–57) | ns |
| ALT* IU/ml | 43.37 (7–325) | 42.38 (7–554) | ns |
| ALT* IU/ml (women) | 42.32 (2–456) | 29.85 (2–373) | 0.003 |
values expressed as mean (range)
excludes 9 post-liver transplant patients
Risk factors for Liver Disease
Approximately half of the cohort reported a history of occupational exposure to dust or fumes (Table 1). As expected, a large proportion (66.9%) of the cohort previously smoked cigarettes, with a mean 20.1 pack/year history (range 0.5–92 pack/year). No difference in tobacco use was found between those with and without liver disease. Use of alcohol among this cross-section of adults with AAT deficiency was high with 82.4% of individuals reporting either current or previous use. Significant differences in alcohol use between those with and without liver disease were found. On average, the number of drinks per week was higher in those with liver disease (10.62, range 1–84 vs. 7.46, range 1–126; p<0.003). No differences were noted in the presence of diabetes or hypertension, repeated illnesses, or occupational exposures. A significantly higher proportion of adults with liver disease reported a childhood history of liver disease, the majority of which was jaundice within the first month of life (Table 1). A multivariate analysis performed to assess risk factors of developing liver disease demonstrated male gender and childhood history of liver disease as risk factors for adult liver disease (Table 5).
Table 5.
Univariate and Multivariate Analysis for Risk of Liver Disease
| Variable | No Liver Disease n=596 | Liver Disease n = 51 | Univariate Analysis p-value | Multivariate analysis p-value | RR (95% CI) |
|---|---|---|---|---|---|
| Age | 54.8 | 52.8 | 0.182 | ||
| Gender | |||||
| Male | 278 | 35 | 0.003 | 0.015 | 2.19(1.17–4.09) |
| Female | 318 | 16 | |||
| Alcohol Use | |||||
| Never | 99 | 12 | 0.245 | ||
| Ever | 494 | 39 | |||
| Colds per year | |||||
| <2 | 336 | 30 | 0.77 | ||
| ≥2 | 260 | 21 | |||
| Childhood History | |||||
| Yes | 22 | 12 | <0.001 | <0.001 | 6.99 (3.18–15.36) |
| No | 574 | 39 | |||
| Family History | |||||
| Yes | 100 | 10 | 0.564 | ||
| No | 496 | 41 |
Discussion
The mechanisms responsible for liver disease in AAT deficiency are being unraveled and translated into new therapeutic opportunities, but there is still a lack of knowledge as to which patients should be targeted for intervention.13, 14 A better understanding of the overall burden and risk for liver disease in AAT PI*ZZ adults is needed to direct screening, counseling, and appropriate therapies towards patients who could benefit most. As such, this study aimed to define the prevalence of ALT abnormalities and liver disease present in adult participants in the Alpha-1 DNA and Tissue Bank Registry.
Our major observations are: (1) Screening AAT deficient individuals for liver disease with simple liver function test may not be sufficient to detect disease (2) Childhood liver disease may identify those at risk of developing liver disease as an adult. (3) A large proportion (> 80%) of adults with AAT deficiency consume alcohol, although it was not associated with an increased risk of liver disease as defined by an elevated ALT.
The typical demographic features of the patients in this study are similar to the NHLBI registry: middle aged, Caucasian, previous smokers with moderate to severe pulmonary disease.15 The overall prevalence of liver disease at 7.9% which is similar to other survey and registry reports from the United States.16, 17 Cirrhosis was present in only 3.4% of the AAT individuals in the registry, and this may be related to the population studied and methods of ascertainment. The diagnosis of AAT deficiency is often made after respiratory symptoms persist over many years, giving those individuals a greater chance of being recruited to participate in a registry. In contrast, the mean survival is 2 years or less in individuals diagnosed with AAT deficiency after presenting with cirrhosis and portal hypertension.18 We can postulate that after the diagnosis of cirrhosis, individuals may not have an extended duration of recruitment for participation in the registry. We can also speculate that the mechanisms that damage the liver and lead to cirrhosis in AAT adults are asymptomatic. Unimpressive clinical symptoms lead to under detection by physicians and under reporting of liver disease by patients.
Currently the only prospective data available to evaluate the natural history of liver disease comes from the Swedish cohort of PI*ZZ individuals (n=127) identified at birth.5 Systematic reporting on these individuals has identified a pattern of liver test abnormalities that peak in early childhood, and then return to essentially normal by adolescence in the absence of clinical liver disease.6–8 Followed out to age 30, the prevalence of an abnormal ALT has steadily remained less than 10%.9 Our cross sectional data from a much larger number of AAT deficient patients suggests the prevalence of an abnormal ALT remains at 10% or less across all age groups, including a large number of patients over age 50 which should be at the highest risk for liver disease.19–21 Our data showed a relatively stable prevalence of liver disease across all age groups. The exception was in the 20–29 year old group that reported 50% with cirrhosis. The reason for this unexpected finding is not clear. We considered recall bias, but none of these individuals reported childhood liver disease. We also considered family history of AAT leading to early detection, but only one person noted this. More likely it is a reflection of the small numbers in this age group.
