Abstract
Although nonviral gene therapy has great potential for use in the lung, the relative lack of cell-specific targeting has limited its applications. We have developed a new approach for cell-specific targeting based on selective nuclear import of plasmids in non-dividing cells. Using a microinjection and in situ hybridization approach, we tested several potential DNA sequences for the ability to mediate plasmid nuclear import in alveolar type II epithelial (ATII) cells. Of these, only a sequence within the human surfactant protein C (SP-C) promoter was able to mediate nuclear localization of plasmid DNA specifically in ATII cells but not in other cell types. We have mapped the minimal import sequence to the proximal 318 nucleotides of the promoter, and demonstrate that binding sites for NFI, TTF-1, and GATA-6 and the proteins themselves are required for import activity. Using intratracheal delivery of DNA followed by electroporation, we demonstrate that the SP-C promoter sequence will enhance gene expression specifically in ATII cells in mouse lung. This represents a novel activity for the SP-C promoter and thus ATII cell-specific nuclear import of DNA may prove to be a safe and effective method for targeted and enhanced gene expression in ATII cells.
Introduction
The inability to selectively target genes to specific cell types remains a significant limitation to most methods of gene transfer to the lung or any tissue. Although a few approaches have been developed to restrict expression to desired cell types, the two means that are routinely used are the regulation of cell entry by cell surface receptor-ligand interactions to promote cell-specific internalization of the DNA into the cytoplasm and the use of cell-specific promoters to preferentially drive transcription. In addition, regulation of the site of delivery (e.g., luminal vascular delivery of vectors for endothelial cells or airway delivery for the pulmonary epithelium) is also used in vivo to limit gene delivery to desired sites. We have developed a different approach that exploits the mechanisms by which plasmids are transported into the nucleus of nondividing cells. It has been shown that in the absence of mitosis, plasmids are imported into the nucleus in a sequence-specific manner, and we and others have identified several DNA sequences that mediate this nuclear import 1-6. The common feature to these DNA nuclear targeting sequences (DTSs) is that they contain binding sites for transcription factors. Since transcription factors, like all proteins, are synthesized in the cytoplasm, they contain nuclear localization signals (NLSs) that interact with the nuclear protein import machinery for transport into the nucleus. If a plasmid containing the transcription factor binding site within the DTS is present in the cytoplasm, a newly synthesized transcription factor may bind to this site before nuclear import. The NLS import machinery will then bind to the DNA-bound transcription factors and translocate the DNA-protein complex into the nucleus 7,8. One sequence that acts as a DTS is the SV40 enhancer which is known to bind to over 10 distinct, ubiquitously expressed, transcription factors and mediates plasmid nuclear entry in all cell types tested to date 1,3. The other identified DTSs act in a cell-specific manner by binding to a unique set of cell-specific transcription factors resulting in nuclear import in only those cells in which the transcription factors are expressed 9. One such sequence that acts in smooth muscle cells only is the smooth muscle gamma actin promoter 4. This promoter is regulated transcriptionally by the complement of positive and negative transcriptional regulators present within smooth muscle cells including SRF and Nkx factors 10,11, and we have demonstrated that binding of these factors to the DNA are needed for DNA nuclear import activity in cultured smooth muscle cells 12. Thus, cell-specific gene delivery and expression can be regulated at the level of nuclear import of the vector DNA.
In order to identify a DNA nuclear import sequence that is active in alveolar type II epithelial (ATII) cells, a cell that makes up roughly 5% of the alveolar surface, mediates much of the fluid balance within the lung, and which likely serves as a progenitor cell for type I cells, the thin cells that line the remainder of the alveoli and are responsible for gas exchange, we screened the transcriptional regulatory elements of several alveolar epithelial cell-specific genes. In this study, we show that the 318 bp fragment of the SP-C proximal promoter acts as a type II alveolar epithelial cell-specific DNA nuclear targeting sequence whose activity is dependent on binding sites for a number of cell-specific transcription factors. Additionally, we show that the SP-C promoter when included on a plasmid will enhance gene expression specifically in ATII cells in mouse lung.
Materials and Methods
Plasmids
The 5′ flanking sequences and promoters for SP-A, SP-B, SP-C, SP-D, and cytokeratin 8 were amplified by PCR from human genomic DNA (Promega, Madison, WI; Table 1). The SV40 DTS was amplified by PCR from SV40 genomic DNA. Amplified sequences were inserted into the pCRII-TOPO plasmid (Invitrogen, San Diego, CA). The 3.7 kb human SP-C promoter plasmid was a generous gift from Dr. J. Whitsett (Cincinatti Childrens Hospital, Cincinnati, OH). The 215bp SP-C promoter truncation was made by digesting pCRII-SPC with the restriction enzyme ApaI and religating the digested plasmid upon removal of the intervening ApaI fragment. The plasmids used for in vivo delivery were created by removing the multiple cloning site of the pEGFP-N1 vector (Clontech, Mountain View, CA) by BgIII and BamHI digestion/religation and subsequent deletion of the SV40 enhancer/DTS from the vector using PCR mutagenesis to flank the DTS with BglII sites and delete it by digestion and religation. The EGFP gene of this plasmid was replaced with the EYFPnuc gene of the pEYFPnuc vector (Clontech, Mountain View, CA) by digestion with NheI and AfIII, creating pCMV-YFPnuc-ΔDTS. The 365 bp fragment of the human SP-C promoter was inserted downstream of YFPnuc into the BgIII site of pCMV-YFPnuc-ΔDTS, creating pCMV-YFPnuc-SPC. The PCR-based Quickchange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) was used to mutate desired sites within the SP-C promoter. Plasmids were purified by Promega Wizard Maxiprep kits (Promega, Madison, WI). All plasmids were confirmed by DNA sequencing.
