Abstract
Symptomatic individuals presenting to their primary care providers may need further evaluation and/or testing to determine whether a cancer is present. A number of issues arise in determining who needs further testing, what tests are needed, which specialists need to be involved, and how the testing can be organized and supported within a specific health-care system within a timely, coordinated, and cost-efficient manner. This article explores the challenges in the interface of primary care providers and specialists, includes evidence from prior research, and proposes research opportunities to understand and improve this phase of care.
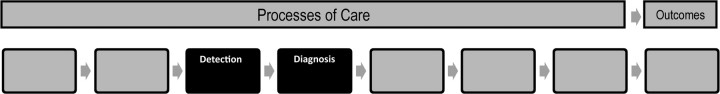
Symptomatic individuals presenting to their primary care providers may need further evaluation and/or testing to determine whether a cancer is present. A number of issues arise in determining who needs further testing, what tests are needed, which specialist or specialists need to be involved, how the testing can be organized and supported within a specific health-care system, and how to achieve desired health outcomes, including those important to patients. This article explores the challenges in the interface of primary care providers and specialists along the cancer care continuum (Figure 1), provides research context for the issues involved, and identifies research opportunities to enhance the interaction between the medical providers in the care of patients from symptoms through cancer diagnosis. Two other articles (1,2) in this supplement address issues in screening and follow-up after abnormal screening tests.
Challenges at the Interface of Primary and Specialty Care
Primary Care Perspective
Patients commonly see primary care providers before being diagnosed with cancer (3), although the vast majority of patients seen by primary care providers present with symptoms that do not lead to a cancer diagnosis. Therefore, some patients with symptoms will need additional evaluation and others may be appropriately reassured at the time of initial presentation. The first step for the primary care provider is to differentiate when symptoms are likely to be benign from when they may suggest a malignancy (“alarm” symptoms); decide when to proceed with additional testing and when to stop; and balance the potential of missing a cancer diagnosis against proceeding with possibly unnecessary, costly, and anxiety-provoking evaluations. To our knowledge, the largest study to address the question of frequency of alarm symptoms in general medical settings and their likelihood of cancer was conducted by Jones et al. (4). The study found that while patients commonly presented to their primary care providers with symptoms, such as hematuria, hemoptysis, dysphasia, and rectal bleeding, more than 90% of the patients did not have cancer (Table 1). Likewise, another study, Barton et al. (5) found that most women who present with a breast mass do not have breast cancer. Table 1 presents the positive predictive values of selected alarm symptoms based on studies conducted in primary care settings.
Table 1.
Positive predictive value (PPV)*
| Symptom | Cancer | PPV (95% CI) | Men | Women |
| Rectal bleeding† | Gastrointestinal cancer | 1.8 (1.5 to 2.2) | 1.5 (1.3 to 1.8) | |
| Hemoptysis† | Respiratory tract cancer | 5.8 (5.0 to 6.7) | 3.3 (2.6 to 4.3) | |
| Breast mass‡ | Breast cancer | Not available | 10.7 (4.6 to 16.9) |
* PPV calculated by the number of patients with symptoms who were diagnosed with cancer divided by the number of patients with symptoms, multiplied by 100%. CI = confidence interval.
Jones et al. (4) and based on observed diagnoses of cancer in the 6 months after first alarm symptom. PPVs varied by gender and age and increased when patients were followed for 3 years after presentation of symptoms.
Barton et al. (5) followed patients for at least 12 months after the presentation of symptoms.
