Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are characterized by acute respiratory failure and are associated with diverse disorders, such as pulmonary edema, pneumonia, sepsis, trauma, shock and lung contusion. Gene therapy is a potentially powerful approach to treat a variety of diseases related to ALI/ARDS. Numerous viral and non-viral methods for gene delivery to the lung have been developed, although pulmonary architecture and immune activation represent barriers to successful gene transfer. In this review, recent advances in the development of more efficient viral and non-viral gene transfer systems are discussed. In addition, the current status of gene therapy applied to ALI/ARDS-associated pulmonary diseases is reviewed. With the development of more efficient gene therapy vectors, gene therapy is a promising strategy for clinical application in the not too distant future.
Keywords: Gene therapy, acute lung injury, viral vectors, non-viral vectors
I. INTRODUCTION
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are life-threatening conditions of acute respiratory failure, which is induced by direct and indirect injury to lung, such as pneumonia, sepsis and trauma. ALI/ARDS has a mortality rate of up to 40% in the United States, leading to 74,500 deaths and 3.6 million hospital days every year 1. Although many potential therapeutic approaches have been developed to control ALI/ARDS, these treatments have so far proven unable to decrease the mortality of patients with ALI/ARDS. While labs around the world have focused on the disease and uncovered a number of molecular mechanisms involved in its pathogenesis and resolution, translating this into productive treatments has lagged.
Gene therapy is a potentially powerful approach to treat any number of diseases including ALI/ARDS, but most approaches have serious limitations and thus have hampered the use of this technology in clinical medicine. Gene delivery approaches are based on two types of delivery vehicles: those based on viral systems, so-called viral vectors, and those not based on viruses, or non-viral vectors, which are typically plasmid-based. Viral vector systems have been associated with inflammation, immunological responses, and non-specificity of cell targeting, despite very high delivery efficiency in the lung. For example, adenovirus appears to be the most widely used vector for pulmonary gene therapy in the lab because of high efficiency transduction in a variety of target cells and high expression of the delivered genes. However, the use of adenovirus can result in inflammatory responses, which cause cell damage and limit repeated administration. By contrast, much less inflammation and immune responses are generated against non-viral DNA, but the major drawbacks to non-viral gene therapy in the lung are side effects of certain vectors and inefficiency of gene transfer, often leading to expression that is 10 to 1000-fold less than that seen with their viral counterparts.
Although transfer and expression of therapeutic genes to the lung using both viral and non-viral gene therapy technologies has been performed with some success, there is still a long way to go to move this methodology toward clinical use. In this review, we provide an overview of the current status of viral and non-viral gene therapy for ALI/ARDS, focus on issues of mechanism and applications as they influence in vivo gene delivery, and extend the utility of this strategy for future medical treatments.
II. Gene Delivery to the Lung
The lung is a complex organ and can be divided into the conducting large and small airways, including the trachea, bronchi, and bronchioles, and the parenchyma, which consists of gas-exchanging alveolar cells. Gene therapy is notably attractive for many acute and chronic pulmonary diseases. However, with the presence of barriers to lung gene transfer, such as pulmonary architecture, the innate immune system, and immune activation, it is somewhat more difficult and less effective to deliver genes into the parenchyma. As a consequence, many investigations have focused on improving gene transfer to the airway and alveolar epithelium to make it more efficient, less inflammatory, and to have extended duration of expression 2. To date, a number of viral and non-viral vector systems have been used to deliver transgenes into the lung to treat diverse pulmonary diseases 3.
A. Viral vectors for gene delivery to the lung
Adenovirus, perhaps the most widely used of vectors for lung gene therapy, is a double stranded DNA virus which is made to be replication-deficient for gene therapy by deletion of essential genes. The major advantages of adenovirus are the high-efficiency transduction seen in dividing and non-dividing cells and the very high expression of delivered genes. However, inflammation, immunological responses, and non-specificity of cell targeting are just a few of the problems associated with adenovirus vectors. Furthermore, immune responses developed against the viral vector limit the success of repeated administration (thus it can be used only once or twice in an individual for effective gene delivery) 4. Adenovirus can directly deliver genes to the airway and alveolar epithelia and have been be the vector of choice for animal models of many pulmonary diseases in the laboratory, but in clinic trials, the vector results in acute inflammation and innate immune responses, limiting effectiveness 5, 6. Further, the receptors for adenovirus reside primarily on the basolateral surface of epithelial cells in the lower airways, making high level gene transfer dependent on transient barrier dysfunction, which is not desirable in many disease states 7. Much effort has been directed at making vectors that show reduced host immune reactions to the viral gene products, so “gutless” or “helper-dependent” third-generation adenovirus vectors have been developed to extend expression and limit the initial inflammatory responses to administration 8. However, the safety issues surrounding this vector may outweigh its superior ability to transfer genes for widespread clinical use.
Another popular viral vector is based on adeno-associated virus (AAV), a non-pathogenic single stranded DNA virus of the dependovirus genus, which requires a helper virus (typically adenovirus) to complete its lytic life cycle 9. AAV is attractive because it has shown broad specificity of infection and persistent expression in the lung. The vector appears less inflammatory and elicits weaker immune responses than does adenovirus 10. Furthermore, Moss et al have demonstrated successful and safe repeated gene transfer of aerosolized AAV to the lungs of humans with cystic fibrosis 11. However, this vector has been significantly limited for lung gene transfer because of the small cloning capacity of the virus, the difficulty in getting high viral titers during production, and relatively low efficiency in human trials.
Retroviruses provide prolonged gene expression due to integration of the virus into the host genome, but require dividing cells for integration and show no transduction in the terminally differentiated, non-dividing epithelium 12. By contrast, lentiviruses, a subclass of retroviruses that are based on HIV (human immunodeficiency virus) and pseudotyped with the vesicular stomatitis virus (VSV) glycoprotein, can efficiently transfer genes to non-dividing cells and thus have been developed as gene transfer vectors 13. But again, similar to adenovirus, these vectors when pseudotyped with VSV glycoprotein demonstrate inefficient gene transfer to cells due to their receptor being expressed on the basolateral surface of the epithelium in vivo 14. However, Kobinger et al have shown that efficient transduction of the apical surface of the epithelium can be achieved in the mouse lung using an envelope protein derived from the Zaire strain of Ebola virus to pseudotype lentivirus vectors 15. In either case of retroviruses or lentiviruses, the necessity for integration to achieve effective levels of gene expression in vivo may also result in activation of oncogenes depending on the site of random integration.