Table 1. Promoters used in this study.
| Promoter | Promoter Fragment1 | Reference |
|---|---|---|
| SP-A | −1000 to +38 | 37 |
| SP-B | −730 to +39 | 38 |
| SP-C | −318 to +18 | 17 |
| SP-D | −1675 to +864 | 39 |
| Keratin 8 | −1762 to +18 | 40 |
Nucleotide numbers are relative to the transcriptional start site at +1
Cell culture and microinjection
A549 (CCL-185), MLE-12 (CRL-2110), HeLa (CCL-13), Beas-2B (CRL-9609), and NIH3T3 (CRL-1658) cell lines were obtained from American Type Culture Collection (Washington DC). TC7 cells, a subline of African Green Monkey kidney epithelium 1, primary cultures of human pulmonary artery smooth muscle cells (PASMC; Cambrex, East Rutherford, NJ, USA) and rat alveolar type II epithelial cells (a generous gift from K. Ridge, Northwestern University, Chicago, IL), were also used. All cells were grown on etched glass coverslips in Dulbecco's Minimal Essential Media (DMEM) containing 10% fetal bovine serum and supplemented with antibiotics and antimycotics. All cells were cytoplasmically microinjected using an Eppendorf Femtojet system as described previously 13. Purified protein-free DNA was suspended in phosphate-buffered saline and injected at a concentration of 0.5 mg/ml, which corresponds to approximately 20,000 plasmids injected per cell. Rhodamine-labeled bovine serum albumin (BSA; Molecular Probes, Eugene, OR) was co-injected at a concentration of 0.5 mg/ml.
In situ hybridization
In situ hybridizations were performed as described using fluorescently labeled probes were prepared by nick translation of pCRII-TOPO or pCAT and mounted with DAPI and the anti-bleaching reagent DABCO 1. Cells were visualized by fluorescence microscopy on a Leica DMRXA2 and images were captured on a Hamamatsu Orca-ER 12 bit, cooled CCD camera (Hamamatsu, Japan) and OpenLab software (Improvision, Lexington, MA). Confocal microscopy on a Zeiss LCM 510-META was used to confirm nuclear localization.
siRNA delivery and knockdown assessment
siRNAs against mouse TTF-1 (Catalog # L-041979-01), NFIA (Catalog # L-044168-01) and GATA-6 (Catalog #L-065585-00) were purchased from Dharmacon, Inc. (Lafayette, CO) and resuspended to a concentration of 20 μm in 1× siRNA buffer. Cells were counted and approximately 25,000-50,000 cells were plated on glass coverslips in a 12 well plate. After 24 hours incubation in antibiotic-free media, siRNA was transfected at a concentration of 2 μM in antibiotic- and serum-free media with the DharmaFect 1 transfection reagent (Dharmacon, Lafayette, CO). Media with serum was added after 24 hours if extensive cell death was observed. Cells were lysed at 24, 48, and 72 hours post-transfection and protein levels were compared with those seen in cells transfected with a non-targeting siRNA and assayed at each time point by Western blot. TTF-1 was detected using the TTF-1 Ab-1 antibody from Lab Vision/Neomarkers (Catalog #MS-699 Fremont, CA). NFI was detected using the NF-1 antibody from Santa Cruz Biotechnology (Catalog #SC-870, Santa Cruz, CA). GATA-6 levels were detected using the anti-human GATA-6 goat polyclonal antibody from R&D Systems (Catalog #AF1700, Minneapolis, MN). The time point where the greatest knockdown was observed was noted and cells were microinjected with the pCRII-SPC promoter plasmid at this optimum time point. Each siRNA time point optimization was repeated three times.
In vivo gene transfer to the lung
Female Balb/c mice (18-22 g) were anesthetized with sodium pentobarbital (50 mg/kg body weight, IP) and placed in the supine position. An endotracheal tube (20G angiocath cut at an angle 2 inches from the end) was placed through the vocal cords into the trachea using a guide wire. A solution of 100 μl of pCMV-YFPnuc-SPC or pCMV-YFPnuc-ΔDTS DNA (1.5 mg/ml) in 10 mM Tris, pH 8.0, 1 mM EDTA and 140 mM NaCl was instilled into the lungs through the catheter with a Hamilton syringe. Immediately afterwards, an electric field was applied across the animal's chest using 8 square wave pulses of 10 msec duration each at a field strength of 200 V/cm delivered with a BTX ECM 830 electroporator (BTX, San Diego, CA) and pre-gelled pediatric surface electrodes (Medtronic, Minneapolis, MN). After electroporation, mice were mechanically ventilated at a tidal volume of 150 μl (15% total lung capacity) for approximately 1 minute or until they recovered spontaneous breathing. The animals were allowed to recover from anesthesia, returned to the vivarium, and 48 hours later euthanized by a sodium pentobarbital overdose. Blood was perfused from the body by injection of 1 ml of cold PBS into the right atrium of the heart. Lungs were fixed in vivo by inflating the lung with 2% paraformaldehyde for 1 hour, then removed, inflated to total lung capacity with a 50% mixture of OCT medium and PBS, and snap frozen in liquid nitrogen. All experiments were conducted in accordance with institutional guidelines in compliance with the recommendations of the Guide for Care and Use of Laboratory Animals.
Lung immunofluorescence
Eight micron frozen thin sections were permeabilized with 0.2% triton-X and immunostained with goat anti-mouse lamellar body 180 antibody as a marker for ATII cells (Chemicon International, Temecula, CA). Antibodies were detected using Alexa 555 anti-mouse secondary antibodies (Molecular Probes, Eugene, OR). Direct fluorescence of YFP or antibodies was visualized using a Zeiss LCM 510-META confocal microscope.
BrdU incorporation
Animals were injected with 0.5ml of 2 mg/ml Bromo-deoxyuridine at 24 and1 hour prior to gene delivery and 1, 24, and 45 hours post-delivery. Lungs were harvested at 48 hours and processed for frozen thin sections. BrdU incorporation was detected using the BrdU In-Situ Detection Kit (BD Biosciences, San Diego, CA).