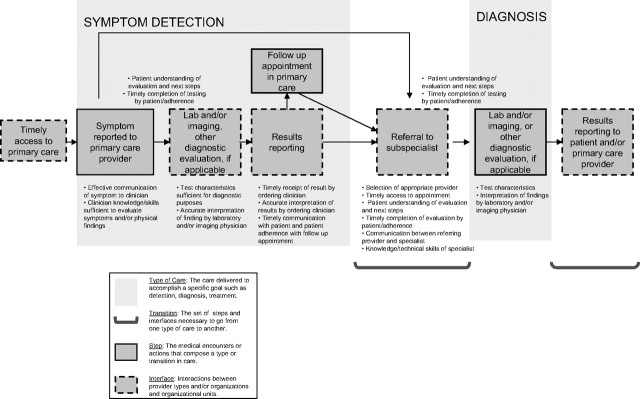
A timely and accurate initial evaluation of a presenting symptom is a critical step in the diagnostic pathway outlined in Figure 2. Once this step occurs, the primary care provider may take on additional evaluation, such as laboratory testing, imaging, and/or other diagnostic tests. At this step, the provider needs to select an appropriate diagnostic test and accurately interpret its findings. Informal or “curbside” consultations with specialists are common (6,7) and are often used to help primary care providers select appropriate diagnostic testing and treatment plans. Because of the limitations of such consultations, a formal referral is usually indicated. In referring to a specialist, the primary care provider needs to decide to which specialist the patient should be referred.
Figure 2.
Steps and interfaces from symptoms to diagnosis.
This decision is based on several factors, such as clinicians’ use and personal knowledge of specific specialists, prior experience with the specialist and the quality of prior evaluations, the availability of a preferred specialist during the desired time frame, and the patients’ insurance coverage, and often, patient preferences (8,9).
Commentary
Do patients even know whether conversations or consultations occur between primary care and specialty providers? Very little conversation occurs with patients about the mechanics of referrals.
From a Survivor
Figure 1.
Types and transitions in care that constitute the process of care across the cancer continuum.
Whether the primary care provider refers a patient for additional testing or to a specialist, he/she must explain the referral process to the patient and clarify what the patient may expect in the course of the evaluation. Once a referral is made, the primary care provider must be certain that the patient follows through with the diagnostic testing and that he/she communicates a clear rationale for the referral and the specific questions to be addressed by the specialist.
Once the patient makes the transition from detection to diagnosis, the technical details of the diagnostic process become important. Primary care providers and patients expect the specialist to provide a clear explanation about the evaluation, diagnosis, and management of the patient (10). Although delays in cancer diagnosis have been mostly attributed to clinician inaction or to reassurance following a false-negative evaluation, patient and system factors also play a role (11,12). Communication between the specialist and the provider is needed to close the loop in the evaluation process. In this supplement, Zapka (2) reviews issues relating to follow-up after an abnormal screening examination.
In summary, primary care providers face several challenges in the symptom phase of cancer diagnosis (Figure 2). First, symptoms that may be attributed to cancer are common in primary care settings, but cancer is relatively rare. As a result, primary care providers must have the knowledge and skills to determine which patients to refer and which to reassure. Second, they must decide on the most appropriate test and/or specialist needed for the diagnostic evaluation. Third, they must be sure that follow-up has been completed and the problem has come to an accurate diagnosis and/or an acceptable resolution. In each of these steps, communication with the patient and among providers is critical.
Perspective From Specialty Care
Specialists also recognize the challenge of how to best sort out those with disease from the many with symptoms but no disease. A common concern from the specialist’s perspective is the high number of unnecessary referrals (13,14). At the opposite end of the spectrum, some patients presenting with symptoms to primary care should have been referred earlier but were not. A study by Goodson and Moore (12) examined the causes for diagnostic delay of breast cancer and found that 5% of women with delays were inappropriately reassured that a malignant lump was benign without a biopsy. Korsgaard et al. (15) found that 25% of colorectal cancer patients had a greater than 50-day delay in diagnosis attributed to their primary care physician. Reasons for delay included incomplete use of guidelines, and referral letters that did not clearly state that a colorectal cancer was suspected. Jage et al. (16) found that 27% of melanoma patients at their initial presentation to primary care were given a nonmelanoma diagnosis with no further steps being initiated from that visit.
Other concerns include inappropriate investigations initiated by primary care providers that are unhelpful, increase costs, and may delay diagnoses, as well as incomplete investigations that might have made referral to a specialist unnecessary or resulted in a quicker referral. A common scenario that illustrates both these issues is a young woman with a painful breast lump referred to a surgeon, imaging or both without an attempt at fine needle aspiration. This simple test should be within the capabilities of a trained primary care physician (17,18) and can immediately identify the most likely diagnosis of a benign cyst if fluid is obtained and the mass disappears. A successful aspiration is diagnostic, therapeutic, and reduces the need for referral. In summary, the interval between symptoms and diagnosis involves several steps, each with potential challenges (Figure 2) for the specialist as for the primary care provider.
