Abstract
Background
Menopausal hormone therapy (MHT) increases risk of coronary heart disease (CHD) in older women with elevated low-density lipoprotein (LDLC) levels. The endogenous estrogen receptor antagonist 27-hydroxycholesterol (27OHC) is correlated with LDLC levels and may block beneficial effects of estrogen on the cardiovascular system.
Methods and Results
We conducted a nested case-control study in the Women’s Health Initiative trials of 350 CHD cases and 813 matched controls to explore potential mediation by 27OHC of the dependence of the CHD risk elevation with MHT on LDLC. Baseline levels of 27OHC were not associated with CHD risk when LDLC was included in the multivariable models. The odds ratio for CHD associated with increased LDLC was 1.15 (95% confidence interval 1.08, 1.23) and was unchanged at 1.14 (1.07, 1.22) when 27OHC was added to the model. Baseline 27OHC did not interact with MHT on CHD risk (p = 0.81). In contrast, LDLC levels modified the effect of MHT on CHD risk (p for interaction = 0.02), and adding 27OHC did not affect this result. Using log scales the MHT effect on CHD increased linearly with increasing level of baseline LDLC, with a transition from no risk to increased risk at approximately 3.36 mmol/L (130 mg/dl).
Conclusions
27OHC does not independently increase risk of CHD, does not modify the increased risk of CHD due to MHT, and does not mediate the interaction of LDLC with MHT. Measuring blood lipids may aid in counseling individual women about initiating MHT and cardiovascular risk mitigation.
Keywords: acute coronary syndrome, atherosclerosis, estrogen, low-density lipoprotein (LDL)-cholesterol, pathophysiology
Introduction
Primary and secondary prevention trials of menopausal hormone therapy (MHT) have demonstrated an early increased risk for coronary heart disease (CHD).1–3 This finding stands in contrast to the CHD benefit associated with MHT use in observational studies.4,5 A plausible explanation for the apparent discrepancy is that the observational studies missed the early increase in CHD events; when time since initiation of MHT is taken into account the studies agree closely.6–9
However, these and other analyses also provided some evidence that women initiating MHT relatively close to the menopause may not be at increased CHD risk while those more distant from the menopause have increased risk.5,6,8–10 A “timing hypothesis” has gained currency by which MHT is thought to have protective effects in the healthier arteries in younger women and adverse effects in the potentially atherosclerotic arteries of older women.11 Older women are also more likely to have elevated levels of risk factors such as high blood cholesterol. In this respect, a possible mechanism by which high levels of blood cholesterol might block a beneficial effect of estrogen on arteries has recently been postulated.12 Specifically, in rat models 27-hydroxycholesterol (27OHC), an abundant cholesterol oxidation product which is correlated with LDLC levels, acts as an endogenous estrogen antagonist in the vasculature. In mice, 27OHC decreased estrogen-dependent expression of nitric oxide synthase and inhibited carotid re-endothelialization. These investigators hypothesize that the trials of MHT have failed to demonstrate benefit because they included mostly older women in whom a substantial proportion of participants had elevated LDLC levels and therefore high 27OHC levels.12,13
There is some support for a role for lipid metabolism in modifying the effect MHT on CHD. Risk attenuation was observed in women receiving statins in the Heart and Estrogen/Progestin (HERS) trial, while risk exacerbation was seen among women with elevated baseline low-density lipoprotein (LDLC) levels in the Women’s Health Initiative (WHI) trials.2,14–16
In this contribution we examine the association of 27OHC with CHD, and for our primary hypothesis we test whether the modification of the effect of MHT on CHD by baseline LDLC level is due to variation in the levels of 27OHC.
Methods
Details of the design, recruitment, randomization, data collection, intervention, and outcomes ascertainment procedures in the WHI MHT trials, including CONSORT diagrams, have been published previously.17,18
Study population and interventions
The WHI hormone trials enrolled 27 347 postmenopausal women aged 50–79 from 1993 to 1998 at 40 US clinical centers. Based on hysterectomy status women were randomized to either a trial of conjugated equine estrogens plus progestin (CEE+MPA) (n=16 608 without hysterectomy) or in a trial of conjugated equine estrogens (CEE) alone (n=10 739 with hysterectomy). At baseline, women completed screening and baseline questionnaires by interview and self-report and a physical examination was done. Blood specimens were collected at baseline and the one-year visit. The study was approved by the human subjects review committee at each participating institution, and all participants provided written informed consent.
Participants were randomly assigned to take a single daily tablet containing a placebo or active medication: women without hysterectomy took 0.626 mg CEE plus 2.5 mg MPA (Prempro), and women with hysterectomy took 0.625 mg CEE (Premarin). Study drugs and placebo were supplied by Wyeth-Ayerst, St. Davids, PA. The planned end-date of the trials was 2005 for a total follow up of 8.4 years; however, CEE+MPA trial medications were stopped in 2002 and CEE medications were stopped in 2004 after mean follow-up periods of 5.6 and 7.1 years, respectively.2,3
All cases of CHD (n=359), stroke and venous thromboembolism during the first 4 years of follow up were included in a set of CVD biomarker studies. Controls (n=820) were matched on age, randomization date, hysterectomy status, and prevalent cardiovascular disease at baseline.16 This study used CHD cases and all combined controls, with all case-control matching variables included in analyses.
Follow-up and outcome ascertainment
Clinical outcomes were identified by semi-annual questionnaires and classified by central adjudicators following medical record review.19 CHD included nonfatal myocardial infarction (MI), CHD death, and silent MI. Definite and probable nonfatal MI required overnight hospitalization and was defined according to an algorithm based on standardized criteria using cardiac pain, cardiac enzymes and troponin levels, and electrocardiographic findings, and included MI occurring during surgery and aborted MI. CHD death was defined as death consistent with underlying cause of CHD plus one or more of the following: hospitalization for myocardial infarction within 28 days prior to death, previous angina or myocardial infarction, death due to a procedure related to CHD, or a death certificate consistent with underlying cause of atherosclerotic CHD. Definite silent myocardial infarction was diagnosed from baseline and year 3 and 6 electrocardiograms performed in the WHI field centers.