Results
Human SP-C promoter mediates plasmid nuclear import in MLE-12 cells
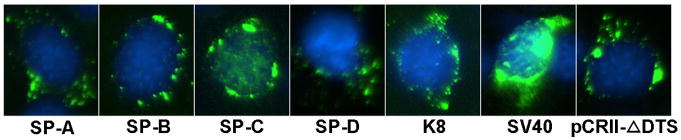
In order to identify a nuclear import sequence that is active in alveolar epithelial type II cells (ATII), we screened the transcriptional regulatory elements of several ATII cell-specific genes. Based on the literature, we chose the minimal sequence that gave cell-specific transcription, with the assumption that these sequences contain binding sites for ATII-specific transcription factors. These promoter sequences included surfactant protein genes A, B, C and D, and a general epithelial cell-specific gene, cytokeratin 8 (Table1). These promoter fragments were PCR amplified from human genomic DNA and cloned into the pCRII-TOPO plasmid, which does not contain a DNA nuclear targeting sequence or eukaryotic promoter 3. To assay for nuclear import, the promoter-containing plasmids were microinjected along with rhodamine-labeled bovine serum albumin (Rh-BSA) into the cytoplasm of MLE-12 cells, a transformed mouse alveolar type II epithelial cell line that expresses high levels of surfactant proteins like primary type II cells 14. Cells were fixed at eight hours post-injection and the DNA was visualized using fluorescence in situ hybridization. Cells showing any nuclear Rh-BSA were not scored, since the injected labeled protein should not have access to the nucleus unless the cells had divided or had been accidentally injected in the nucleus. Immediately following injection, all plasmids showed diffuse staining throughout the cytoplasm (not shown). By 8 hours, all plasmids were still restricted to the cytoplasm except the plasmid carrying the SP-C promoter (Fig. 1). When pCR-SPC was injected into the cytoplasm of MLE-12 cells, all of the injected DNA (>90%) migrated to the nucleus in 25-30% of the cells showing fluorescent in situ hybridization signal. An additional 20% of the cells with fluorescent signal showed > 50% of the DNA in the nucleus (not shown). By contrast, none of the cells injected with the other promoter-containing plasmids showed any nuclear localization of the plasmids. Similarly, the plasmid backbone of these plasmids (pCR) showed very little (≤5%) nuclear localization when the DNA was cytoplasmically injected (Fig. 1). As a positive control, the same plasmid backbone containing the SV40 DTS in place of the SP-C promoter (pCR-SV40) was cytoplasmically injected and visualized at 8 hours, and had nuclear import activity nearly identical to that of the SP-C promoter.
Figure 1. Plasmids containing the SP-C promoter show nuclear import activity in MLE-12 cells in the absence of cell division.
MLE-12 cells were cytoplasmically injected with plasmids carrying either the SP-A, SP-B, SP-C, SP-D, or keratin 8 promoter, the SV40 enhancer, or no eukaryotic promoter (pCRII-ΔDTS) . Eight hours later, the location of the injected DNA was detected by in situ hybridization. Injected plasmid is shown in green and nuclear DNA is counterstained with DAPI (blue). Between 100 and 200 cells were injected for each construct and the experiments were repeated three times for all DNAs; representative cells are shown.
Human SP-C promoter does not mediate plasmid nuclear import in non-alveolar epithelial type II cells
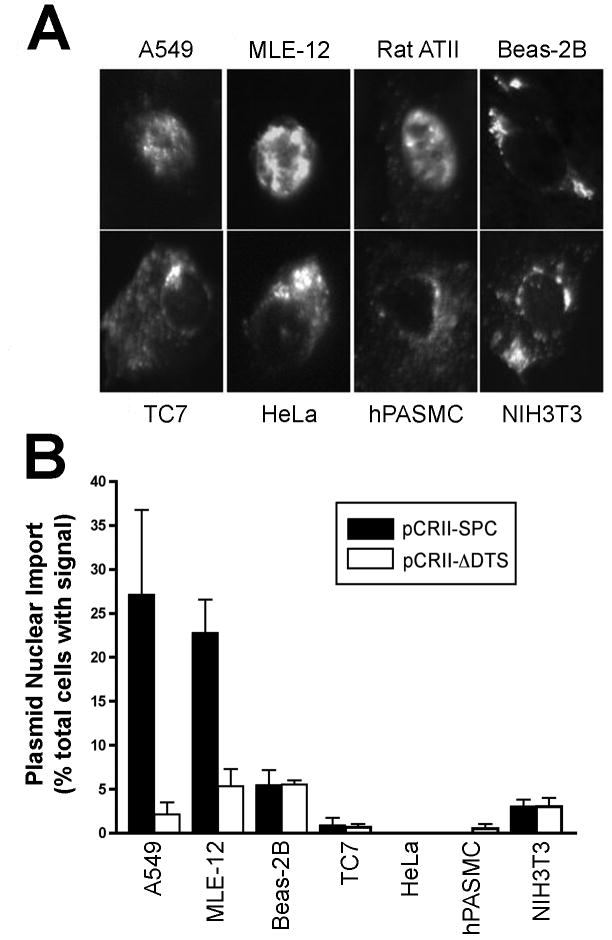
To determine whether the nuclear import activity of the SP-C promoter was restricted to type II cells, the plasmid was microinjected into the cytoplasm of a variety of cell types and localized by in situ hybridization. These included TC7 cells (green monkey kidney epithelium), HeLa cells (human cervical carcinoma), Beas-2B cells (human bronchial epithelium), NIH3T3 cells (fibroblasts), and primary human pulmonary artery smooth muscle cells. Additionally, A549 cells, another alveolar type II-like epithelial cell line and freshly isolated primary rat ATII cells were also tested. All of these cells were injected with pCR-SV40 which showed DNA nuclear localization similar to that seen in MLE-12 cells, demonstrating that all of these cell types have the ability to import plasmid DNA into the nucleus in a sequence-specific manner (not shown). Further, none of the cells showed nuclear localization of the promoter-less plasmid, pCRII-TOPO (not shown). While the pCR-SPC plasmid localized entirely to the nucleus in ∼30% of the MLE-12, A549, and primary rat ATII cells (Fig. 2). By contrast, pCR-SPC showed no nuclear localization in the absence of cell division in any of the other cell types, suggesting that the SP-C promoter mediates nuclear import specifically in alveolar type II epithelial cells, but not in non-type II cells.
Figure 2. SP-C promoter plasmids localize to the nuclei of alveolar type II epithelial cell lines but not of other cell types.
Different cell lines were cytoplasmically injected with equal numbers of plasmids carrying the human SP-C promoter and the location of the DNA was detected by in situ hybridization eight hours later. A. Representative cells displaying pCR-SPC nuclear or cytoplasmic localization. B. The percentage of cells showing greater than 90% nuclear signal was quantified for each cell type. The pCRII-ΔDTS plasmid is included as a negative control for background nuclear import. Data are plotted as mean ± st. dev. from three or more independent experiments. Approximately 100 cells per experiment were imaged for each cell type.