Potential Solutions: Insights From Research
The challenges outlined above represent quality-improvement opportunities in the symptoms to diagnosis phase. Donabedian's (19) structure–process–outcomes paradigm (Table 2) may be used to organize and explore what is already known about the primary care provider–specialist interface at this phase of care and to identify where opportunities might lie. In this model, structure refers to having the equipment, resources, and provider experience necessary to provide care. Process refers to the examination of the technical and interpersonal elements of care that transpire between the clinician and patient, such as the extent of the history and physical examination, use of tests, and treatments received. The outcomes include complications, survival, and patient-reported outcomes, such as health-related quality of life (20). Below, we elaborate on this paradigm in the context of the symptoms to diagnosis phase of cancer.
Table 2.
Application of the structure–process–outcomes paradigm to the symptom to diagnosis interval
| Structure |
| Sufficient primary care providers and specialists |
| Practice setting |
| Diagnostic equipment |
| Information technology |
| Process |
| Appropriate/timely referral |
| Appropriate/timely investigations |
| Appropriate communication |
| Outcomes |
| Cancer mortality |
| Morbidity (treatment, cancer, psychological) |
| Provider satisfaction/burden |
| System costs |
Structure includes aspects of health-care systems that are of major interest to policy makers across the cancer control continuum. First, the health-care system's financial structure may have implications on access, costs, patterns of care, and outcomes (21). In the United Kingdom, the simplicity of a single-payer system has been credited with helping to organize quality-improvement activities resulting in quality gains at the primary care level (22,23). However, comparisons of managed care and fee-for-service plans in the United States have not shown, on average, significant differences in the cancer stage at presentation or patterns of care for colorectal, breast, and prostate cancers (21,24). Pay-for-performance approaches did not result in quality gains in the United Kingdom, perhaps a ceiling effect, because of their previous other quality performance activities (22,25) but are being promoted as possible methods of improving quality within primary care in the United States (26). Second, information technology is an important aspect of the structure. As reviewed by Shekelle et al. (27), information technology has the potential to transform health care, making it safer, more effective, and more efficient. However, widespread implementation has been limited by a lack of generalizable knowledge about what types of information technology and implementation methods will both improve care and also manage costs for specific health systems. As suggested by Hesse et al. in this supplement (28), information technology alone will not be able to solve the challenges in the interface between primary care and specialists but can enhance in the retrieval of relevant information, communication between providers and/or patients, and monitor for the resolution of the evaluation process.
More specific to the symptoms to diagnosis phase of cancer care, structure includes having sufficient physicians with the appropriate training and/or experience as well as sufficient diagnostic equipment in place to perform the appropriate tests within the benchmarked time. Delays in diagnosis often occur because of physician misdiagnosis (29), and, thus, educational interventions to improve the recognition of symptoms or interpretation of reports by physicians may be required. The appropriateness, training, and use of different diagnostic procedures in primary care, including fine needle aspiration, sigmoidoscopy, endometrial biopsy, and even dermatoscopy for skin lesions, have been published (30–33), but research into the need, training, and competency testing of these skills may need to be better defined. Specialized diagnostic centers have been created, particularly for patients with breast and prostate problems, yet despite their proliferation, little information is available on how they improve process measures, cost-effectiveness, or patient satisfaction (34–37).
We have outlined several process issues in Figure 2 that are addressed below. First, process issues in the model of Donabedian would include appropriate evaluation and testing of symptoms that might be related to an underlying malignancy. One potential approach to improve the evaluation process is to implement diagnostic practice guidelines. Many guidelines exist to assist clinicians how to evaluate symptoms, who should be tested, and what tests to order (38–44). Conceptual tools for development of new guidelines (45) and processes to achieve consensus on guidelines have been described (46,47). Guidelines have been shown to improve structure and processes of care, but with much more modest effects on patient outcomes (48,49). Monitoring for adherence with guidelines and subsequent improvements in the desired outcomes is needed (50–53). It is important to note that the same guideline may not work in different health-care settings (13,54); thus, success requires careful planning, implementation, and evaluation within a given system (55).