Biomarker analysis
Valid 27OHC measurements were obtained in 350 cases and 813 controls (N=1163). Data on blood lipids and several other biomarkers in this dataset have been published.15,16 Blood samples were collected from all participants at baseline and stored at −70° Celsius. Analyses were run in single batches including both cases and controls and 10% blind duplicates within 8 years of collection. Lipid profiles were analyzed in EDTA plasma with high density lipoprotein (HDL) precipitation by heparin manganese (Dade-Behring, Deerfield Illinois, United States) and were measured at Medical Research Laboratories (Highland Heights, KY). Intra-class correlations of 70 blind duplicates were 0.99 for each of LDLC and total cholesterol (TC). The Atherosclerosis Research Laboratory at Baylor College of Medicine, Houston, TX performed the assay of 27OHC on serum using a modified isotope-dilution gas-chromatography-mass spectrometry (GC-MS) method as previously described.20 Briefly, lipids were extracted from plasma using a modified Folch technique with the addition of 0.2% butylated hydroxytoluene (BHT) and 200 ng of deuterium-labeled 27OHC (Avanti Polar Lipids, Alabaster, AL) as internal standard.21 Lipid extracts were subjected to alkaline hydrolysis for 2 hours at room temperature and oxysterols were purified from other lipids by solid-phase extraction using silica columns (AccuBondII, 200mg/3mL cartridge). Following solid-phase extraction, oxysterols were derivatized using pyridine-bis(trimethylsilyl)trifluoroacetamide (BSTFA)(1:1) at 60 °C for 30 min and analyzed by GC-MS using a Hewlett Packard GC6890 connected to an HP 5973 Mass selective detector. The reference range for 27OHC in this laboratory is 13.64–68.2 nmol/L (conversion factor to ng/mL= division by 2.48). In the current study the between-run precision (or inter-assay coefficient of variance) was 16.6% and intra-class correlation of 55 blind duplicates was 0.47.
Statistical methods
All baseline marker values were log-transformed for statistical tests due to skewed distributions and for consistency with earlier analyses. The statistical approach examines the association of 27OHC and LDLC with CHD, and the interaction of 27OHC and LDLC with MHT (CEE, CEE Placebo, CEE+MPA, CEE+MPA Placebo) on CHD separately and jointly. If the effect of LDLC on CHD is mediated through 27OHC, then as a more proximal factor, one might expect 27OHC to show a stronger association with CHD than LDLC, and entering 27OHC in the model would attenuate or remove the association of LDLC with CHD. Similarly, 27OHC might show a comparatively stronger interaction with MHT on CHD, such that entering 27OHC into interaction analysis would attenuate or remove the interaction of LDLC with MHT effect on CHD. Additional analyses examined the association of the 27OHC/LDLC ratio to CHD risk. In preliminary analyses there were no significant interactions between biomarker and MHT trial assignment (i.e., hysterectomy status) for CHD, suggesting that it was appropriate to combine the trials and use a stratified parametric test for the interaction between biomarker and MHT (active vs. placebo) in relation to CHD risk. This increases the statistical power of the analyses.
Fully adjusted unconditional logistic regression models of case versus control status were controlled for baseline age, hysterectomy status, race/ethnicity, history of diabetes mellitus, smoking status, history of cardiovascular disease, alcohol consumption, systolic blood pressure, diastolic blood pressure, body mass index (BMI), waist circumference, C-reactive protein (CRP), blood glucose, treatment for high blood cholesterol, left ventricular hypertrophy (LVH) on electrocardiogram, and use of antihypertensive medication, aspirin, or statins. Even though cases and controls were matched in the study design, an unconditional logistic regression analysis is justified by the fact that controls can be regarded as random samples from trial cohorts conditional on matching factors, each of which was included in regression modeling.
The interactions between LDLC and 27OHC with MHT effect on CHD risk were also examined non-parametrically with generalized additive models that also used the full covariate adjustment described above. For these models, the biomarkers and interactions were summarized by penalized regression splines with smoothing parameters selected objectively via generalized cross-validation.22 Interactions that were common to the two hysterectomy/treatment groups were tested to enhance statistical efficiency, and selected two degrees of freedom interaction tests were also carried out for completeness.
Secondary analyses were performed within strata defined by age (50–64, 65–69, and 70–79 years), and by time since randomization (<2 years, ≥2 years). Sensitivity analyses were performed after excluding the 99 subjects reporting baseline statin use. We tested for nominal significance at two-sided P<0.05 without adjustment for multiple testing. Statistical analyses were performed using SAS statistical software (version 9; SAS Institute, Cary, North Carolina), generalized additive models and corresponding figure were computed with R (version 2.11; R Development Core Team (2010) - http://www.R-project.org.).
Results
As previously reported, in unadjusted analyses several baseline characteristics including total-, LDL- and HDL-cholesterol levels were associated with CHD risk (Table 1).16 Baseline 27OHC levels did not differ between cases and controls but the 27OHC/LDLC ratio was lower in cases than controls. Levels of 27OHC were correlated significantly (p < 0.001) with HDLC (r=0.12), LDLC (r=0.24), TC (r=0.29). Levels of 270HC were directly associated with alcohol intake but not with age or smoking and inversely associated with BMI, waist circumference, diabetes, fasting blood glucose, CRP, IL-6, and prothrombin F1.2 (p<0.05 for marginal association, Table 2). Though 27OHC levels did not vary significantly with statin use, the median (IQR) of the 27OHC/LDLC ratio was 10.96 (4.53) among statin users and 9.93 (3.90) in non-users, p=0.005. Levels of 27OHC were not associated with E-selectin, matrix metalloproteinase-9, D-dimer, fibrinogen, factor VIII, von Willebrand factor, leukocyte count, hematocrit, homocysteine, or insulin (data not shown).
Table 1.