Current ATS guidelines recommend regular LFT testing to screen for liver disease in adults with AAT deficiency and pulmonary symptoms or those who are asymptomatic.4 However, data supporting this recommendation is lacking. The results of this study would suggest that ALT testing is not helpful to determine if liver disease is present. Average ALT values for the PI*ZZ individuals in the study fall within established normal limits for both men and women. The mean ALT was higher in men with liver disease; however, the values fell within the normal range, suggesting a statistically significant finding that was not clinically significant. Additionally, we found a background prevalence of ALT abnormalities of 4.5% in our control group without liver disease. When compared to the AAT deficient population, only the GGT was significantly higher. The significance of this is unknown. It could be that GGT is a more sensitive marker for liver disease in AAT deficiency, or it could be a reflection of the widespread alcohol use reported by this patient population. We do not have data on alcohol use in the control group for comparison.
The sensitivity of an ALT above the upper limit of normal was poor for the detection of liver disease. Sensitivity is defined as the ability of a test to correctly identify those who have the disease, and in this case, the sensitivity of an elevated ALT was only 11.9%. As a result, the positive predictive value (PPV) and LR+ were both low. Lowering the cutoff for a “normal” ALT improves the sensitivity of an elevated ALT to 52.4% at the expense of lowering the specificity. As a consequence, the PPV of an elevated ALT is actually lower (7.0%), and there is no advantage to using a lower threshold to detect liver disease. The results should not be surprising given that in many other liver diseases, ALT does not correlate with histology and can be normal in the presence of cirrhosis.22, 23
The genetic and environmental factors that place some patients at risk are ill defined. Age >50 and male gender have been consistently identified as risk, whereas the results on viral hepatitis and alcohol use are conflicting.16, 19, 24 Our findings support the increased risk for males. However, we were unable to confirm age as a risk factor for liver disease, which is at odds with most of the previous literature. This is especially important when considering that the study population includes a large number of people over age 50, who should be at the highest risk for cirrhosis. Despite the widespread use of alcohol in the entire cohort, alcohol use was not associated with liver disease. Significant differences in the usage patterns between those with and without liver disease were present, but the clinical impact of this could not be determined. Chronic alcohol use as a factor in the progression of other liver diseases is well known. Until the risks are further delineated, caution should be used in AAT patients. Interestingly, childhood liver disease was identified as a risk factor, which has not been reported before. If this result is confirmed with additional studies, it could be a simple way to identify and target asymptomatic adults for liver biopsy to stage fibrosis.
Our study has limitations. The Alpha-1 Foundation DNA and Tissue Bank Registry is not population based, which may restrict generalization of the results. Second, the diagnosis of liver disease and cirrhosis is based on reported medical histories and no liver histology was available to confirm the diagnosis and use as a reference standard. Therefore, our findings regarding validity of ALT testing in this population must be reproduced before a strong recommendation could be made against LFT testing in adults with AAT deficiency, especially since there are not good alternatives. As a cross sectional study, our findings represent a single time point, and perhaps serial testing could be informative. Finally, there is a possibility of recall bias when evaluating risk factors like childhood history of liver disease and alcohol use.
In summary, our results provide some interesting clinical insights into AAT deficiency and liver disease by using a large registry of patients with a rare disease. However, in order to confirm these findings and expand our knowledge about the natural history of liver disease in adults with AAT deficiency, a prospective study including histology must be performed.
Acknowledgments
Grant Support: This work supported in part by NIH/NCRR CTSA award to the University of Florida UL1 RR029890, NIH/NCI award K24CA139570, the Alpha-1 Foundation, and the Fundacion Leopoldo Fernandez Pujals
Abbreviations
- AAT
Alpha-1 Antitrypsin
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GGT
γ-glutamyl transpeptidase
- PI*ZZ
protease inhibitor homozygous
- LFT
liver function test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose.