Another process issue noted in Figure 2 is appropriate and timely communication between primary care providers and specialists. Primary care providers share anecdotal accounts of referring patients to specialists, who then evaluate, perform procedures, and make additional referrals but neglect to communicate with the referring physician. Often, the patients are left to update the primary care providers with the details of the evaluation process (56). Consult letters often omit relating important information to the primary care provider, and referral letters from the specialist are equally deficient (57). Primary care providers have reported that written communication is inadequate for the complexity of cancer care and would prefer face-to-face or telephone communication (58). A Cochrane review of intervention studies found gaps in this area of research but concluded that distributing guidelines with standard forms for referrals combined with involving specialists in education would be most likely to improve the overall referral process (59). Another systematic review found that educational materials aimed at health-care providers alone may have a modest effect on process outcomes, but not on patient outcomes, and that information about how to optimize educational materials is insufficient (60). Gagliardi (61) found that primary care physicians are receptive to educational interventions attached to consult letters; however, this is probably not commonly done in clinical settings. Furthermore, the use of standardized letters has been found to be useful (62). Communication between the specialists and the primary care providers is crucial in relaying the evaluation process and whether the process has led to a definitive diagnosis, a resolution of the problem, or need for additional evaluation. Gaps in relaying this information may lead not only to suboptimal care but also in delays in diagnosis and possible litigation.
Communication between providers as well as with patients is important in the interval between symptoms to diagnosis phase (Figure 2). It is critical that patients understand the goal of the evaluation and the implications of not completing the diagnostic process. Health literacy needs to be taken into account when communicating with patients (63).
COMMENTARY
We're talking about a “triangle of communication” between the primary care provider, the cancer specialists, and the patients. How can we eliminate this triangle by having a single point of common information and communication?
From a Supplement Author
Understanding the financial restrictions and insurance limitations, particularly in the US health-care system, cannot be underestimated when evaluating a patient for a possible cancer diagnosis and advising diagnostic evaluation. Such evaluation is often costly and time-consuming, requiring even insured patients to pay co-pays and miss work days. Lack of insurance has been cited as a barrier to early diagnosis of cancer among adolescents and young adults (64).
Reaching a timely diagnosis is another process measure that needs to be considered. In this phase of care, the patient often contributes to the delay (29). To reduce waiting times, several studies have suggested the implementation of a facilitated referral process or one-stop investigation clinics (65–67). Although minimizing delays in diagnosis is often desired, the objectives of timely diagnosis need to be clear. Most patients and many physicians believe that delays in diagnosis will result in worse cancer survival outcomes; however, a systematic review found incomplete evidence regarding the impact on cancer-related mortality of waiting for surgery (68). Of the 27 studies found that could address the question, “Do delays in surgery of greater than 12 weeks affect survival?”, the answer was “No” in 21 (78%). Extrapolating this information to diagnostic delay, it is unlikely that the relatively small improvements that could possibly be made in reducing waiting times from presentation to diagnosis would result in measurable improvements in cancer mortality. A greater challenge, but perhaps a greater potential in changing mortality outcomes, could come from improvements in patient delay in presenting to the clinical setting for their initial evaluation.
Improvements in diagnostic delay, however, may have impact on psychological morbidity outcomes. Few studies have assessed this outcome with established quality of life tools, although several have reported the effects of waiting on patient dissatisfaction and distress (68–72). Interestingly, there was a much stronger correlation between waiting time from diagnosis to surgery and satisfaction (72). Efforts by primary care physicians and specialists working together to reduce diagnostic delays are important, but probably more so for patient psychosocial well-being and satisfaction with the health-care system than with medical outcomes.