B. Non-viral vectors for gene delivery to the lung
To combat inflammatory and immune responses mediated by viral vectors, non-viral vectors are an attractive alternative for gene therapy because of their ability to be repeatedly administered and their generally good safety profile 16. The primary non-viral vector systems constitute plasmids complexed with cationic lipids as lipoplexes or with polymers, such as diethylaminoethyl-dextran (DEAE-dextran) and polyethylenimine (PEI) to form polyplexes protecting naked DNA from degradation 17. Lipoplexes have proven to be an attractive tool for gene transfer into cells with respect to simplicity of use, ease of production, and low immunogenicity since their initial development by Felgner and colleagues in 1987 18, 19. However, the results from clinical trials have shown that lipoplexes do indeed generate inflammatory responses, albeit much less than do their viral counterparts, when they are used for gene therapy approaches in the lung 20, 21. This, taken together with their low efficiency for in vivo gene delivery in the lung, has limited their clinical potential. Similarly, the use of DEAE-dextran for gene delivery to the lung has largely been abandoned due to low levels of gene transfer, cellular toxicity and lack of biodegradability 22. PEI has shown some promise to enhance levels of gene transfer. In the presence of PEI, plasmid DNA condenses to form a DNA-PEI complex, which can protect DNA from degradation by serum nucleases during gene transfer to the lung in vivo. Once endocytosed into the cell, PEI induces osmotic swelling to promote endosomal escape and releases DNA into the cytoplasm, so it achieves greater levels of gene transfer to the lung. Unfortunately, PEI has been shown to interfere with transcriptional and translational processes and show mild to moderate toxicity (primarily due to low molecular weight PEI contaminants) and innate immune responses 23, 24. More recent, and as yet not completely vetted, systems include the use of glycoconjugates, targeting serpin-enzyme complex receptors, and nanoparticle formulations 25. Some groups have even combined viral vectors with liposomes containing dexamethasone-spermine (DS)/dioleoylphosphatidylethanolamine (DOPE) to improve targeting to the apical airway epithelium in vivo and to attenuate vector-induced inflammation 26.
The simplest non-viral vectors are naked plasmid DNA in which the specific sequences can uniquely manipulate transgene expression 27. It is an attractive approach for gene transfer to the lung due to reduced inflammatory and immune responses compared to liposome or polymer complexes 28. However, unprotected DNA is susceptible to nuclease degradation and thus it needs to rapidly cross the plasma membrane of target cells in lung tissue 17. Therefore, the efficiency of gene expression is restricted by quantity of naked DNA that reaches its target cells. Recent research from our laboratory and others has demonstrated that electroporation can be used to efficiently deliver DNA to various tissues, including the lung, with high-level gene expression and without damage 29-32. The method is simple, fast and safe: naked DNA is administered to the lungs of anesthetized animals and a series of eight consecutive, 10 millisecond square-wave electric pulses is applied to the lungs using electrodes placed on either side of the chest over a 10-second period (Figure 1). The application of electric pulses to the lung transiently opens pores in the cell membrane that allow plasmid DNA to enter the cell 29. Electroporation leads to safe, efficient, and reproducible transgene expression in the lung 28. Besides electroporation, effort has also been made to develop several other physical methods for non-viral gene delivery, including jet injection, the gene gun, hydrodynamic delivery, and sonoporation 33-36. As yet, their use in the lung has been limited.
Figure 1. Common elements of a gene therapy plasmid.

A typical plasmid is depicted showing control elements and expressed genes. Promoters that express for a short period of time (e.g., the CMViep), a long period of time (e.g., the UbC promoter), or in specific cell types (e.g., the SP-C promoter for expression in AT2 cells or the CC10 promoter for expression in clara cells) are used to drive expression of the gene of interest. In almost all cases, cDNA for the gene to be expressed is used instead of genomic sequence, thus eliminating all introns to make the gene smaller in size and allow for more efficient expression that does not require mRNA splicing and maturation. A DTS can be included downstream of the transgene to aid in general or cell-specific DNA nuclear import, and an antibiotic resistance gene is carried on the plasmid for maintenance and production of the plasmids in bacteria.
Although many investigators have demonstrated that DNA can be directly delivered to the lung to express genes for short periods, an effective delivery system should be able to express a transferred gene for long-term periods at therapeutic levels and with specificity of cell targeting if desired. To date, we and others have used electroporation to noninvasively deliver plasmid DNA into the lung parenchyma and shown either short term (1 to 7 days using the CMV immediate early promoter/enhancer) or long term (greater than 30 days using the UbC promoter) transgene expression 31, 37, 38. Thus, by choosing the appropriate promoters, the duration of expression can be well controlled (Figure 2). As for controlling the distribution of gene expression, electroporation is unlike all other gene delivery approaches in that delivery is not restricted to the surface layer of cells with which the vector comes into contact. Rather, it has the ability to transfer the non-viral vector across the epithelial barrier and into sub-epithelial cells below, reaching interstitial fibroblasts, airway and vascular smooth muscle cells, and even endothelial cells following DNA administration via the airways 31. Despite the ability to transfer genes to all cell types within a tissue, it is often very desirable, especially in the lung, to limit gene delivery and expression to specific cell targets. We have used two approaches to restrict delivery and expression to specific cell types in the lung: use of cell specific promoters to drive expression only in desired cells (ie, the smooth muscle gamma actin promoter for expression in smooth muscle cells) and the use of cell-specific DNA nuclear targeting sequences to limit delivery of plasmids to the nuclei of desired cell types. A number of years ago, our lab demonstrated that plasmids can gain entry into the nuclei of non-dividing cells only if they carry unique DNA sequences termed DNA nuclear targeting sequences (DTS) 39-41. These sequences interact with cytoplasmically localized transcription factors and the protein nuclear import machinery to traffic the plasmid-protein complex into the nucleus through the nuclear pore complex. Several such sequences that act in all cell types have been identified. Further, a number of sequences that act in specific cell types due to the fact that they bind cell-specific transcription factors that are only expressed in those cells have also been identified. For example, we have demonstrated that a sequence within the human surfactant protein C (SP-C) promoter is able to mediate nuclear localization of plasmid DNA specifically in type II alveolar epithelial (AT2) cells but not in other cell types both in cultured cells and in the mouse lung 42. More recently, we have identified additional DTS that restrict plasmid delivery to multiple cell types in the lung, including smooth muscle cells 43-45, endothelial cells 46, and type I alveolar epithelial cells, all potential targets for gene therapy for ALI/ARDS and other lung diseases.