Specific sequences of the human SP-C promoter are required for alveolar epithelial cell-specific nuclear import
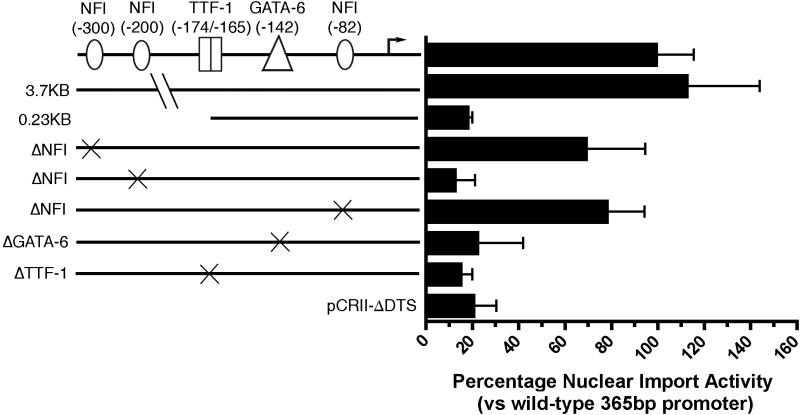
In order to identify which sequences within the human SP-C promoter were required for ATII-specific nuclear import, plasmids containing the large 3.7 Kb fragment of the SP-C promoter as well as several truncations were tested for import activity using the microinjection and in situ hybridization strategy. It has been shown that the developmental expression of the human 3.7 Kb SP-C promoter mimics that of the endogenous SP-C gene, indicating that the cis-active regulatory elements for cell-specific and developmental expression are located within this region while the 336 bp (-318 to +18) proximal promoter shows alveolar type II cell specific expression in cultured cells that is dependent, suggesting that even this short segment maintains control elements for appropriate cell-specific expression 15,16. Both the full-length and the 336 bp proximal SP-C promoter showed similar levels of nuclear import activity in MLE12 cells (Fig. 3). However, truncation of the promoter to nucleotide –215 abolished DNA nuclear import activity to that seen with plasmids lacking any promoter fragment, suggesting that sequences between –215 and –318 are required for DNA nuclear import activity.
Figure 3. Sequence requirements for SP-C promoter nuclear import.
Plasmids containing the wild type 365 bp SP-C promoter, full-length 3.7 Kb human SP-C promoter, 0.23 Kb truncation, and the SP-C promoter containing the designated mutations were microinjected into the cytoplasm of MLE-12 cells and the localization of the DNA was detected by in situ hybridization after eight hours. Plasmids containing no promoter (pCRII-ΔDTS) were injected as a negative control, Nuclear import activity is shown relative to that of the wild type promoter (mean ± st. dev.), which showed complete nuclear localization in 28% of injected cells. Between 100 and 200 cells were injected and visualized for each construct in both cell types and the experiments were repeated three times.
Multiple transcription factor binding sites are required for SP-C promoter nuclear import in alveolar type II epithelial cell lines
Based on the published literature, we mutated several transcription factor binding sites shown to be important for type II cell-specific transcription, including nuclear factor I (NFI), GATA-6, and thyroid transcriptional factor 1 (TTF-1) in order to determine which are required for SP-C nuclear import activity 15-21. Mutations within the TTF-1 and GATA-6 sites reduced nuclear import activity to background levels (i.e., similar to the promoter-less pCRII-ΔDTS plasmid) as did mutation of the NFI binding site located at nucleotide –200 (Fig 3). Interestingly, mutations within the two other NFI sites (located at –300 and –82) retained ∼75% nuclear import activity when compared to the wild-type promoter sequence. In combination with the deletion analysis, these results suggest that the region between –219 and –82 are required for DNA nuclear import activity and further that NFI, TTF-1, and GATA-6, which bind in this region, play a role in the ATII-specific DNA nuclear import.
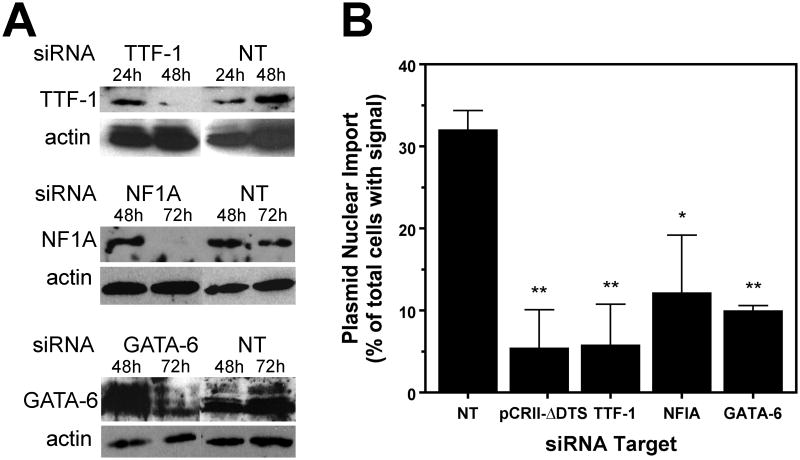
TTF-1, NFIA, and GATA-6 are required for SP-C nuclear import activity in MLE-12 cells
In order to determine whether NFI, TTF-1, and GATA-6 do indeed play a role in the nuclear import of plasmids containing the SP-C promoter, the levels of these factors in MLE-12 cells were reduced using siRNA and nuclear import of pCR-SPC was evaluated. Since the timing and efficacy of siRNA-mediated reduction of proteins varies for each individual protein, cells were transfected with siRNAs and the window of greatest protein reduction was determined by Western blotting (Figure 4A). Cells treated with TTF-1 siRNA were tested for import activity 48 hours after siRNA transfection, while cells treated with NF1A or GATA-6 siRNAs were subsequently microinjected at 72 hours post-transfection. In all cases, siRNA treatment resulted in >90% reduction of the various transcription factors (Fig. 4A). Knock-down of TTF-1, NFIA, or GATA-6 resulted in a substantial inhibition of SP-C nuclear import, whereas treatment of cells with a control, non-targeting siRNA did not affect the level of nuclear import compared to untreated cells (Fig. 4B). Taken together with the DNA mutagenesis studies, these results demonstrate that these three factors are required for the nuclear import of SP-C DTS containing plasmids.
Figure 4. Nuclear import of the SP-C plasmid in MLE-12 cells is abrogated following siRNA knockdown of TTF-1, NFIA, or GATA-6.