Other factors, such as system costs and/or burden, also need to be taken into account when trying to reduce delays in diagnosis. Thus, an important challenge for multiple stakeholders within a health-care system, including primary care providers, specialists, and patients, is to agree upon an appropriate wait time, given all of the considerations. In 1999, in response to outcries for timely evaluation of breast symptoms, the United Kingdom put into place a 2-week referral rule to reduce diagnostic delays. The rule stipulated that patients who presented to their primary care providers with breast-related “alarm” symptoms are expeditiously referred to a breast clinic within a 2-week period of time; those presenting with other breast-related symptoms were advised to be fully evaluated by their primary care providers or referred for nonurgent consultation. Potter et al. (73) found that since the imposition of this rule, the number of 2-week wait referrals increased significantly but the routine referrals remained the same, suggesting that more women were being evaluated for breast symptoms following the rule than before the rule. Interestingly, there were no differences in the rates of breast cancer diagnosis among those referred under the 2-week rule and those referred routinely, suggesting that women triaged to the expeditious referrals were not at a higher risk for breast cancer than were women who remained in the routine referral group. Finally, the authors found that the 2-week rule had an untoward effect on clinic capacity and increased waiting times for routine referrals.
The effect of the 2-week rule for referring patients suspected to have colorectal cancer was examined in a systematic review (74) that found no difference in the rates of cancer diagnosed among patients referred expeditiously. Furthermore, the 2-week rule did not appear to identify patients at earlier stages of cancer (4). Although the United Kingdom's “2-week rule” example did not show unequivocal success, based on this initial experience, this health system is now likely better equipped to evaluate the structure–process–outcomes and make the necessary changes.
Implications for Future Research
The existing literature describing “alarm” symptoms has been informative. However, more research addressing the frequency of such symptoms in primary care settings and the risks of associated cancers is needed. Because of selection bias, it is not sufficient to provide information on the likelihood of symptoms among patients seen in referral centers (75–78) nor is it helpful to use information from studies without a comparison group (79,80). Once sufficient data in appropriate settings are collected, it would be informative to develop predictive models that may be used in clinical settings to risk stratify patients and guide further evaluation.
The diagnostic pathway includes a number of steps that may affect the quality of care (Figure 2) and may be improved through research. Research opportunities in most health-care systems aimed at improving the provider interplay in the symptoms to diagnosis phase may follow the principles of quality improvement. Primary care physicians and specialists within a given system need to come to consensus on what they feel are the important structure–process–outcomes measures for a given cancer and then determine what needs to change. Guidance on the collection of important indicators, for example, days to resolution of findings, patient satisfaction, and/or others may come from national initiatives in quality improvement (81,82). Testing whether a change leads to an improvement can be done using the Plan-Do-Study-Act (PDSA) cycle—by planning it, trying it, observing the results, and acting on what is learned (83). Although this research opportunity is rather obvious, a survey of health-care systems would likely find few that can identify specific guidelines, wait time information, or benchmarks that are used consistently across their own system.
Improvements in physician communication with other physicians can come from evaluating the effect of disseminating guidelines with structured referral sheets and involvement of consultants in educational activities, and perhaps the effect of financial incentives around referrals (59). Patient–physician communication in this phase is critical and could be improved with the use of user-friendly tools, electronic and/or paper communication. Finally, as with all parts of the cancer control continuum, it is challenging to move forward in the symptoms to diagnosis phase without investment in quality measurement systems, including information technology (82). This topic is further addressed by Hesse et al. (28) in this supplement.
Conclusions
The phase in the cancer care continuum from symptoms to diagnosis presents a number of issues that could be fruitfully explored by research. First, symptoms are common, but diagnoses are relatively rare, so methods to assist primary care providers to make appropriate diagnoses and avoid unnecessary testing and referrals are needed. Second, the referral process includes several steps and requires adequate communication between primary care providers, specialists, and patients. Third, it is important to define appropriate wait times that balance better psychological outcomes with lower system costs and/or burden. And finally, primary care providers and specialists need to implement and examine the quality improvement strategies in structures, processes, or outcomes within their own health-care system and/or practice.