Figure 2. In-vivo pulmonary electroporation.
A. Cartoon for in vivo plasmid delivery and electroporation for the lungs showing endotracheal tube and electrodes. B and C. GFP-β1 expressing plasmids (600 μg) were administered to the lungs and electroporated (200 V/cm, 8 pulses at 10 μsec each). Three days later, the lungs were visualized in situ (B) and GFP-β1 expression was detected by fluorescence microscopy (C). Taken with permission from ref. 54.
III. Gene Therapy for ALI/ARDS
ALI and ARDS are clinically characterized by acute hypoxemic respiratory failure, caused by direct injury, including viral or bacterial infections, gastric aspiration, trauma, and hyperoxia, and indirect injury to the lung, such as in sepsis. ALI/ARDS is subdivided into the acute exudative phase and the later fibroproliferative phase 47. The exudative phase of ALI/ARDS is initially characterized by disruption of the alveolar-capillary interface and leakage of protein-rich edema fluid into the interstitium and alveolar space, followed by extensive release of multiple inflammatory cytokines and chemokines and neutrophil infiltration. The later phase of ALI/ARDS is characterized by fibroproliferation and organization of previously deposited exudates. Over the past 20 years, the feasibility of using gene transfer to treat ALI/ARDS has been demonstrated using a variety of viral and non-viral vectors to deliver various transgenes to the lung in multiple animal models, although none have moved to clinical trials.
A. Gene Therapy for Improved alveolar fluid clearance
ALI/ARDS are characterized by abnormal accumulation of protein-rich edema fluid in the alveolar spaces, caused by increased movement of fluid from the capillaries to the lung interstitium, and decreased fluid transport out of alveolar air spaces 48. Injury to the alveolar epithelium plays an important role in the pathogenesis of increased alveolar fluid because 99% of the surface area of lung is comprised by the alveolar epithelium, which is the major site for the removal of excess alveolar fluid 49. Therefore, treatments to improve alveolar epithelial function might become one of the key therapeutic strategies to accelerate recovery and decrease the mortality of ALI/ARDS patients.
The alveolar epithelial monolayer consists of flat type I alveolar epithelial (AT1) cells, which comprise approximately 90% of the alveolar surface area, and cuboidal type II alveolar epithelial (AT2) cells, which produce surfactant proteins, vectorially transport ions, and act as progenitor cells to regenerate the epithelium after injury. The presence of multiple ion transporters, including the epithelial Na+ channel (ENaC), the Na+, K+ transporting adenosine-5′-triphosphatase (Na,K-ATPase), the cystic fibrosis trans-membrane conductance regulator (CFTR), and several K+ channels in both AT1 and AT2 cells creates a trans-epithelial osmotic gradient formed by the active movement of Na+, which is needed for the transit of edema fluid from the alveolar airspace. It is well accepted that alveolar fluid clearance is driven by sodium transport entering the alveolar epithelial cell via ENaC on the apical surface and then being pumped out by the Na,K-ATPase within the basolateral surface into the interstitium and the pulmonary circulation 50. Studies have demonstrated that overexpression of genes such as ENaC and Na,K-ATPase regulated by β-adrenergic receptor or cAMP can enhance alveolar active transport to clear pulmonary edema 51-54. In order for gene transfer to be useful, it is necessary to transduce the alveolar epithelium. Both viral and non-viral approaches for the overexpression of Na,K-ATPase and CFTR channels in the lung as well as the delivery of keratinocyte growth factor and hemoxygenase-1 to the lung have been tested with the aim of increasing alveolar fluid clearance. Gene therapy may prove to be an important alternative for the treatment and prevention of pulmonary edema by restoring alveolar epithelial function.
It has been demonstrated previously that transfer of the α2- or β1-subunit of Na,K-ATPase using recombinant adenoviruses can increase Na,K-ATPase expression and alveolar fluid clearance function in normal rat lungs and in rat lungs injured by ventilation 55-58. Factor and colleagues showed that adenovirus-mediated gene transfer of the Na,K-ATPase β1-subunit to the alveolar epithelium improved alveolar fluid clearance in rat models of ALI induced by ventilation 59, acutely elevated left atrial pressure 60and hyperoxia 56. Similarly, Qiao et al. demonstrated that lentivirus-mediated gene transfer of the β1, not α 1, subunit of the Na,K-ATPase to rat primary alveolar epithelial cells can augment the activity of the Na,K-ATPase, indicating augmentation of alveolar fluid clearance 61. Adenoviruses were able to transfer genes to the pulmonary epithelium after hyperoxia-mediated acute lung injury as efficiently as they could in healthy rat lungs, a prerequisite for any potential treatment option that would have to be given to patients with pre-existing disease 62. In addition, adenovirus-mediated transfer of the β2-adrenergic receptor gene to rat lungs enhanced their sensitivity to catecholamines and resulted in increases in both ENaC and Na,K-ATPase expression, leading to increased alveolar fluid clearance 52, 63, 64. Transfer of CFTR gene by adenovirus to the alveolar epithelium of normal rats and mice accelerated alveolar fluid clearance due to increased expression and function of components of the active Na+ transport machinery as well 65. Taking a different approach to alter endogenous expression of ion channels, another group showed that adenoviral overexpression of superoxide dismutase could prevent the hypoxia-mediated decrease in alveolar fluid reabsorption due to decreased Na,K-ATPase levels at the membrane (since hypoxia normally inhibits Na,K-ATPase activity, preventing ROS production and accumulation by gene transfer of superoxide dismutase abrogated the effects of hypoxia) 66.