Baseline Characteristics of Women in the Nested Case-Control Study (n=1163)
| CEE+MPA Trial | CEE Trial | P-Value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | ||||||
| N | % | N | % | N | % | N | % | ||
| Age group at screening^ | 0.46 | ||||||||
| 50–59 yrs | 41 | 20.6 | 86 | 17.9 | 14 | 9.3 | 51 | 15.3 | |
| 60–69 yrs | 88 | 44.2 | 216 | 45.0 | 75 | 49.7 | 168 | 50.5 | |
| 70–79 yrs | 70 | 35.2 | 178 | 37.1 | 62 | 41.1 | 114 | 34.2 | |
| Race/ethnicity | 0.73 | ||||||||
| White | 177 | 88.9 | 422 | 87.9 | 116 | 76.8 | 251 | 75.4 | |
| Black | 12 | 6.0 | 28 | 5.8 | 26 | 17.2 | 58 | 17.4 | |
| Other/Unspecified | 10 | 5.0 | 30 | 6.3 | 9 | 6.0 | 24 | 7.2 | |
| Smoking status | <0.001 | ||||||||
| Never | 88 | 45.8 | 268 | 56.7 | 70 | 47.3 | 167 | 51.5 | |
| Past | 63 | 32.8 | 169 | 35.7 | 48 | 32.4 | 128 | 39.5 | |
| Current | 41 | 21.4 | 36 | 7.6 | 30 | 20.3 | 29 | 9.0 | |
| Alcoholic drinks per day | 0.001 | ||||||||
| Non drinker | 103 | 52.3 | 220 | 46.4 | 100 | 67.6 | 171 | 51.7 | |
| ≤1 drink/day | 77 | 39.1 | 191 | 40.3 | 41 | 27.7 | 133 | 40.2 | |
| >1 drink/day | 17 | 8.6 | 63 | 13.3 | 7 | 4.7 | 27 | 8.2 | |
| Total expenditure from physical activity | 0.001 | ||||||||
| Inactive | 40 | 23.8 | 60 | 14.5 | 32 | 24.1 | 68 | 23.1 | |
| <5 METs/week | 41 | 24.4 | 89 | 21.5 | 44 | 33.1 | 72 | 24.4 | |
| 5–<12 METs/week | 41 | 24.4 | 103 | 24.9 | 25 | 18.8 | 65 | 22.0 | |
| ≥12 METs/week | 46 | 27.4 | 162 | 39.1 | 32 | 24.1 | 90 | 30.5 | |
| Body-mass index (kg/m2) | 0.01 | ||||||||
| <25 | 51 | 25.6 | 173 | 36.3 | 30 | 19.9 | 70 | 21.0 | |
| 25 – <30 | 73 | 36.7 | 149 | 31.3 | 49 | 32.5 | 125 | 37.5 | |
| ≥30 | 75 | 37.7 | 154 | 32.4 | 72 | 47.7 | 138 | 41.4 | |
| Treated diabetes (pills or shots) | 27 | 13.6 | 22 | 4.6 | 34 | 22.5 | 20 | 6.0 | <0.001 |
| History of hypertension | <0.001 | ||||||||
| Never hypertensive | 84 | 50.3 | 272 | 65.7 | 54 | 41.2 | 172 | 59.1 | |
| Untreated hypertensive | 19 | 11.4 | 37 | 8.9 | 15 | 11.5 | 18 | 6.2 | |
| Treated hypertensive | 64 | 38.3 | 105 | 25.4 | 62 | 47.3 | 101 | 34.7 | |
| History of high cholesterol requiring pills† | 48 | 24.1 | 72 | 15.0 | 39 | 25.8 | 60 | 18.0 | <0.001 |
| Baseline statin use | 30 | 15.1 | 34 | 7.1 | 22 | 14.6 | 35 | 10.5 | <0.001 |
| Baseline aspirin use | 56 | 28.1 | 109 | 22.7 | 50 | 33.1 | 79 | 23.7 | 0.01 |
| LVH on electrocardiography | 9 | 4.5 | 22 | 4.7 | 21 | 14.2 | 23 | 7.0 | 0.05 |
| History of CVD‡,^ | 26 | 13.1 | 40 | 8.3 | 24 | 15.9 | 41 | 12.3 | 0.04 |
|
| |||||||||
| Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | P-Value | |
|
| |||||||||
| Age at screening | 67.0 | (11.0) | 67.0 | (10.0) | 69.0 | (9.0) | 67.0 | (9.0) | 0.70 |
|
| |||||||||
| Body-mass index (kg/m2) | 27.9 | (7.5) | 27.3 | (7.2) | 29.7 | (8.4) | 28.8 | (7.3) | 0.005 |
|
| |||||||||
| Waist circumference (cm) | 90.0 | (20.0) | 85.0 | (20.0) | 94.0 | (21.8) | 91.0 | (16.7) | <0.001 |
|
| |||||||||
| Systolic BP (mm Hg) | 132.0 | (28.0) | 129.0 | (24.0) | 139.0 | (28.0) | 130.0 | (21.0) | <0.001 |
|
| |||||||||
| Diastolic BP (mm Hg) | 77.0 | (15.0) | 75.0 | (13.0) | 77.0 | (15.0) | 75.5 | (12.0) | 0.03 |
|
| |||||||||
| C-reactive protein (nmol/L) | 28.1 | (41.4) | 16.6 | (33.2) | 35.7 | (52.6) | 24.9 | (34.4) | <0.001 |
|
| |||||||||
| HDLC (mmol/L)§ | 1.2 | (0.4) | 1.4 | (0.5) | 1.2 | (0.4) | 1.3 | (0.4) | <0.001 |
|
| |||||||||
| LDLC (mmol/L)§ | 3.9 | (1.1) | 3.6 | (1.1) | 3.8 | (1.2) | 3.6 | (1.2) | <0.001 |
|
| |||||||||
| Total Cholesterol (mmol/L)§ | 6.1 | (1.1) | 5.7 | (1.3) | 6.0 | (1.3) | 5.9 | (1.3) | <0.001 |
|
| |||||||||
| Triglyceride (mmol/L)** | 1.6 | (1.2) | 1.5 | (0.9) | 1.8 | (1.2) | 1.6 | (1.0) | <0.001 |
|
| |||||||||
| 27- OH Cholesterol (nmol/L)†† | 37.7 | (11.4) | 36.5 | (11.9) | 37.0 | (11.7) | 36.5 | (11.4) | 0.39 |
|
| |||||||||
| 27OHC (nmol/L)/LDLC (mmol/L) | 9.6 | (3.7) | 10.2 | (4.3) | 9.4 | (3.9) | 10.2 | (3.7) | 0.02 |
|
| |||||||||
| Glucose (mmol/L) | 5.6 | (1.4) | 5.3 | (0.9) | 5.6 | (1.8) | 5.4 | (1.0) | <0.001 |
|
| |||||||||
| IL-6(pg/mL) | 3.4 | (2.3) | 2.8 | (2.2) | 3.7 | (3.7) | 2.8 | (2.3) | <0.001 |
|
| |||||||||
| Prothrombin Fragment 1 + 2 (nmol/L) | 1.3 | (0.5) | 1.3 | (0.4) | 1.4 | (0.6) | 1.3 | (0.5) | 0.11 |
The P values quantify the marginal association of each baseline characteristic with incident CHD and are obtained from a logistic regression model adjusted for treatment assignment (CEE, CEE placebo, CEE+MPA, or CEE+MPA placebo) using a 1-df test for trend or association except for the categorical variables race/ethnicity, smoking, and history of hypertension.