MLE-12 cells were treated with siRNAs against the transcription factors TTF-1, NFIA, and GATA-6, or a non-targeted siRNA as a negative control. A. Western blots were performed on cell extracts at the indicated times following siRNA transfection. B. Forty-eight (TTF-1) or 72 (NF1 and GATA-6) hours post-transfection, pCR-SPC was microinjected into the cytoplasm of the cells and its cellular localization was determined 8 hours later by situ hybridization. The pCRII-ΔDTS plasmid is included as a negative control for background nuclear import. Data are plotted as mean ± st. dev. from three or more independent experiments. (*) indicates p<0.05 when compared to the non-targeting control.
Use of the SP-C promoter DNA targeting sequence enhances transgene expression specifically in ATII cells in vivo
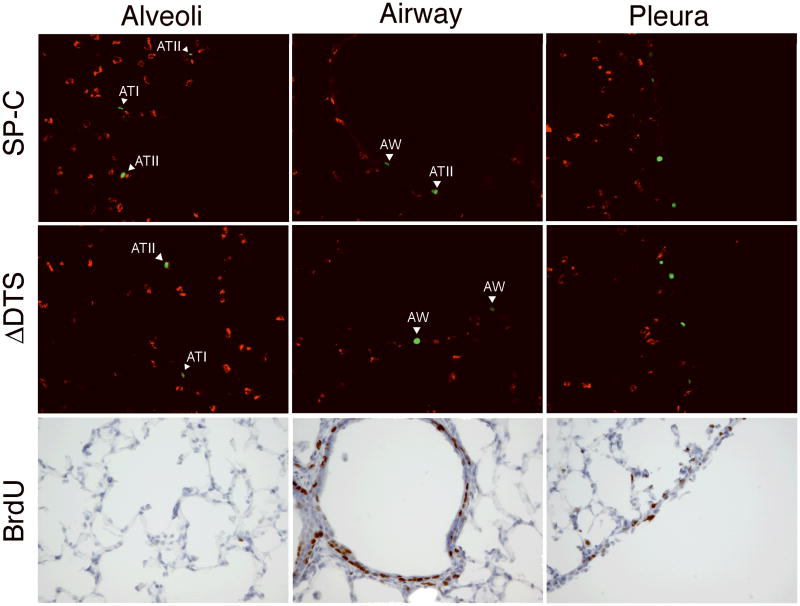
In order to assay the nuclear import activity of the SP-C promoter in vivo, we constructed plasmids that express nuclear-targeted yellow fluorescent protein (YFPnuc) under the control of the CMV promoter and which contain or lack the 336 bp SP-C promoter/DTS downstream of YFP. The SP-C promoter was placed downstream of the expression cassette to minimize the transcriptional effects of the SP-C promoter and to isolate its nuclear import activity. Plasmids were delivered via the airways to the lungs of mice using transthoracic electroporation, ventilated briefly following electroporation, and sacrificed 48 hours post-delivery 22. YFPnuc was detected in multiple cell types throughout the airways, alveoli, and pleura (Fig. 5A). To determine whether there was any preference for delivery and expression in type II cells, they were visualized with an ATII-specific lamellar body protein antibody. Indeed, most of the cells in the parenchyma that expressed YFPnuc also were positive for lamellar body protein staining. Based on our cell culture results, we were surprised by the relative lack of specific targeting to type II cells and the amount of cells in the airways and pleura that also expressed gene product. We hypothesized that the unusually high number of airway and pleural YFPnuc positive cells could be due to cell division in these cells. Since YFPnuc is under the transcriptional control of the CMV promoter, if the plasmids are able to reach the nucleus of any cell type during cell division, YFPnuc will be expressed regardless of any DTS activity. To test this hypothesis, two approaches were taken. First, a plasmid lacking any DTS sequence (pCMV-YFPnuc-ΔDTS) that has been shown to be transcriptionally equivalent to DTS containing plasmids in dividing cells but which has very limited expression in non-dividing cells and tissues was delivered to the mice 3,23. Large numbers of YFPnuc positive cells were detected in the airways and pleura of mice receiving pCMV-YFPnuc-ΔDTS, but fewer cells were found in the alveoli (Fig. 5). This suggests that cell division is likely playing a role in gene delivery to the lung. Thus, BrdU incorporation was used to visualize cell division in these animals (Fig. 5). BrdU was injected into animals at several time points before and after DNA delivery, and the animals were sacrificed and prepared as described above. Significant numbers of BrdU positive cells were detected in the airways and the pleura but were scarce in the alveoli, suggesting that significant numbers of cells in the airway and periphery of the lung are actively dividing, while the cells in the alveoli are largely quiescent (Fig. 5).
Figure 5. Identification of lung cells expressing YFPnuc and BrdU incorporation.
Plasmids (50 μg) expressing YFPnuc from the CMViep and carrying the SP-C DTS downstream of YFPnuc were delivered to the lungs of Balb/c mice (n=3) by transthoracic electroporation (8 pulses of 10 msec duration at 200 V/cm). The mice were also injected with BrdU (1 mg) 24 and 1 hour prior to gene delivery and 1, 24, and 45 hours after gene delivery. Two days after gene delivery, lungs were harvested and embedded in OCT for frozen sections. Cryosections containing YFPnuc positive cells (green) following BrdU injections, intratracheal delivery and electroporation were counterstained with an ATII-specific antibody, LB180 (red). Separate sections were also reacted with an anti-BrdU antibody and counterstained with hematoxylin. Representative images of specified lung areas are shown.