However, a drawback to all these in vivo viral vector studies is that the effects of gene transfer on protection from injury (none tested whether gene therapy could be used to treat existing ALI/ARDS) could only be measured experimentally 7 days after gene delivery because of the induction of inflammatory responses by the viral vector. In other words, use of these viral vectors caused profound inflammatory responses in healthy animals; using similar vectors to treat what is largely an inflammatory disease could trip the balance to endanger animals or patients with pre-existing disease. As such, the use of viral vectors for ALI/ARDS may not be the most promising approach, and the much less inflammatory non-viral approaches may be better suited for this disease.
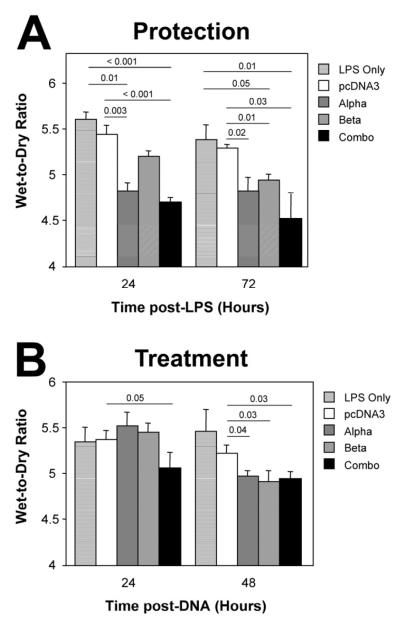
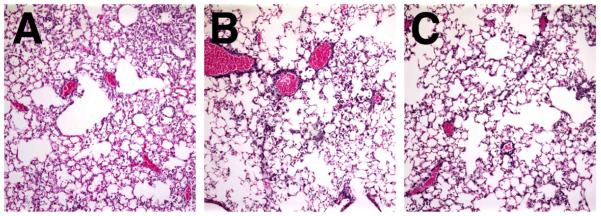
By contrast, the liposome-DNA complexes were primarily thought to induce weaker immunological responses than viral vectors. Indeed, Eric Alton’s group showed that liposome-mediated gene transfer of α- and β-subunits of the Na,K-ATPase can protect from subsequent thiourea-induced pulmonary edema in mice due to increased Na,K-ATPase activity 67. In this study, very little inflammation was noted following delivery of the plasmid-liposome complexes. Another approach to upregulate edema clearance has been to use growth factors. Keratinocyte growth factor (KGF) is a growth factor that has been shown to be an AT2 mitogen and can increase active ion transport in the lung through transcriptional upregulation of the Na+-K+-ATPase. Work from Alton’s group has shown that gene transfer of KGF using liposomes into mouse lungs protected animals from oleic acid-induced lung injury, albeit much less than administration of recombinant protein itself, suggesting that the efficacy of gene delivery is still not optimal 68. In support of this, the use of adenovirus to transfer KGF resulted in greater levels of KGF expression, significant proliferation of AT2 cells, and greater protection in a hyperoxia model of ALI 69. Research from our lab and others has demonstrated that electroporation can be used to effectively deliver “naked” DNA to living animal lungs with no damage and high-level gene expression 70-74. Indeed, after electroporation-mediated transfer of β1-subunit of Na,K-ATPase to the lungs of healthy rats, alveolar fluid clearance rates can be increased almost twofold, similar to using adenovirus expressing the same gene 54. More recently, we have also shown that transfer of genes encoding α and/or β subunits of the Na,K-ATPase using electroporation not only protects from subsequent endotoxin-induced lung injury in mice, but also can be used to treat previously injured lungs with existing pulmonary edema and neutrophil infiltrates by up-regulating mechanisms of pulmonary edema clearance (Figure 3) 51. Not only were rates of alveolar fluid clearance increased in LPS-treated mice at the height of injury when plasmids expressing the Na,K-ATPase subunit genes were delivered by electroporation, but the number of infiltrating neutrophils and alveolar macrophages were decreased in these treated animals and the lungs appeared less injured by histology (Figure 4) 51.
Figure 3. Electroporation-mediated gene transfer of subunits of the Na+,K+-ATPase can both protect from subsequent LPS-induced lung injury and reverse pre-existing LPS-induced lung injury.
A. Protection Studies. One hundred μg of plasmid in 50 μl were administered intratracheally to mice by electroporation. One day later, LPS (4 mg/kg) was administered to the lungs and 24 or 27 hours after this, lungs were removed for wet-to-dry ratio analysis (mean ± sem; n=5). B. Treatment Studies. LPS (4 mg/kg) was administered intratracheally to Balb/c mice and one day later, 100 μg of plasmid was delivered to the lungs by electroporation. Twenty-four or forty-eight hours later, lungs were removed for gravimetric analysis. Wet-to-dry ratios are shown as mean ± SEM (n=5). Statistical analysis was by non-parametric Mann-Whitney U test. Taken with permission from ref ,51.
Figure 4. Electroporation-mediated gene transfer of the Na+,K+-ATPase shows reduced pulmonary infiltrates in LPS-injured lungs.
LPS (4 mg/kg) was administered intratracheally to Balb/c mice and one day later, 100 μg of plasmid was delivered to the lungs by electroporation. Forty-eight hours later, lungs were removed and processed for histology using hematoxylin and eosin staining. A. LPS only. B. LPS followed by pCMV-β1. C. LPS followed by a mixture of pCMV-α1 and pCMV-β1(50 μg of each).