References
- 1.Anhang Price R, Zapka J, Edwards H, Taplin SH. Organizational factors and the cancer screening process. J Natl Cancer Inst Monogr. 2010;(40):52–71. doi: 10.1093/jncimonographs/lgq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zapka J, Taplin SH, Anhang Price R, Cranos C, Yabroff R. Factors in quality care—the case of follow-up to abnormal cancer screening tests—problems in the steps and interfaces of care. J Natl Cancer Inst Monogr. 2010;(40):38–51. doi: 10.1093/jncimonographs/lgq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allgar VL, Neal RD. General practitioners’ management of cancer in England: secondary analysis of data from the National Survey of NHS Patients-Cancer. Eur J Cancer Care (Engl) 2005;14(5):409–416. doi: 10.1111/j.1365-2354.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 4.Jones R, Latinovic R, Charlton J, Gulliford MC. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ. 2007;334(7602):1040. doi: 10.1136/bmj.39171.637106.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med. 1999;130(8):651–657. doi: 10.7326/0003-4819-130-8-199904200-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kuo D, Gifford DR, Stein MD. Curbside consultation practices and attitudes among primary care physicians and medical subspecialists. JAMA. 1998;280(10):905–909. doi: 10.1001/jama.280.10.905. [DOI] [PubMed] [Google Scholar]
- 7.Keating NL, Zaslavsky AM, Ayanian JZ. Physicians’ experiences and beliefs regarding informal consultation. JAMA. 1998;280(10):900–904. doi: 10.1001/jama.280.10.900. [DOI] [PubMed] [Google Scholar]
- 8.Forrest CB, Nutting PA, Starfield B, von Schrader S. Family physicians’ referral decisions: results from the ASPN referral study. J Fam Pract. 2002;51(3):215–222. [PubMed] [Google Scholar]
- 9.Franks P, Clancy CM. Referrals of adult patients from primary care: demographic disparities and their relationship to HMO insurance. J Fam Pract. 1997;45(1):47–53. [PubMed] [Google Scholar]
- 10.Piterman L, Koritsas S. Part II. General practitioner-specialist referral process. Intern Med J. 2005;35(8):491–496. doi: 10.1111/j.1445-5994.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 11.Caplan LS, Helzlsouer KJ, Shapiro S, Wesley MN, Edwards BK. Reasons for delay in breast cancer diagnosis. Prev Med. 1996;25(2):218–224. doi: 10.1006/pmed.1996.0049. [DOI] [PubMed] [Google Scholar]
- 12.Goodson WH, III, Moore DH. Causes of physician delay in the diagnosis of breast cancer. Arch Intern Med. 2002;162(12):1343–1348. doi: 10.1001/archinte.162.12.1343. [DOI] [PubMed] [Google Scholar]
- 13.Charles RJ, Chak A, Cooper GS, Wong RC, Sivak MV., Jr Use of open access in GI endoscopy at an academic medical center. Gastrointest Endosc. 1999;50(4):480–485. doi: 10.1016/s0016-5107(99)70069-6. [DOI] [PubMed] [Google Scholar]
- 14.Hanna SJ, Muneer A, Khalil KH. The 2-week wait for suspected cancer: time for a rethink? Int J Clin Pract. 2005;59(11):1334–1339. doi: 10.1111/j.1368-5031.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 15.Korsgaard M, Pedersen L, Laurberg S. Delay of diagnosis and treatment of colorectal cancer—a population-based Danish study. Cancer Detect Prev. 2008;32(1):45–51. doi: 10.1016/j.cdp.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Jage G, Kuhne KH, Gollnick H. Observations on the “pretherapeutic phase” of malignant melanoma of the skin 1988–1990 and 1993–1995. Hautarzt. 1998;49(11):844–849. doi: 10.1007/s001050050836. [DOI] [PubMed] [Google Scholar]
- 17.Lucas JH, Cone DL. Breast cyst aspiration. Am Fam Physician. 2003;68(10):1983–1986. [PubMed] [Google Scholar]
- 18.Heisey R, Mahoney L, Watson B. Management of palpable breast lumps. Consensus guideline for family physicians. Can Fam Physician. 1999;45(August):1926–1932. [PMC free article] [PubMed] [Google Scholar]
- 19.Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q. 2005;83(4):691–729. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litwin MS. Health services research. Semin Radiat Oncol. 2008;18(3):152–160. doi: 10.1016/j.semradonc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Riley GF, Warren JL, Potosky AL, Klabunde CN, Harlan LC, Osswald MB. Comparison of cancer diagnosis and treatment in Medicare fee-for-service and managed care plans. Med Care. 2008;46(10):1108–1115. doi: 10.1097/MLR.0b013e3181862565. [DOI] [PubMed] [Google Scholar]