Self-report or use of antihyperlipidemic medications at baseline.
Includes stroke, MI and VTE.
To convert to mg/dL multiply by 38.7.
To convert to mg/dL multiply by 88.6.
To convert to ng/ml divide by 2.48.
Matching variables
Table 2.
Baseline Characteristics by Quartiles of 27-OH Cholesterol (n=1163)
| Quartiles of 27-OH Cholesterol (nmol/L)* | P-Value† | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥ 42.90 | 36.70 –< 42.90 | 31.25 –< 36.70 | < 31.25 | ||||||
| N | % | N | % | N | % | N | % | ||
| Age group at screening^ | 0.56 | ||||||||
| 50–59 yrs | 46 | 15.4 | 47 | 16.4 | 51 | 17.1 | 48 | 17.2 | |
| 60–69 yrs | 142 | 47.7 | 135 | 47.0 | 137 | 45.8 | 133 | 47.7 | |
| 70–79 yrs | 110 | 36.9 | 105 | 36.6 | 111 | 37.1 | 98 | 35.1 | |
| Race/ethnicity | 0.40 | ||||||||
| White | 237 | 79.5 | 247 | 86.1 | 253 | 84.6 | 229 | 82.1 | |
| Black | 42 | 14.1 | 24 | 8.4 | 29 | 9.7 | 29 | 10.4 | |
| Other/Unspecified | 19 | 6.4 | 16 | 5.6 | 17 | 5.7 | 21 | 7.5 | |
| Smoking status | 0.28 | ||||||||
| Never | 147 | 50.3 | 143 | 51.3 | 154 | 52.6 | 149 | 54.6 | |
| Past | 108 | 37.0 | 96 | 34.4 | 105 | 35.8 | 99 | 36.3 | |
| Current | 37 | 12.7 | 40 | 14.3 | 34 | 11.6 | 25 | 9.2 | |
| Alcoholic drinks per day | <0.001 | ||||||||
| Non drinker | 133 | 44.8 | 143 | 50.5 | 156 | 52.9 | 162 | 58.9 | |
| ≤ 1 drink/day | 122 | 41.1 | 107 | 37.8 | 120 | 40.7 | 93 | 33.8 | |
| >1 drink/day | 42 | 14.1 | 33 | 11.7 | 19 | 6.4 | 20 | 7.3 | |
| Total expenditure from physical activity | 0.37 | ||||||||
| Inactive | 49 | 18.7 | 51 | 20.9 | 46 | 17.6 | 54 | 22.3 | |
| <5 METs/week | 64 | 24.4 | 58 | 23.8 | 57 | 21.8 | 67 | 27.7 | |
| 5–<12 METs/week | 60 | 22.9 | 60 | 24.6 | 66 | 25.2 | 48 | 19.8 | |
| ≥12 METs/week | 89 | 34.0 | 75 | 30.7 | 93 | 35.5 | 73 | 30.2 | |
| Body-mass index (kg/m2) | 0.004 | ||||||||
| <25 | 97 | 32.7 | 79 | 27.5 | 71 | 23.9 | 77 | 27.7 | |
| 25 – <30 | 112 | 37.7 | 98 | 34.1 | 103 | 34.7 | 83 | 29.9 | |
| ≥30 | 88 | 29.6 | 110 | 38.3 | 123 | 41.4 | 118 | 42.4 | |
| Treated diabetes (pills or shots) | 14 | 4.7 | 19 | 6.6 | 32 | 10.7 | 38 | 13.6 | <0.001 |
| History of hypertension | 0.66 | ||||||||
| Never hypertensive | 156 | 59.3 | 148 | 61.9 | 134 | 51.5 | 144 | 59.8 | |
| Untreated hypertensive | 22 | 8.4 | 25 | 10.5 | 23 | 8.8 | 19 | 7.9 | |
| Treated hypertensive | 85 | 32.3 | 66 | 27.6 | 103 | 39.6 | 78 | 32.4 | |
| History of high cholesterol requiring pills‡ | 62 | 20.8 | 55 | 19.2 | 55 | 18.4 | 47 | 16.8 | 0.21 |
| Baseline statin use | 28 | 9.4 | 26 | 9.1 | 34 | 11.4 | 33 | 11.8 | 0.23 |
| Baseline aspirin use | 77 | 25.8 | 75 | 26.1 | 73 | 24.4 | 69 | 24.7 | 0.64 |
| LVH on electrocardiography | 18 | 6.1 | 18 | 6.3 | 17 | 5.8 | 22 | 8.0 | 0.47 |
| History of CVD§^ | 38 | 12.8 | 26 | 9.1 | 33 | 11.0 | 34 | 12.2 | 0.99 |
|
| |||||||||
| Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | ||
|
| |||||||||
| Age at screening | 67.0 | (9.0) | 67.0 | (10.0) | 67.0 | (10.0) | 67.0 | (10.0) | 0.92 |
|
| |||||||||
| Body-mass index (kg/m2) | 27.1 | (6.5) | 28.2 | (7.9) | 28.9 | (7.1) | 28.3 | (8.4) | <0.001 |
|
| |||||||||
| Waist circumference (cm) | 86.4 | (19.0) | 88.0 | (20.0) | 91.0 | (17.4) | 90.5 | (22.0) | 0.005 |
|
| |||||||||
| Systolic BP (mm Hg) | 130.0 | (23.0) | 130.0 | (23.0) | 131.0 | (24.0) | 133.0 | (27.0) | 0.11 |
|
| |||||||||
| Diastolic BP (mm Hg) | 75.0 | (13.0) | 76.0 | (13.0) | 76.0 | (13.0) | 77.0 | (14.0) | 0.13 |
|
| |||||||||
| C-reactive protein (nmol/L) | 17.2 | 36.3) | 22.3 | (35.7) | 26.4 | (42.4) | 27.7 | (44.2) | <0.001 |
|
| |||||||||
| HDLC (mmol/L) | 1.4 | (0.5) | 1.3 | (0.4) | 1.3 | (0.4) | 1.3 | (0.4) | <0.001 |
|
| |||||||||
| LDLC (mmol/L) | 3.9 | (1.1) | 3.9 | (1.1) | 3.6 | (1.2) | 3.3 | (1.1) | <0.001 |
|
| |||||||||
| Total Cholesterol (mmol/L) | 6.2 | (1.3) | 6.1 | (1.3) | 5.7 | (1.2) | 5.4 | (1.2) | <0.001 |
|
| |||||||||
| Triglyceride (mmol/L) | 1.5 | (1.2) | 1.6 | (1.1) | 1.6 | (1.0) | 1.5 | (0.9) | 0.16 |
|
| |||||||||
| Glucose (mmol/L) | 5.4 | (0.9) | 5.4 | (0.9) | 5.5 | (1.2) | 5.4 | (1.2) | <0.001 |
|
| |||||||||
| IL-6 (pg/ml) | 2.9 | (2.6) | 2.9 | (2.2) | 3.1 | (2.4) | 3.1 | (2.4) | 0.05 |
|
| |||||||||
| Prothrombin Fragment 1 + 2 (nmol/l) | 1.3 | (0.5) | 1.3 | (0.4) | 1.3 | (0.5) | 1.3 | (0.6) | 0.04 |
Quartiles based on the entire analytic sample (n=1163). Quartiles (25th/50th/75th percentiles) of cases and controls were 31.74/37.32/43.15 and 31.25/36.46/42.66 (nmol/L), respectively.