B. Gene Therapy Targeted at Sepsis and Pulmonary Inflammation
The development of ALI results from both direct insults to the lung such as pneumonia, aspiration or lung contusion, as well as from indirect pulmonary insults such as extra-pulmonary sepsis, trauma, and shock. Consequently, indirect treatment of ALI could be achieved by gene therapy approaches to limit or prevent these insults. Pneumonia is common, resulting from a variety of causes, including infection with bacteria, virus, and fungi and can contribute to the morbidity and mortality associated with ALI/ARDS. Multiple cytokines and chemokines are released during infections resulting in acute respiratory syndromes, such as hemophagocytic syndrome and lymphoid depletion. It is possible that these elevated levels of cytokines and chemokines are associated with increased severity of pneumonia and subsequent ALI. Gene therapy may be a powerful method to deliver cytokines and chemokines to the lung to modulate the inflammatory environment to treat pneumonia and the ensuing ALI.
A number of groups have used gene delivery to overexpress various cytokines and chemokines in the lung to directly attenuate inflammation caused by infection. These include the anti-inflammatory cytokines interleukin-10 75, IFN protein-10 (IP-10) 76, interleukin-12 77, and transforming growth factor beta-1 (TGF-β1)78. Despite being transferred with adenoviral vectors which can exacerbate the inflammatory response, delivery of all of these anti-inflammatory cytokines have improved survival in mice and reduced inflammation, as predicted. Several other less obvious approaches have also been taken to limit inflammation or control immune responses in the lung in response to infection. For example, one group used 4-1BB ligand (4-1BBL) to protect from lung injury caused by influenza infection in the lung 79. 4-1BBL binds to 4-1BB (CD137), a member of the TNF receptor family expressed on activated T cells that is rapidly up-regulated on T cells following viral infection. Binding and signaling of 4-1BBL leads to increased T cell expansion, and T cell trafficking to and retention in the lungs. Gene transfer of 4-1BBL in a mouse influenza infection model showed greater control of the infection and reduced lung injury. Another approach has been to use hemoxygenase-1 (HO-1) to modulate neutrophil activity. While the main function of hemoxygenase-1 (HO-1) is to regulate heme metabolism, evidence suggests that the enzyme is also involved in controlling infiltration of neutrophils into the injured lung and in the resolution of inflammation by modulating apoptotic cell death and cytokine expression. Consequently, several groups have delivered HO-1 expressing adenoviruses to the lungs in both pneumonia and hyperoxia models and have shown significant reductions in inflammation and subsequent lung injury 80-82. At least part of this effect appears to be mediated through the anti-inflammatory cytokine IL-10 whose expression is greatly upregulated when HO-1 is overexpressed 82. Finally, Weiss et al. demonstrated that adenovirus-mediated gene delivery of heat shock protein-70 to the pulmonary epithelium can protect from cecal ligation and double puncture, a clinically relevant model of sepsis and ARDS, mediated lung injury in mice 83.
As alluded to above, although a number of studies indicate that viral vectors are promising candidates for gene therapy to control these direct or indirect pulmonary infections, inflammation can be actually be accelerated by the viral vector systems carrying the desired transgenes. Therefore, major efforts have been directed toward developing non-viral vectors for these purposes. For example, the β-defensin-2 delivered to rat lungs by polyethylenimine can effectively inhibit Pseudomonas aeruginosa infection in the lung 84. However, certain non-viral lipid or polymer formulations can also induce varying levels of inflammatory cytokines and their efficiency of gene transfer remains low. Recently, electroporation has gained increasing attention for development of non-viral gene therapies and vaccines. Using skeletal muscle as bioreactors, it has been shown that vaccines can be delivered by electroporation to protect from bacteria- or virus-induced pneumonia 85, 86. Taken together, these non-viral approaches may indeed show great promise for treating pulmonary infections leading to and/or resulting from ALI/ARDS as long as gene transfer efficiencies are improved.
Summary and Conclusions
Extensive research on ALI/ARDS, using animal models and humans, has revealed a variety of molecular mechanisms that contribute directly and indirectly to the pathogenesis of these diseases. Consequently, the opportunities for the use of gene therapy are great. It is likely that gene therapy will become a novel therapeutic approach in clinical medicine for treating a variety of inherited and acquired lung diseases in the near future, if concerns for safety and high level gene expression are kept in balance. Although the technology is rapidly evolving, the limitation in development of gene therapy for ALI/ARDS is not at the level of the understanding of molecular mechanisms of the disease but rather at the level of drug delivery technology. Improved vectors and delivery systems that are nontoxic, non-inflammatory, allow repeated dosing, produce therapeutic levels of gene product, and do so in specific cells are needed. While each technology may address one or more of these problems, none to date have solved all of them. It is likely that combinations of approaches will be used for successful treatment. With the development of these more efficient gene therapy approaches, the first clinical use of therapeutic gene therapy shown to be safe and efficacious for treatment of ALI/ARDS, is not far off.