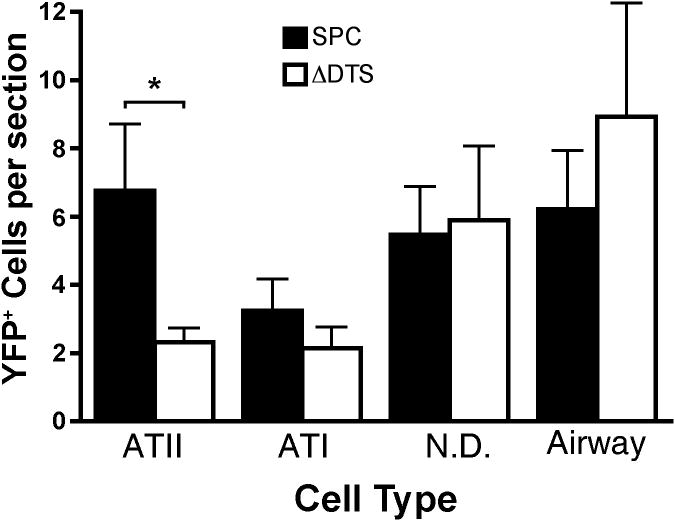
In order to quantify enhancement of gene expression, we decided to focus on the effect of the SP-C promoter on gene expression in the non-dividing alveolar cells. Fifteen slides per animal were counted spanning the whole lung and each expressing cell type was classified as ATII (positive staining with antibody marker), ATI, airway, and non-type I/non-type II cells (Fig. 6). These latter cells are present in the alveolus but do not have the flat phenotype of ATI cells and do not stain positively for ATII, macrophage, or fibroblast markers (data not shown). Plasmids containing the SP-C promoter/DTS showed statistically significantly more expression in type II cells compared to type I cells (6.80 ± 1.93 cells/section vs 3.13 ± 0.80 cells/section, p ≤ 0.01). By contrast, equal numbers of type I and type II cells took up and expressed the DTS-lacking plasmid (2.33 ± 0.40 cells/section vs 2.20 ± 0.60 cells/section). Non-type I/non-type II cells also showed equivalent numbers of YFPnuc positive cells following delivery of plasmids carrying or lacking the SP-C DTS. Since the ΔDTS plasmid expresses only in dividing cells, these results suggest that the expression from SP-C promoter/DTS plasmids seen in type I and non-type I/non-type II cells (and ∼ 30% of type II cells) is most likely due to cell division. Taken together, these results suggest that the SP-C promoter/DTS enhances gene delivery and expression preferentially in ATII cells.
Figure 6. Classification and quantification of lung cells expressing YFPnuc.
Cryosections (15 per animal; spanning the whole lung) containing YFPnuc positive cells following intratracheal delivery and electroporation were counterstained with an ATII-specific antibody. Each cell expressing YFPnuc was defined as an ATII, ATI, airway, or unclassified cell. ATII cells were identified by positive antibody marker staining. ATI cells were identified by location in the lung and a flat nuclear morphology. Airway cells were identified by location in the lung. Cells expressing YFPnuc in the periphery were not scored. (*) indicates statistical differences with p<.05 by a paired one-tail t-test. Data are plotted as mean ± st. dev. from three or more animals.
Discussion
One of the more important requirements for safe and effective gene therapy is the limitation of gene expression to specific cell types. To date, the majority of techniques developed are directed at the transcriptional and cell entry levels. We have shown in previous studies that specific DNA sequences can be included on plasmids to mediate nuclear import in non-dividing cells and that this paradigm can be used to promote and/or restrict gene expression in desired cells 4,23,24. In this report, we demonstrate that the human SP-C promoter is a cell-specific DTS that acts in alveolar type II epithelial cells. The sequences needed for nuclear import map to the proximal 318 nucleotides of the SP-C promoter, and mutagenesis has revealed that multiple transcription factor binding sites within this region are required for nuclear import activity. Moreover, following gene transfer to the non-dividing cell populations in the lungs of mice, we have shown that the SP-C promoter enhances gene delivery and expression in preferentially in alveolar type II cells.
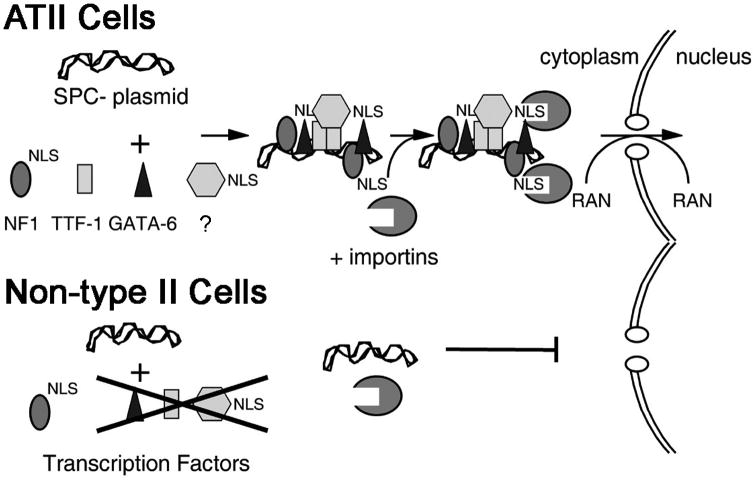
We have proposed a model for ATII-specific plasmid DNA nuclear import based on important transcription factor binding sites (Fig. 7). Based on the requirement of multiple transcription factor binding sites for nuclear import activity, our results suggest that a multimeric protein complex forms on the promoter to drive transport through the nuclear pore complex via importinα and/or importinβ, the NLS receptors 7,25. Whether inclusion of multiple copies of the SP-C DTS will increase plasmid nuclear import beyond that seen with one copy has not been evaluated, but it would follow that by increasing the number of potential transcription factor binding sites, the likelihood of forming productive DNA-protein complexes would increase as would nuclear localization of the plasmid. This remains to be seen. Although the nature of the complex is not known, our data indicates it is dependent on a highly specific set of proteins, including NFI, GATA-6, and TTF-1. TTF-1 has been shown to bind to the SP-C promoter and to be an important regulator of SP-C promoter transcription 16. It also has been shown that TTF-1 binding is required for the activation of the promoters of surfactant protein A and B 26,27. According to our data, since the promoters of surfactant protein A and B do not mediate nuclear import in ATII cells, TTF-1 binding alone may not be sufficient to drive plasmid nuclear import.
Figure 7. Model of alveolar epithelial cell-specific nuclear import.
According to the mutagenesis studies in MLE-12 cells, it appears that the central three sites binding NFI, TTF-1, and GATA-6 are required for SP-C promoter nuclear import activity. The most proximal and distal NFI sites seem to be least important. Although it is unknown why certain sequences act as DNA nuclear targeting sequences while others do not, our hypothesis is that certain sequences will bind transcription factors and other proteins in distinct orientations where their nuclear localization signals are either exposed and accessible to importin-β binding, or masked from this interaction by another protein or the DNA itself. For example, based on the structure of the TTF-1 protein 28, the NLS is located within the DNA binding site, which likely renders the NLS inaccessible to the importin machinery when DNA is bound. However, since it has been shown that other factors such as GATA-6, TAZ, and the NFI proteins directly interact with TTF-1 and synergistically activate the SP-C promoter 19,21,29, it is possible that the NLSs of these other proteins are exposed and thus involved in plasmid nuclear import while TTF-1 acts only as a required scaffold protein.