The P values quantify the marginal association of each baseline characteristic with quartiles of 27-OH cholesterol and are obtained from an ordinal logistic regression model adjusted for age,^ hysterectomy status (trial),^ and history of cardiovascular disease^ using a 1-df test for trend or association except for the categorical variables race/ethnicity, smoking, and history of hypertension.
Self-report or use of antihyperlipidemic medications at baseline.
Includes stroke, MI and VTE.
Matching variables.
Consistent with the published trial results, cases and controls included these analyses showed ORs of 1.45 (95% CI 1.04, 2.03) for CEE+MPA vs placebo and 1.16 (0.79, 1.71) for CEE vs placebo during the first 4 years of the trial follow-up. The direct associations of baseline total- and LDL-cholesterol with CHD risk, and the inverse association for HDLC, were not attenuated by adding 27OHC to the logistic regression models (Table 3). In fully adjusted models 27OHC was marginally associated with CHD (OR per 10% increment 1.07, 95% CI 1.00, 1.14) and the association was more clearly significant when HDLC was included in the model (OR 1.08, CI 1.01, 1.15). However this association was reduced and non-significant upon inclusion of either total- or LDL-cholesterol in the models (Table 3). Secondary analyses by age and time since randomization showed similar results within these strata (data not shown). The 27OHC/LDLC ratio was marginally and inversely associated with CHD in multivariable-adjusted models (OR per 10% increment 0.96, 95% CI 0.92, 1.00). Analyses excluding statin users did not change the association of LDLC or 27OHC/LDLC with CHD risk.
Table 3.
Risk of Coronary Heart Disease Associated with Increment in Levels of Total Cholesterol, LDL Cholesterol, HDL Cholesterol, and 27-OH Cholesterol
| Biomarker | Odds Ratio per 10% Increment in Biomarker Level (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 3‡ | Model 4§ | Model 5** | Model 6†† | Model 7‡‡ | |
|
| |||||||
| Total cholesterol | 1.12 (1.03, 1.21) | 1.19 (1.09, 1.31) | 1.18 (1.07, 1.29) | ||||
| LDL-cholesterol | 1.11 (1.05, 1.17) | 1.15 (1.08, 1.23) | 1.14 (1.07, 1.22) | ||||
| HDL-cholesterol | 0.87 (0.82, 0.91) | 0.93 (0.87, 0.99) | 0.92 (0.87, 0.99) | ||||
|
| |||||||
| 27-OH cholesterol | 1.02 (0.97, 1.08) | 1.07 (1.00, 1.14) | 1.08 (1.01, 1.15) | 1.04 (0.97, 1.11) | 1.04 (0.97, 1.11) | 1.04 (0.98, 1.11) | |
Estimated odds ratio for CHD during trial compared with controls for a 10% increment in log-transformed biomarker value from a logistic regression model adjusted for treatment assignment (CEE, CEE placebo, CEE+MPA, CEE+MPA placebo) and age
Adjusted for treatment assignment, age,^ race/ethnicity, diabetes mellitus, smoking status, history of cardiovascular disease,^ alcohol consumption, systolic blood pressure, diastolic blood pressure, body mass index, waist circumference, C-reactive protein, glucose, history of high blood cholesterol, LVH, use of antihypertensive medication, aspirin, or statins.
Adjusted for the aforementioned covariates included in model 2, and 27-OH cholesterol
Adjusted for the aforementioned covariates included in model 2, and HDL-cholesterol
Adjusted for the aforementioned covariates included in model 2, and LDL-cholesterol
Adjusted for the aforementioned covariates included in model 2, and total cholesterol
Adjusted for the aforementioned covariates included in model 2, and HDL-cholesterol and LDL-cholesterol.
Matching variables.