Acknowledgments
This project was supported by grants HL81148, HL92801, EB9903, and GM94228
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Kolb M, Martin G, Medina M, Ask K, Gauldie J. Gene therapy for pulmonary diseases. Chest. 2006 Sep;130(3):879–884. doi: 10.1378/chest.130.3.879. [DOI] [PubMed] [Google Scholar]
- 3.Geiger J, Aneja MK, Rudolph C. Vectors for pulmonary gene therapy. Int J Pharm. 2010 May 5;390(1):84–88. doi: 10.1016/j.ijpharm.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Kushwah R, Cao H, Hu J. Potential of helper-dependent adenoviral vectors in modulating airway innate immunity. Cell Mol Immunol. 2007 Apr;4(2):81–89. [PubMed] [Google Scholar]
- 5.Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004 Dec;15(12):1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 6.Innate immune response induced by gene delivery vectors. Int J Pharm. 2008 Apr 16;354(1-2):9–15. doi: 10.1016/j.ijpharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Grubb BR, Pickles RJ, Ye H, et al. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994 Oct 27;371(6500):802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- 8.Koehler DR, Martin B, Corey M, et al. Readministration of helper-dependent adenovirus to mouse lung. Gene Ther. 2006 May;13(9):773–780. doi: 10.1038/sj.gt.3302712. [DOI] [PubMed] [Google Scholar]
- 9.Conway JE, Zolotukhin S, Muzyczka N, Hayward GS, Byrne BJ. Recombinant adeno-associated virus type 2 replication and packaging is entirely supported by a herpes simplex virus type 1 amplicon expressing Rep and Cap. J Virol. 1997 Nov;71(11):8780–8789. doi: 10.1128/jvi.71.11.8780-8789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tal J. Adeno-associated virus-based vectors in gene therapy. J Biomed Sci. 2000 Jul-Aug;7(4):279–291. doi: 10.1007/BF02253246. [DOI] [PubMed] [Google Scholar]
- 11.Moss RB, Rodman D, Spencer LT, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004 Feb;125(2):509–521. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt JF, Yankaskas JR, Wilson JM. In vivo retroviral gene transfer into human bronchial epithelia of xenografts. J Clin Invest. 1992 Dec;90(6):2598–2607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trono D. Lentiviral vectors: turning a deadly foe into a therapeutic agent. Gene Ther. 2000 Jan;7(1):20–23. doi: 10.1038/sj.gt.3301105. [DOI] [PubMed] [Google Scholar]
- 14.Pickles RJ. Physical and biological barriers to viral vector-mediated delivery of genes to the airway epithelium. Proc Am Thorac Soc. 2004;1(4):302–308. doi: 10.1513/pats.200403-024MS. [DOI] [PubMed] [Google Scholar]
- 15.Kobinger GP, Weiner DJ, Yu QC, Wilson JM. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001 Mar;19(3):225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- 16.Lam AP, Dean DA. Progress and prospects: nuclear import of nonviral vectors. Gene Ther. 2010 Apr;17(4):439–447. doi: 10.1038/gt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller AM, Dean DA. Tissue-specific and transcription factor-mediated nuclear entry of DNA. Adv Drug Deliv Rev. 2009 Jul 2;61(7-8):603–613. doi: 10.1016/j.addr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Liggitt D, Zhong W, Tu G, Gaensler K, Debs R. Cationic liposome-mediated intravenous gene delivery. J Biol Chem. 1995 Oct 20;270(42):24864–24870. doi: 10.1074/jbc.270.42.24864. [DOI] [PubMed] [Google Scholar]
- 20.Caplen NJ, Alton EW, Middleton PG, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995 Jan;1(1):39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 21.Gill DR, Southern KW, Mofford KA, et al. A placebo-controlled study of liposome-mediated gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 1997 Mar;4(3):199–209. doi: 10.1038/sj.gt.3300391. [DOI] [PubMed] [Google Scholar]
- 22.De Smedt SC, Demeester J, Hennink WE. Cationic polymer based gene delivery systems. Pharm Res. 2000 Feb;17(2):113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- 23.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001 Mar;22(5):471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 24.Chollet P, Favrot MC, Hurbin A, Coll JL. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J Gene Med. 2002 Jan-Feb;4(1):84–91. doi: 10.1002/jgm.237. [DOI] [PubMed] [Google Scholar]
- 25.Davis PB, Cooper MJ. Vectors for airway gene delivery. AAPS J. 2007;9(1):E11–17. doi: 10.1208/aapsj0901002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price A, Limberis M, Gruneich JA, Wilson JM, Diamond SL. Targeting viral-mediated transduction to the lung airway epithelium with the anti-inflammatory cationic lipid dexamethasone-spermine. Mol Ther. 2005 Sep;12(3):502–509. doi: 10.1016/j.ymthe.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Herweijer H, Wolff JA. Progress and prospects: naked DNA gene transfer and therapy. Gene Ther. 2003 Mar;10(6):453–458. doi: 10.1038/sj.gt.3301983. [DOI] [PubMed] [Google Scholar]
- 28.Zhou R, Norton JE, Zhang N, Dean DA. Electroporation-mediated transfer of plasmids to the lung results in reduced TLR9 signaling and inflammation. Gene Ther. 2007 May;14(9):775–780. doi: 10.1038/sj.gt.3302936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somiari S, Glasspool-Malone J, Drabick JJ, et al. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000;2:178–187. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 30.Conwell CC, Huang L. Recent Advances in Non-viral Gene Delivery. Adv Genet. 2005;53PA:1–18. doi: 10.1016/S0065-2660(05)53001-3. [DOI] [PubMed] [Google Scholar]
- 31.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther. 2003 Sep;10(18):1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou R, Norton JE, Dean DA. Electroporation-mediated gene delivery to the lungs. Methods Mol Biol. 2008;423:233–247. doi: 10.1007/978-1-59745-194-9_17. [DOI] [PubMed] [Google Scholar]