Controlling gene expression at the level of nuclear import provides several advantages over other strategies. Limitation of transcription using cell-specific promoters is effective in restricting gene expression to specific cell types, yet the levels of expression are limited by the endogenous transcriptional control of the given promoter. Using DNA nuclear targeting sequences, any promoter can be used to drive expression of the transgene (provided it is active in these cells), including strong viral promoters like the cytomegalovirus immediate early enhancer/promoter, long-term expressing promoters such as the ubiquitin ligase C promoter 30,31, or inducible promoters such as the tet on/off system 32. In this way, it is possible to use the most transcriptionally suitable promoter to drive transcription while limiting nuclear import via sequence-specific nuclear import of plasmid DNA to desired cell types. Although the utility of this strategy is dependent on targeting cells that are not undergoing active cell division, considering that the vast majority of cells in vivo are either slowly or non-dividing, including those of the pulmonary epithelium, this strategy represents a novel strategy for cell-specific targeting of gene expression in vivo.
While we have shown that inclusion of the SP-C promoter on a plasmid will enhance gene transfer and expression preferentially in nondividing ATII cells in the lung, several other cell types also take up and express transgenes from these plasmids. The fact that the vast majority of these other cells are BrdU-positive suggests that they have likely undergone cell division between the time the plasmids were delivered by electroporation and assayed for gene expression two days later. We and others have shown that in the presence of cell division, there is no sequence-specificity to nuclear localization of plasmids 3,33,34, and since a ubiquitously active CMV promoter is used to drive the YFPnuc transgene, gene expression would be expected in any cell that divides prior to analysis. However, it is also possible that some of the gene expression seen in non-type II cells may be due to specific nuclear import of the SP-C promoter containing plasmids in these cells. Indeed, bronchial and large airway columnar epithelial cells express levels of NF1, GATA-6, and TAZ detectable by immunohistochemistry in human lung sections 35 and TTF-1 is expressed in bronchial epithelial and goblet cells as well 36. Regardless of the mechanism, this highlights the utility, and limitations, of using nuclear import sequences to restrict gene delivery to desired non-dividing cells.
Acknowledgments
Funding: NIH grants EB00903 and HL81148 and a predoctoral training fellowship from the Midwest Affiliate of the American Heart Association (JVD).
We thank Josh Gasiorowski for technical advice and members of the Dean lab for stimulating discussions. We also thank Lee Davies (Oxford) for help with EGFP visualization in cryosections. This work was supported in part by NIH grants EB00903 and HL81148 and a predoctoral training fellowship from the Midwest Affiliate of the American Heart Association (JVD).
References
- 1.Dean DA. Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res. 1997;230:293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- 2.Langle-Rouault F, Patzel V, Benavente A, Taillez M, Silvestre N, Bompard A, et al. Up to 100-fold increase of apparent gene expression in the presence of Epstein-Barr virus oriP sequences and EBNA1: implications of the nuclear import of plasmids. J Virol. 1998;72:6181–6185. doi: 10.1128/jvi.72.7.6181-6185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean DA, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear entry. Exp Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vacik J, Dean BS, Zimmer WE, Dean DA. Cell-specific nuclear import of plasmid DNA. Gene Therapy. 1999;6:1006–1014. doi: 10.1038/sj.gt.3300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesika A, Grigoreva I, Zohar M, Reich Z. A regulated, NFkappaB-assisted import of plasmid DNA into mammalian cell nuclei. Mol Ther. 2001;3:653–657. doi: 10.1006/mthe.2001.0312. [DOI] [PubMed] [Google Scholar]
- 6.Goncalves C, Ardourel MY, Decoville M, Breuzard G, Midoux P, Hartmann B, et al. An optimized extended DNA kappa B site that enhances plasmid DNA nuclear import and gene expression. J Gene Med. 2009;11:401–411. doi: 10.1002/jgm.1312. [DOI] [PubMed] [Google Scholar]
- 7.Wilson GL, Dean BS, Wang G, Dean DA. Nuclear import of plasmid DNA in digitonin-permeabilized cells requires both cytoplasmic factors and specific DNA sequences. J Biol Chem. 1999;274:22025–22032. doi: 10.1074/jbc.274.31.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colin M, Moritz S, Fontanges P, Kornprobst M, Delouis C, Keller M, et al. The nuclear pore complex is involved in nuclear transfer of plasmid DNA condensed with an oligolysine-RGD peptide containing nuclear localisation properties. Gene Ther. 2001;8:1643–1653. doi: 10.1038/sj.gt.3301572. [DOI] [PubMed] [Google Scholar]
- 9.Miller AM, Dean DA. Tissue-specific and transcription factor-mediated nuclear entry of DNA. Adv Drug Deliv Rev. 2009;61:603–613. doi: 10.1016/j.addr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Carson JA, Fillmore RA, Schwartz RJ, Zimmer WE. The smooth muscle gamma-actin gene promoter is a molecular target for the mouse bagpipe homologue, mNkx3-1, and serum response factor. J Biol Chem. 2000;275:39061–39072. doi: 10.1074/jbc.M006532200. [DOI] [PubMed] [Google Scholar]
- 11.Browning CL, Culberson DE, Aragon IV, Fillmore RA, Croissant JD, Schwartz RJ, et al. The developmentally regulated expression of serum response factor plays a key role in the control of smooth muscle-specific genes. Dev Biol. 1998;194:18–37. doi: 10.1006/dbio.1997.8808. [DOI] [PubMed] [Google Scholar]