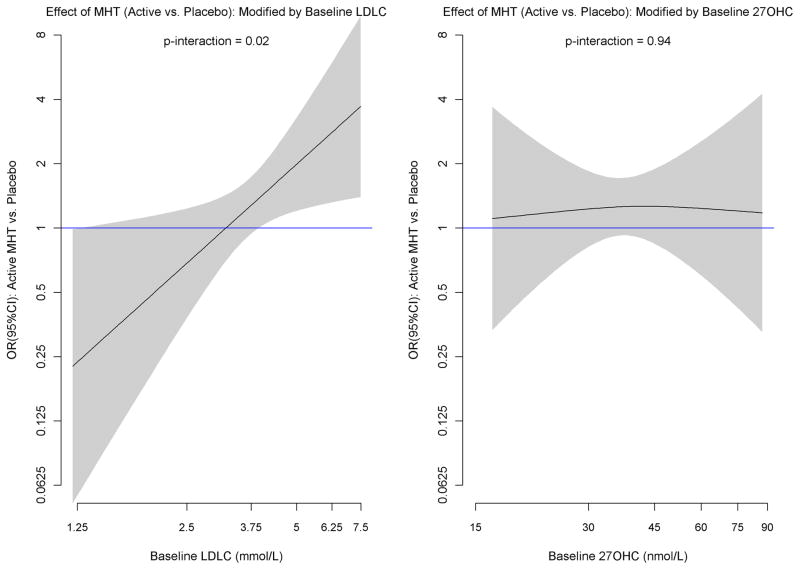
Baseline 27OHC did not interact with MHT in relation to CHD risk (p for interaction = 0.81) and further adjustment for total-, LDL-, or HDL-cholesterol did not affect this result (Table 4). As previously described, LDLC levels modified the effect of MHT on CHD risk (p for interaction = 0.02) such that higher levels of LDLC were associated with increased risk in women randomized to MHT.16 After excluding statin users, the interaction with LDLC was stronger (p = 0.009). There was no attenuation of the interaction with LDLC when 27OHC was added to the logistic regression models. Higher 27OHC/LDL ratio was associated with lower CHD risk due to MHT (p for interaction = 0.02). The modification of MHT effect on CHD by level of LDLC, and lack of modification by level of 27OHC, are illustrated by non-parametric spline fits where the effect of MHT is allowed to vary by baseline biomarker value (Figure 1). The figure shows how the log OR for CHD increases linearly with increasing level of baseline log LDLC, with a transition from no risk to increased risk at about 3.36 mmol/L (130 mg/dl). By way of contrast, the effect of MHT shows little evidence of variation across levels of 27OHC. The findings were similar for both LDLC and 27OHC when the trials were examined separately. The findings for 27OHC did not vary significantly by age (p for 3-way interaction = 0.11) or by time since randomization (p for heterogeneity = 0.57). The interactions of LDLC with MHT on CHD did not vary significantly by age (p for 3-way interaction = 0.28), but the LDLC interaction was more marked for CHD events occurring 2 or more years since randomization (p for heterogeneity = 0.003).
Table 4.
Associations of Baseline Total Cholesterol, LDL Cholesterol, HDL Cholesterol, and 27-OH Cholesterol with CHD Risk by Treatment Assignment
| Biomarker | Odds Ratio* per 10% Increment in Biomarker Level (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|
| CEE | CEE Placebo | CEE+MPA | CEE+MPA Placebo | Partially Adjusted P Value† | Fully Adjusted P Value | |
|
| ||||||
| Total cholesterol | 1.22 (1.01, 1.48) | 1.10 (0.91, 1.32) | 1.29 (1.09, 1.52) | 1.09 (0.91, 1.31) | 0.11 | 0.12‡ |
|
| ||||||
| LDL-cholesterol | 1.19 (1.05, 1.36) | 1.09 (0.96, 1.25) | 1.25 (1.11, 1.40) | 1.02 (0.90, 1.16) | 0.02 | 0.02§ |
|
| ||||||
| HDL-cholesterol | 0.91 (0.80, 1.04) | 0.94 (0.83, 1.08) | 0.88 (0.79, 0.98) | 0.98 (0.88, 1.10) | 0.14 | 0.18** |
|
| ||||||
| 27-OH cholesterol | 1.02 (0.89, 1.16) | 1.04 (0.91, 1.20) | 1.13 (1.01, 1.26) | 1.09 (0.98, 1.23) | 0.81 | 0.89†† |
| 27-OH cholesterol | 0.97 (0.85, 1.11) | 0.99 (0.86, 1.14) | 1.09 (0.97, 1.22) | 1.07 (0.95, 1.20) | 0.94‡‡ | |
| 27-OH cholesterol | 0.98 (0.85, 1.12) | 0.99 (0.86, 1.14) | 1.09 (0.97, 1.21) | 1.07 (0.95, 1.20) | 0.92§§ | |
| 27-OH cholesterol | 0.97 (0.85, 1.11) | 1.00 (0.87, 1.16) | 1.09 (0.98, 1.22) | 1.07 (0.95, 1.20) | >0.99*** | |
From fully adjusted logistic regression models that included treatment assignment, biomarker, corresponding interaction, and adjusted for age,^ race/ethnicity, diabetes mellitus, smoking status, history of cardiovascular disease,^ alcohol consumption, systolic blood pressure, diastolic blood pressure, body mass index, waist circumference, C-reactive protein, glucose, history of high blood cholesterol, LVH, use of antihypertensive medication, aspirin, or statins. and either 27OHC, LDLC, or total cholesterol as noted in footnotes 3, 4, 5 and 6.
From logistic regression models that included treatment(active vs. placebo), biomarker, corresponding interaction, and adjusted for hysterectomy status,^ age,^ race/ethnicity, diabetes mellitus, smoking status, history of cardiovascular disease,^ alcohol consumption, systolic blood pressure, diastolic blood pressure, body mass index, waist circumference, C-reactive protein, glucose, history of high blood cholesterol, LVH, use of antihypertensive medication, aspirin, or statins. P values corresponds to a 1-df test of the interaction between active treatment/placebo × biomarker (log scale) on CHD risk.
Model additionally adjusted for 27-OH cholesterol.
Model additionally adjusted for 27-OH cholesterol.
Model additionally adjusted for 27-OH cholesterol.
Model additional adjusted for HDL-cholesterol.
Model additionally adjusted for LDL-cholesterol.
Model additionally adjusted for total cholesterol.
Model additionally adjusted for HDL-cholesterol and LDL-cholesterol.
Matching variables
Figure 1.
Non-parametric spline fits of the log OR (95% confidence interval in shaded region) of the effect of MHT (active vs. placebo) by level of baseline log LDLC (left panel) and 27OHC (right panel). The smoothness of each fit was chosen objectively by generalized cross-validation. P-values shown are from the logistic regression interaction tests of Table 4.