- 33.Walther W, Siegel R, Kobelt D, et al. Novel jet-injection technology for nonviral intratumoral gene transfer in patients with melanoma and breast cancer. Clin Cancer Res. 2008 Nov 15;14(22):7545–7553. doi: 10.1158/1078-0432.CCR-08-0412. [DOI] [PubMed] [Google Scholar]
- 34.Goudy KS, Wang B, Tisch R. Gene gun-mediated DNA vaccination enhances antigen-specific immunotherapy at a late preclinical stage of type 1 diabetes in nonobese diabetic mice. Clin Immunol. 2008 Oct;129(1):49–57. doi: 10.1016/j.clim.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007 Dec;15(12):2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 36.Sheyn D, Kimelman-Bleich N, Pelled G, Zilberman Y, Gazit D, Gazit Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2008 Feb;15(4):257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 37.Machado-Aranda D, Adir Y, Young JL, et al. Gene transfer of the Na+,K+-ATPase b1 subunit using electroporation increases lung liquid clearance in rats. Am J Respir Crit Care Med. 2005;171:204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazdhar A, Bilici M, Pierog J, et al. In vivo electroporation and ubiquitin promoter--a protocol for sustained gene expression in the lung. J Gene Med. 2006 Jul;8(7):910–918. doi: 10.1002/jgm.911. [DOI] [PubMed] [Google Scholar]
- 39.Dean DA. Import of plasmid DNA into the nucleus is sequence specific. Exp. Cell Res. 1997;230:293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- 40.Dean DA, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear entry. Exp. Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean DA, Strong DD, Zimmer WE. Nuclear entry of nonviral vectors. Gene Ther. 2005 Jun;12(11):881–890. doi: 10.1038/sj.gt.3302534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degiulio JV, Kaufman CD, Dean DA. The SP-C promoter facilitates alveolar type II epithelial cell-specific plasmid nuclear import and gene expression. Gene Ther. 2010 Apr;17(4):541–549. doi: 10.1038/gt.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller AM, Dean DA. Cell-specific nuclear import of plasmid DNA in smooth muscle requires tissue-specific transcription factors and DNA sequences. Gene Ther. 2008;15(15):1107–1115. doi: 10.1038/gt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vacik J, Dean BS, Zimmer WE, Dean DA. Cell-specific nuclear import of plasmid DNA. Gene Therapy. 1999;6:1006–1014. doi: 10.1038/sj.gt.3300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young JL, Zimmer WE, Dean DA. Smooth muscle-specific gene delivery in the vasculature based on restriction of DNA nuclear import. Exp Biol Med. 2008;233(7):840–848. doi: 10.3181/0712-RM-331. [DOI] [PubMed] [Google Scholar]
- 46.Dean DA. Nucleocytoplasmic trafficking. In: Mahato RI, editor. Pharmaceutical perspectives of nucleic acid-based therapeutics. Harwood Academic Publishers; London: 2002. pp. 229–260. [Google Scholar]
- 47.Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem. 2008;15(19):1911–1924. doi: 10.2174/092986708785132942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu KD, Matthay MA. Advances in critical care for the nephrologist: acute lung injury/ARDS. Clin J Am Soc Nephrol. 2008 Mar;3(2):578–586. doi: 10.2215/CJN.01630407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol. 2005 Nov;289(5):L685–695. doi: 10.1152/ajplung.00247.2005. [DOI] [PubMed] [Google Scholar]
- 50.Budinger GR, Sznajder JI. The alveolar-epithelial barrier: a target for potential therapy. Clin Chest Med. 2006 Dec;27(4):655–669. doi: 10.1016/j.ccm.2006.06.007. abstract ix. [DOI] [PubMed] [Google Scholar]
- 51.Mutlu GM, Machado-Aranda D, Norton JE, et al. Electroporation-mediated gene transfer of the Na+,K+ -ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med. 2007 Sep 15;176(6):582–590. doi: 10.1164/rccm.200608-1246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutlu GM, Dumasius V, Burhop J, et al. Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling. Circ Res. 2004 Apr 30;94(8):1091–1100. doi: 10.1161/01.RES.0000125623.56442.20. [DOI] [PubMed] [Google Scholar]
- 53.Snyder PM. Liddle’s syndrome mutations disrupt cAMP-mediated translocation of the epithelial Na(+) channel to the cell surface. J Clin Invest. 2000 Jan;105(1):45–53. doi: 10.1172/JCI7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machado-Aranda D, Adir Y, Young JL, et al. Gene transfer of the Na+,K+-ATPase beta1 subunit using electroporation increases lung liquid clearance. Am J Respir Crit Care Med. 2005 Feb 1;171(3):204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Factor P, Saldias F, Ridge K, et al. Augmentation of lung liquid clearance via adenovirus-mediated transfer of a Na,K-ATPase beta1 subunit gene. J Clin Invest. 1998 Oct 1;102(7):1421–1430. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Factor P, Dumasius V, Saldias F, Brown LA, Sznajder JI. Adenovirus-mediated transfer of an Na+/K+-ATPase beta1 subunit gene improves alveolar fluid clearance and survival in hyperoxic rats. Hum Gene Ther. 2000 Nov 1;11(16):2231–2242. doi: 10.1089/104303400750035753. [DOI] [PubMed] [Google Scholar]
- 57.Ridge KM, Olivera WG, Saldias F, et al. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003 Mar 7;92(4):453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 58.Adir Y, Welch LC, Dumasius V, Factor P, Sznajder JI, Ridge KM. Overexpression of the Na-K-ATPase alpha2-subunit improves lung liquid clearance during ventilation-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2008 Jun;294(6):L1233–1237. doi: 10.1152/ajplung.00076.2007. [DOI] [PubMed] [Google Scholar]
- 59.Adir Y, Factor P, Dumasius V, Ridge KM, Sznajder JI. Na,K-ATPase gene transfer increases liquid clearance during ventilation-induced lung injury. Am J Respir Crit Care Med. 2003 Dec 15;168(12):1445–1448. doi: 10.1164/rccm.200207-702OC. [DOI] [PubMed] [Google Scholar]
- 60.Azzam ZS, Dumasius V, Saldias FJ, Adir Y, Sznajder JI, Factor P. Na,K-ATPase overexpression improves alveolar fluid clearance in a rat model of elevated left atrial pressure. Circulation. 2002 Jan 29;105(4):497–501. doi: 10.1161/hc0402.102848. [DOI] [PubMed] [Google Scholar]