Author Contributions: Virginia C. Clark, MD, MS: study concept and design, analysis and interpretation of the data, drafting of the manuscript; funding
Renumathy Dhanasekaran, MD: statistical analysis and interpretation of the data
Mark Brantly, MD: critical revision of the manuscript for intellectual content; funding
Farshid Rouhani, MS: acquisition of the data and technical support
Pam Schreck, RN, MSN: acquisition of the data and administrative support
David R. Nelson, MD: study concept and design, critical revision of the manuscript for intellectual content
Reference List
- 1.Blank CA, Brantly M. Clinical features and molecular characteristics of alpha 1-antitrypsin deficiency. Ann Allergy. 1994;72:105–120. [PubMed] [Google Scholar]
- 2.Teckman JH, Lindblad D. Alpha-1-antitrypsin deficiency: diagnosis, pathophysiology, and management. Curr Gastroenterol Rep. 2006;8:14–20. doi: 10.1007/s11894-006-0059-8. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1-antitrypsin deficiency. N Engl J Med. 1986;314:736–739. doi: 10.1056/NEJM198603203141202. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- 5.Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976;294:1316–1321. doi: 10.1056/NEJM197606102942404. [DOI] [PubMed] [Google Scholar]
- 6.Sveger T. Prospective study of children with alpha 1-antitrypsin deficiency: eight-year-old follow-up. J Pediatr. 1984;104:91–94. doi: 10.1016/s0022-3476(84)80599-5. [DOI] [PubMed] [Google Scholar]
- 7.Sveger T. The natural history of liver disease in alpha 1-antitrypsin deficient children. Acta Paediatr Scand. 1988;77:847–851. doi: 10.1111/j.1651-2227.1988.tb10767.x. [DOI] [PubMed] [Google Scholar]
- 8.Sveger T, Eriksson S. The liver in adolescents with alpha 1-antitrypsin deficiency. Hepatology. 1995;22:514–517. doi: 10.1002/hep.1840220221. [DOI] [PubMed] [Google Scholar]
- 9.Bernspang E, Carlson J, Piitulainen E. The liver in 30-year-old individuals with alpha(1)-antitrypsin deficiency. Scand J Gastroenterol. 2009;44:1349–1355. doi: 10.3109/00365520903296669. [DOI] [PubMed] [Google Scholar]
- 10.Browne RJ, Mannino DM, Khoury MJ. Alpha 1-antitrypsin deficiency deaths in the United States from 1979–1991. An analysis using multiple-cause mortality data. Chest. 1996;110:78–83. doi: 10.1378/chest.110.1.78. [DOI] [PubMed] [Google Scholar]
- 11.Stoller JK, Tomashefski J, Jr., Crystal RG, et al. Mortality in individuals with severe deficiency of alpha1-antitrypsin: findings from the National Heart, Lung, and Blood Institute Registry. Chest. 2005;127:1196–1204. doi: 10.1378/chest.127.4.1196. [DOI] [PubMed] [Google Scholar]
- 12.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 13.Ekeowa UI, Freeke J, Miranda E, et al. Defining the mechanism of polymerization in the serpinopathies. Proc Natl Acad Sci U S A. 2010;107:17146–17151. doi: 10.1073/pnas.1004785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidvegi T, Ewing M, Hale P, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–232. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- 15.McElvaney NG, Stoller JK, Buist AS, et al. Baseline characteristics of enrollees in the National Heart, Lung and Blood Institute Registry of alpha 1-antitrypsin deficiency. Alpha 1-Antitrypsin Deficiency Registry Study Group. Chest. 1997;111:394–403. doi: 10.1378/chest.111.2.394. [DOI] [PubMed] [Google Scholar]
- 16.Bowlus CL, Willner I, Zern MA, et al. Factors associated with advanced liver disease in adults with alpha1-antitrypsin deficiency. Clin Gastroenterol Hepatol. 2005;3:390–396. doi: 10.1016/s1542-3565(05)00082-0. [DOI] [PubMed] [Google Scholar]
- 17.Strange C, Stoller JK, Sandhaus RA, Dickson R, Turino G. Results of a survey of patients with alpha-1 antitrypsin deficiency. Respiration. 2006;73:185–190. doi: 10.1159/000088061. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson S. Alpha 1-antitrypsin deficiency and liver cirrhosis in adults. An analysis of 35 Swedish autopsied cases. Acta Med Scand. 1987;221:461–467. [PubMed] [Google Scholar]
- 19.Cox DW, Smyth S. Risk for liver disease in adults with alpha 1-antitrypsin deficiency. Am J Med. 1983;74:221–227. doi: 10.1016/0002-9343(83)90615-0. [DOI] [PubMed] [Google Scholar]
- 20.Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta Med Scand. 1978;204:345–351. doi: 10.1111/j.0954-6820.1978.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 21.Tanash HA, Nilsson PM, Nilsson JA, et al. Clinical course and prognosis of never-smokers with severe alpha-1-antitrypsin deficiency (PiZZ) Thorax. 2008;63:1091–1095. doi: 10.1136/thx.2008.095497. [DOI] [PubMed] [Google Scholar]
- 22.Hay JE, Czaja AJ, Rakela J, et al. The nature of unexplained chronic aminotransferase elevations of a mild to moderate degree in asymptomatic patients. Hepatology. 1989;9:193–197. doi: 10.1002/hep.1840090205. [DOI] [PubMed] [Google Scholar]
- 23.Haber MM, West AB, Haber AD, et al. Relationship of aminotransferases to liver histological status in chronic hepatitis C. Am J Gastroenterol. 1995;90:1250–1257. [PubMed] [Google Scholar]
- 24.Propst T, Propst A, Dietze O, et al. High prevalence of viral infection in adults with homozygous and heterozygous alpha 1-antitrypsin deficiency and chronic liver disease. Ann Intern Med. 1992;117:641–645. doi: 10.7326/0003-4819-117-8-641. [DOI] [PubMed] [Google Scholar]