- 12.Miller AM, Dean DA. Cell-specific nuclear import of plasmid DNA in smooth muscle requires tissue-specific transcription factors and DNA sequences. Gene Ther. 2008;15:1107–1115. doi: 10.1038/gt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasiorowski JZ, Dean DA. Postmitotic Nuclear Retention of Episomal Plasmids Is Altered by DNA Labeling and Detection Methods. Mol Ther. 2005;12:460–467. doi: 10.1016/j.ymthe.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, et al. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci U S A. 1993;90:11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasser SW, Korfhagen TR, Wert SE, Bruno MD, McWilliams KM, Vorbroker DK, et al. Genetic element from human surfactant protein SP-C gene confers bronchiolar-alveolar cell specificity in transgenic mice. Am J Physiol. 1991;261:L349–356. doi: 10.1152/ajplung.1991.261.4.L349. [DOI] [PubMed] [Google Scholar]
- 16.Kelly SE, Bachurski CJ, Burhans MS, Glasser SW. Transcription of the lung-specific surfactant protein C gene is mediated by thyroid transcription factor 1. J Biol Chem. 1996;271:6881–6888. doi: 10.1074/jbc.271.12.6881. [DOI] [PubMed] [Google Scholar]
- 17.Glasser SW, Burhans MS, Eszterhas SK, Bruno MD, Korfhagen TR. Human SP-C gene sequences that confer lung epithelium-specific expression in transgenic mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L933–945. doi: 10.1152/ajplung.2000.278.5.L933. [DOI] [PubMed] [Google Scholar]
- 18.Bachurski CJ, Kelly SE, Glasser SW, Currier TA. Nuclear factor I family members regulate the transcription of surfactant protein-C. J Biol Chem. 1997;272:32759–32766. doi: 10.1074/jbc.272.52.32759. [DOI] [PubMed] [Google Scholar]
- 19.Bachurski CJ, Yang GH, Currier TA, Gronostajski RM, Hong D. Nuclear factor I/thyroid transcription factor 1 interactions modulate surfactant protein C transcription. Mol Cell Biol. 2003;23:9014–9024. doi: 10.1128/MCB.23.24.9014-9024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korfhagen TR, Glasser SW, Wert SE, Bruno MD, Daugherty CC, McNeish JD, et al. Cis-acting sequences from a human surfactant protein gene confer pulmonary-specific gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1990;87:6122–6126. doi: 10.1073/pnas.87.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Glasser SW, Wan H, Whitsett JA. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem. 2002;277:4519–4525. doi: 10.1074/jbc.M107585200. [DOI] [PubMed] [Google Scholar]
- 22.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther. 2003;10:1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young JL, Benoit JN, Dean DA. Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature. Gene Ther. 2003;10:1465–1470. doi: 10.1038/sj.gt.3302021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young JL, Zimmer WE, Dean DA. Smooth muscle-specific gene delivery in the vasculature based on restriction of DNA nuclear import. Exp Biol Med. 2008;233:840–848. doi: 10.3181/0712-RM-331. [DOI] [PubMed] [Google Scholar]
- 25.Miller AM, Munkonge FM, Alton EW, Dean DA. Identification of Protein Cofactors Necessary for Sequence-specific Plasmid DNA Nuclear Import. Mol Ther. 2009 doi: 10.1038/mt.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno MD, Bohinski RJ, Huelsman KM, Whitsett JA, Korfhagen TR. Lung cell-specific expression of the murine surfactant protein A (SP-A) gene is mediated by interactions between the SP-A promoter and thyroid transcription factor-1. J Biol Chem. 1995;270:6531–6536. doi: 10.1074/jbc.270.12.6531. [DOI] [PubMed] [Google Scholar]
- 27.Bohinski RJ, Di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol. 1994;14:5671–5681. doi: 10.1128/mcb.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christophe-Hobertus C, Duquesne V, Pichon B, Roger PP, Christophe D. Critical residues of the homeodomain involved in contacting DNA bases also specify the nuclear accumulation of thyroid transcription factor-1. Eur J Biochem. 1999;265:491–497. doi: 10.1046/j.1432-1327.1999.00796.x. [DOI] [PubMed] [Google Scholar]
- 29.Park KS, Whitsett JA, Di Palma T, Hong JH, Yaffe MB, Zannini M. TAZ interacts with TTF-1 and regulates expression of surfactant protein-C. J Biol Chem. 2004;279:17384–17390. doi: 10.1074/jbc.M312569200. [DOI] [PubMed] [Google Scholar]
- 30.Gill DR, Smyth SE, Goddard CA, Pringle IA, Higgins CF, Colledge WH, et al. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1alpha promoter. Gene Ther. 2001;8:1539–1546. doi: 10.1038/sj.gt.3301561. [DOI] [PubMed] [Google Scholar]
- 31.Yew NS, Przybylska M, Ziegler RJ, Liu D, Cheng SH. High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiquitin promoter. Mol Ther. 2001;4:75–82. doi: 10.1006/mthe.2001.0415. [DOI] [PubMed] [Google Scholar]
- 32.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Therapy. 2000;7:401–407. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- 34.Escriou V, Carriere M, Bussone F, Wils P, Scherman D. Critical assessment of the nuclear import of plasmid during cationic lipid-mediated gene transfer. J Gene Med. 2001;3:179–187. doi: 10.1002/jgm.174. [DOI] [PubMed] [Google Scholar]
- 35.Berglund L, Bjorling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA, et al. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008;7:2019–2027. doi: 10.1074/mcp.R800013-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Park KS, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 2007;117:978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kouretas D, Karinch AM, Rishi A, Melchers K, Floros J. Conservation analysis of rat and human SP-A gene identifies 5′ flanking sequences of rat SP-A that bind rat lung nuclear proteins. Exp Lung Res. 1993;19:485–503. doi: 10.3109/01902149309064359. [DOI] [PubMed] [Google Scholar]
- 38.Bohinski RJ, Huffman JA, Whitsett JA, Lattier DL. Cis-active elements controlling lung cell-specific expression of human pulmonary surfactant protein B gene. J Biol Chem. 1993;268:11160–11166. [PubMed] [Google Scholar]
- 39.Rust K, Bingle L, Mariencheck W, Persson A, Crouch EC. Characterization of the human surfactant protein D promoter: transcriptional regulation of SP-D gene expression by glucocorticoids. Am J Respir Cell Mol Biol. 1996;14:121–130. doi: 10.1165/ajrcmb.14.2.8630261. [DOI] [PubMed] [Google Scholar]
- 40.Oshima RG, Baribault H, Caulin C. Oncogenic regulation and function of keratins 8 and 18. Cancer Metastasis Rev. 1996;15:445–471. doi: 10.1007/BF00054012. [DOI] [PubMed] [Google Scholar]