- 61.Qiao R, Zhou B, Harboe-Schmidt E, et al. Subunit-specific coordinate upregulation of sodium pump activity in alveolar epithelial cells by lentivirus-mediated gene transfer. Hum Gene Ther. 2004 May;15(5):457–468. doi: 10.1089/10430340460745784. [DOI] [PubMed] [Google Scholar]
- 62.Factor P, Mendez M, Mutlu GM, Dumasius V. Acute hyperoxic lung injury does not impede adenoviral-mediated alveolar gene transfer. Am J Respir Crit Care Med. 2002 Feb 15;165(4):521–526. doi: 10.1164/ajrccm.165.4.2101016. [DOI] [PubMed] [Google Scholar]
- 63.Dumasius V, Jameel M, Burhop J, et al. In vivo timing of onset of transgene expression following adenoviral-mediated gene transfer. Virology. 2003 Apr 10;308(2):243–249. doi: 10.1016/s0042-6822(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 64.Dumasius V, Sznajder JI, Azzam ZS, et al. beta(2)-adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ Res. 2001 Nov 9;89(10):907–914. doi: 10.1161/hh2201.100204. [DOI] [PubMed] [Google Scholar]
- 65.Mutlu GM, Adir Y, Jameel M, et al. Interdependency of beta-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res. 2005 May 13;96(9):999–1005. doi: 10.1161/01.RES.0000164554.21993.AC. [DOI] [PubMed] [Google Scholar]
- 66.Litvan J, Briva A, Wilson MS, Budinger GR, Sznajder JI, Ridge KM. Beta-adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na,K-ATPase and alveolar fluid reabsorption. J Biol Chem. 2006 Jul 21;281(29):19892–19898. doi: 10.1074/jbc.M602064200. [DOI] [PubMed] [Google Scholar]
- 67.Stern M, Ulrich K, Robinson C, et al. Pretreatment with cationic lipid-mediated transfer of the Na+K+-ATPase pump in a mouse model in vivo augments resolution of high permeability pulmonary oedema. Gene Ther. 2000 Jun;7(11):960–966. doi: 10.1038/sj.gt.3301193. [DOI] [PubMed] [Google Scholar]
- 68.Ulrich K, Stern M, Goddard ME, et al. Keratinocyte growth factor therapy in murine oleic acid-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005 Jun;288(6):L1179–1192. doi: 10.1152/ajplung.00450.2004. [DOI] [PubMed] [Google Scholar]
- 69.Baba Y, Yazawa T, Kanegae Y, et al. Keratinocyte growth factor gene transduction ameliorates acute lung injury and mortality in mice. Hum Gene Ther. 2007 Feb;18(2):130–141. doi: 10.1089/hum.2006.137. [DOI] [PubMed] [Google Scholar]
- 70.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level nonviral gene transfer to the lung. Gene Ther. 2003 Sep;10(18):1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Somiari S, Glasspool-Malone J, Drabick JJ, et al. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000 Sep;2(3):178–187. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 72.Dean DA. Electroporation of the vasculature and the lung. DNA Cell Biol. 2003 Dec;22(12):797–806. doi: 10.1089/104454903322625000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Favard C, Dean DS, Rols MP. Electrotransfer as a non viral method of gene delivery. Curr Gene Ther. 2007 Feb;7(1):67–77. doi: 10.2174/156652307779940207. [DOI] [PubMed] [Google Scholar]
- 74.Young JL, Zimmer WE, Dean DA. Smooth muscle-specific gene delivery in the vasculature based on restriction of DNA nuclear import. Exp Biol Med (Maywood) 2008 Jul;233(7):840–848. doi: 10.3181/0712-RM-331. [DOI] [PubMed] [Google Scholar]
- 75.Buff SM, Yu H, McCall JN, et al. IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia. Gene Ther. 2010 May;17(5):567–576. doi: 10.1038/gt.2010.28. [DOI] [PubMed] [Google Scholar]
- 76.McAllister F, Ruan S, Steele C, et al. CXCR3 and IFN protein-10 in Pneumocystis pneumonia. J Immunol. 2006 Aug 1;177(3):1846–1854. doi: 10.4049/jimmunol.177.3.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruan S, McKinley L, Zheng M, et al. Interleukin-12 and host defense against murine Pneumocystis pneumonia. Infect Immun. 2008 May;76(5):2130–2137. doi: 10.1128/IAI.00065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mora BN, Boasquevisque CH, Boglione M, et al. Transforming growth factor-beta1 gene transfer ameliorates acute lung allograft rejection. J Thorac Cardiovasc Surg. 2000 May;119(5):913–920. doi: 10.1016/s0022-5223(00)70086-9. [DOI] [PubMed] [Google Scholar]
- 79.Lin GH, Sedgmen BJ, Moraes TJ, Snell LM, Topham DJ, Watts TH. Endogenous 4-1BB ligand plays a critical role in protection from influenza-induced disease. J Immunol. 2009 Jan 15;182(2):934–947. doi: 10.4049/jimmunol.182.2.934. [DOI] [PubMed] [Google Scholar]
- 80.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999 Apr;103(7):1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hashiba T, Suzuki M, Nagashima Y, et al. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 2001 Oct;8(19):1499–1507. doi: 10.1038/sj.gt.3301540. [DOI] [PubMed] [Google Scholar]
- 82.Inoue S, Suzuki M, Nagashima Y, et al. Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Hum Gene Ther. 2001 May 20;12(8):967–979. doi: 10.1089/104303401750195926. [DOI] [PubMed] [Google Scholar]
- 83.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002 Sep;110(6):801–806. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu Q, Zuo P, Shao B, et al. Administration of nonviral gene vector encoding rat beta-defensin-2 ameliorates chronic Pseudomonas aeruginosa lung infection in rats. J Gene Med. 2010 Mar;12(3):276–286. doi: 10.1002/jgm.1435. [DOI] [PubMed] [Google Scholar]
- 85.Thacker EL, Holtkamp DJ, Khan AS, Brown PA, Draghia-Akli R. Plasmid-mediated growth hormone-releasing hormone efficacy in reducing disease associated with Mycoplasma hyopneumoniae and porcine reproductive and respiratory syndrome virus infection. J Anim Sci. 2006 Mar;84(3):733–742. doi: 10.2527/2006.843733x. [DOI] [PubMed] [Google Scholar]
- 86.Saha S, Takeshita F, Sasaki S, et al. Multivalent DNA vaccine protects mice against pulmonary infection caused by Pseudomonas aeruginosa. Vaccine. 2006 Sep 11;24(37-39):6240–6249. doi: 10.1016/j.vaccine.2006.05.077. [DOI] [PubMed] [Google Scholar]