Comment
The major findings from this investigation are that 27OHC was not an independent risk marker for CHD in women, and despite its correlation with serum cholesterol levels it did not modify the effect of MHT on CHD risk. 27OHC is the major oxysterol in plasma and in atheroma lesions and is generated enzymatically from cholesterol in several tissues, including atheroma lesions in direct relation to plasma levels.23,24 Mechanisms by which oxysterols have been postulated to increase risk for CHD include that oxysterols reflect oxidative stress, or oxysterols could have a direct toxic effect via endothelial dysfunction and/or promoting atheromatous plaque formation. Contrary to these pro-atherogenic properties, oxysterols also have an intrinsic role in reverse cholesterol transport and bile acid metabolism, which are important pathways for excretion of excess cholesterol and maintaining cholesterol homeostasis.25,26 In vitro studies have shown that 27OHC is involved in regulating cholesterol efflux from lipid-laden macrophages and thus may have anti-atherogenic properties.27,28
More recently, based on animal biology it has been postulated that estrogen receptor antagonism by 27OHC may block beneficial effects of MHT on CHD particularly in older women with higher levels of cholesterol.12.13 Serum cholesterol is correlated with serum and tissue levels of 27OHC, including levels in arterial walls and atheromatous lesions.22,23 In humans the hypothesis relating to estrogen receptor antagonism can best be tested in a clinical trial setting as we have done here. We did not find any evidence that 27OHC modified the increased risk of CHD conferred by MHT. The absence of evidence for estrogen receptor modulation via 27OHC is consistent with previous findings that estrogen receptor polymorphisms do not influence the effects of MHT on CHD.29 We conclude that variations in 27OHC levels are not responsible for the increased risk of CHD observed in the trials of MHT, and therefore do not explain the discrepant findings compared to observational studies. Overall age differences between observational studies and trials are not likely to explain the discrepancy because they would not apply to observational studies indicating benefit associated with hormone use in older women with existing CHD.30 Even in healthy women age differences may not explain the apparent benefit for hormone use since recent analyses of the Nurses’ Health Study indicate that the average years since menopause in hormone initiators is not dissimilar to that in the WHI trial of estrogen plus progestin.8
Within the WHI trials we have previously shown that the adverse effect of MHT on CHD is more marked in older women.10 In the current study neither LDLC nor 27OHC levels varied across the age range of 50–79 years, but it remains possible that women in the older age groups have a differential susceptibility to increased LDLC levels. The strong association of LDLC with CHD and the fact that LDLC is a major carrier of 27OHC explains why the signal of an association of 27OHC with CHD was removed when LDLC was entered into the model. In contrast, the signal for 27OHC was enhanced when HDLC was entered into the model because of the inverse association of HDLC with CHD. The association of higher levels of LDLC in cases compared controls stands in contrast to the relative lack of variation in 27OHC levels by case/control status and this, rather than the biological role of 27OHC in reverse cholesterol transport, probably explains the finding that the ratio of 27OHC/LDLC was inversely rather than directly associated with CHD risk.
The current study adds new information to our previous reports that baseline LDLC modifies the effects of MHT on CHD in showing that this effect modification is not mediated by 27OHC, is approximately linear across the range of LDLC (using log scales), and that the risk due to MHT becomes adverse at a level of approximately 3.36 mmol/L(130 mg/dl).15,16 Conveniently, that is also a cut-point for risk classification by current cholesterol treatment guidelines.31 The new analyses also suggest that the effect modification by LDLC applies across the age spectrum studied and that it is more pronounced for CHD events occurring more than 2 years after randomization. Since the most striking increases in CHD events occur during the first 2 years of MHT, the current report is consistent with the view that hormone therapy increases the risk of CHD through multiple mechanisms.1–3
The major strength of this study is its setting in a randomized controlled clinical trial. This design minimizes the possibility that confounding due to differences between hormone users and non-users could have influenced the results. Importantly, since all CHD events after hormone initiation were captured, we could account for the early increase in risk after initiation of hormone therapy which has evidently been missed in observational studies. Other strengths are the standardized measurement of participant characteristics, blood collection and storage procedures, laboratory measurements, and the prospective collection of centrally adjudicated clinical outcomes.
We acknowledge some weaknesses. We could not examine oxidative stress directly, but found little evidence that 27OHC was associated with indirect indicators of oxidative stress and inflammation such as age and smoking status, and levels were inversely rather than directly related to C-reactive protein, IL-6, obesity, and diabetes. In part these findings may be due to the previously shown association of 27OHC with HLDC, which is reduced in smokers, obese women, and those with diabetes.32,33 We had limited power to examine differences in association of 27OHC with CHD by age because there was no biomarker increase with age, and the numbers of CHD cases were small in some subgroups. Different results may have been found for women younger than the age range studied (50–79 years). Laboratory measurement of 27OHC is less reproducible than that of serum cholesterol fractions, and the increased measurement error of 27OHC in analyses including both cholesterol and 27OHC could lead to an underestimate of the strength of the association of 27OHC compared to total-and LDL-cholesterol, and an underestimate of the interaction mediation by 27OHC. However, this is an unlikely explanation for the non-significant findings for 27OHC because the intra class correlation for 27OHC was acceptable (0.47) in blind duplicate analyses, and since the suggestion of a 27OHC signal, either as independent risk marker or as interaction mediator, was lacking. We do not have data on change in 27OHC levels due to either endogenous hormone levels or to exogenous MHT. MHT is known to have substantial effects on lipid metabolism and conceivably could also affect 27OHC metabolism.
We conclude that 27OHC does not increase risk of CHD and does not modify the MHT-induced risk of CHD. Our data do not suggest clinical utility for measuring 27OHC for CHD risk assessment in postmenopausal women. On the other hand it would be useful to measure blood lipids before initiating MHT not only because of the risk conferred by LDLC itself, but also because it may aid in counseling individual women about MHT and cardiovascular risk mitigation in general.
Acknowledgments
We acknowledge the contributions of WHI Investigators, see http://www.whiscience.org/publications/WHI_investigators_shortlist.pdf.
Funding Sources: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. Study drugs were provided by Wyeth Research (St. Davids, PA).
Role of the Sponsor: The National Institutes of Health had input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. Wyeth did not participate in any of the aforementioned aspects of the study.
Footnotes
Clinical Trial Registration Information - clinicaltrials.gov; Identifier: NCT00000611.
Conflict of Interest Disclosures: Dr. Hsia is currently a full-time employee of Astra-Zeneca.
References
- 1.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghof E Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Randomized Trial of Estrogen Plus Progestin for Secondary Prevention of Coronary Heart Disease in Postmenopausal Woman. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 2.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 3.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R Women’s Health Initiative Investigators. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 4.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 5.Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at initiation of hormone therapy. J Women’s Health. 2006;15:35–44. doi: 10.1089/jwh.2006.15.35. [DOI] [PubMed] [Google Scholar]
- 6.Prentice RL, Langer R, Stefanick ML, Howard BV, Pettinger M, Anderson G, Barad D, Curb JD, Kotchen J, Kuller L, Limacher M, Wactawski-Wende J Women’s Health Initiative Investigators. Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women’s Health Initiative clinical trial. Am J Epidemiol. 2005;162:404–414. doi: 10.1093/aje/kwi223. [DOI] [PubMed] [Google Scholar]
- 7.Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, Johnson KC, Kuller LH, Lane DS, Wactawski-Wende J, Brzyski R, Allison M, Ockene J, Sarto G, Rossouw JE. Benefits and Risks of Postmenopausal Hormone Therapy When It Is Initiated Soon After Menopause. Am J Epidemiol. 2009;170:12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett KC, Manson JE, Robins JM. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toh S, Hernández-Díaz S, Logan R, Rossouw JE, Hernán MA. Coronary Heart Disease in Postmenopausal Recipients of Estrogen Plus Progestin Therapy: Does the Increased Risk Ever Disappear? A Randomized Trial. Ann Intern Med. 2010;152:211–217. doi: 10.1059/0003-4819-152-4-201002160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 11.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 12.Umetani, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nature Medicine. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 13.Umetani M, Saul PW. 27-Hydroxycholesterol: the first identified endogenous SERM. Trends in Endocrinology and Metabolism. 2011;22:130–135. doi: 10.1016/j.tem.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrington DM, Vittinghoff E, Lin F, Fong J, Harris F, Hunninghake D, Bittner V, Scrott HG, Blumenthal RS, Lew R HERS Study Group. Statin Therapy, Cardiovascular Events, and Total Mortality in the Heart and Estrogen/Progestin Replacement Study (HERS) Circulation. 2002;105:2962–2967. doi: 10.1161/01.cir.0000019406.74017.b2. [DOI] [PubMed] [Google Scholar]
- 15.Bray PF, Larson JC, Lacroix AZ, Manson J, Limacher MC, Rossouw JE, Lasser NL, Lawson WE, Stefanick ML, Langer RD, Margolis KL Women’s Health Initiative Investigators. Usefulness of baseline lipids and C-reactive protein in women receiving postmenopausal hormone therapy as predictors of treatment-related coronary events. Am J Cardiol. 2008;101:1599–1605. doi: 10.1016/j.amjcard.2008.01.043. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T10-4S69H89-4&_user=10&_coverDate=06%2F01%2F2008&_rdoc=13&_fmt=high&_orig=browse&_srch=doc-info(%23toc%234876%232008%23998989988%23689970%23FLA%23display%23Volume)&_cdi=4876&_sort=d&_docanchor=&_ct=33&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=0c0ba293b24a35c5fe9c03375c484cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossouw JE, Cushman M, Greenland P, Lloyd-Jones DM, Bray P, Kooperberg C, Pettinger M, Robinson J, Hendrix S, Hsia J. Inflammatory, lipid, thrombotic, and genetic markers of coronary heart disease risk in the women’s health initiative trials of hormone therapy. Arch Intern Med. 2008;168:2245–2253. doi: 10.1001/archinte.168.20.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 18.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 19.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Duagherty S WHI Morbidity and Mortality Committee. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(suppl):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 20.Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal Biochem. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 21.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22.Wood SN. Fast stable direct fitting and smoothness selection for generalized additive models. Journal of the Royal Statistical Society. 2008;70:495–518. [Google Scholar]
- 23.Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 24.Bertolotti M, Del Puppo M, Corna F, Anzivino C, Gabbi C, Baldelli E, Carulli L, Loria P, Galli Kienle M, Carulli N. Increased appearance rate of 27-hydroxycholesterol in vivo in hypercholesterolemia: A possible compensatory mechanism. Nutr Metab Cardiovasc Dis. 2011 May 3; doi: 10.1016/j.numecd.2011.02.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Seet RC, Lee CY, Loke WM, Huang H, Looi WF, Chew ES, Queck AM, Lim EC, Halliwell B. Biomarkers of oxidative damage in cigarette smokers: which biomarkers might reflect acute versus chronic oxidative stress? Free Radic Biol Med. 2011;50:1787–1793. doi: 10.1016/j.freeradbiomed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Ghosh M, Eftekhari S, Yuan XM. Lipid accumulation and lysosomal pathways contribute to dysfunction and apoptosis of human endothelial cells caused by 7-oxysterols. Biochem Biophys Res Commun. 2011;409:711–716. doi: 10.1016/j.bbrc.2011.05.071. [DOI] [PubMed] [Google Scholar]
- 27.Bjorkhem I, Andersson O, Diczfalusy U, Sevstik B, Xiu RJ, Duan C, Lund E. Atherosclerosis and sterol 27-hydroxylase: evidence for a role of this enzyme in elimination of cholesterol from human macrophages. Proc Natl Acad Sci U S A. 1994;91:8592–8596. doi: 10.1073/pnas.91.18.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiss AB, Martin KO, Javitt NB, Martin DW, Grossi EA, Galloway AC. Sterol 27-hydroxylase: high levels of activity in vascular endothelium. J Lipid Res. 1994;35:1026–1030. [PubMed] [Google Scholar]
- 29.Rossouw J, Bray P, Liu J, Kooperberg C, Hsia J, Lewis C, Cushman M, Bonds D, Hendrix S, Papanicolaou G, Howard T, Herrington D. Estrogen receptor polymorphisms and the vascular effects of hormone therapy. Arterioscler Thromb Vasc Biol. 2011;31:464–469. doi: 10.1161/ATVBAHA.110.215087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]
- 31.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Burkard I, von Eckardstein A, Waeber G, Vollenweider P, Rentsch KM. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis. 2007;194:71–78. doi: 10.1016/j.atherosclerosis.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Babiker A, Diczfalusy U. Transport of side-chain oxidized oxysterols in the human circulation. Biochim Biophys Acta. 1998;1392:333–339. doi: 10.1016/s0005-2760(98)00047-2. [DOI] [PubMed] [Google Scholar]