- 22.Roland M. The future of primary care: lessons from the U.K. N Engl J Med. 2008;359(20):2087–2092. doi: 10.1056/NEJMp0805633. [DOI] [PubMed] [Google Scholar]
- 23.Campbell SM, Roland MO, Middleton E, Reeves D. Improvements in quality of clinical care in English general practice 1998–2003: longitudinal observational study. BMJ. 2005;331(7525):1121. doi: 10.1136/bmj.38632.611123.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst. 2001;93(7):501–515. doi: 10.1093/jnci/93.7.501. [DOI] [PubMed] [Google Scholar]
- 25.Campbell SM, Reeves D, Kontopantelis E, Sibbald B, Roland M. Effects of pay for performance on the quality of primary care in England. N Engl J Med. 2009;361(4):368–378. doi: 10.1056/NEJMsa0807651. [DOI] [PubMed] [Google Scholar]
- 26.Goroll AH. The future of primary care: reforming physician payment. N Engl J Med. 2008;359(20):2087–2090. doi: 10.1056/NEJMp0805765. [DOI] [PubMed] [Google Scholar]
- 27.Shekelle PG, Morton SC, Keeler EB. Costs and benefits of health information technology. Evid Rep Technol Assess (Full Rep) 2006;132(April):1–71. doi: 10.23970/ahrqepcerta132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hesse BW, Hanna C, Massett HA, Hesse NK. Outside the box: will information technology be a viable intervention to improve the quality of cancer care? J Natl Cancer Inst Monogr. 2010;(40):81–89. doi: 10.1093/jncimonographs/lgq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald S, Macleod U, Campbell NC, Weller D, Mitchell E. Systematic review of factors influencing patient and practitioner delay in diagnosis of upper gastrointestinal cancer. Br J Cancer. 2006;94(9):1272–1280. doi: 10.1038/sj.bjc.6603089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatia I, Slade S, Watson WJ, Mahoney L. Teaching family practice residents breast cyst aspiration. Can Fam Physician. 1999;45(August):1910–1915. [PMC free article] [PubMed] [Google Scholar]
- 31.Ashley OS, Nadel M, Ransohoff DF. Achieving quality in flexible sigmoidoscopy screening for colorectal cancer. Am J Med. 2001;111(8):643–653. doi: 10.1016/s0002-9343(01)00959-7. [DOI] [PubMed] [Google Scholar]
- 32.Vigod SN, Stewart DE. Management of abnormal uterine bleeding by northern, rural and isolated primary care physicians: part I—how are we doing? BMC Womens Health. 2002;2(1):10. doi: 10.1186/1472-6874-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Argenziano G, Puig S, Zalaudek I, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J Clin Oncol. 2006;24(12):1877–1882. doi: 10.1200/JCO.2005.05.0864. [DOI] [PubMed] [Google Scholar]
- 34.Castellanos MR, Conte J, Fadel DA, et al. Improving access to breast health services with an interdisciplinary model of care. Breast J. 2008;14(4):353–356. doi: 10.1111/j.1524-4741.2008.00597.x. [DOI] [PubMed] [Google Scholar]
- 35.Patel RS, Smith DC, Reid I. One stop breast clinics—victims of their own success? A prospective audit of referrals to a specialist breast clinic. Eur J Surg Oncol. 2000;26(5):452–454. doi: 10.1053/ejso.1999.0920. [DOI] [PubMed] [Google Scholar]
- 36.Kavanagh AG, Lee JC, Donnelly B. Time to treatment of prostate cancer through the Calgary Prostate Institute rapid access clinic. Can J Urol. 2008;15(2):3975–3979. [PubMed] [Google Scholar]
- 37.Gagliardi A, Grunfeld E, Evans WK. Evaluation of diagnostic assessment units in oncology: a systematic review. J Clin Oncol. 2004;22(6):1126–1135. doi: 10.1200/JCO.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 38.Kvale PA. Chronic cough due to lung tumors: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(supp 1l):147S–153S. doi: 10.1378/chest.129.1_suppl.147S. [DOI] [PubMed] [Google Scholar]
- 39.Choyke PL. Radiologic evaluation of hematuria: guidelines from the American College of Radiology's appropriateness criteria. Am Fam Physician. 2008;78(3):347–352. [PubMed] [Google Scholar]
- 40.Canadian Association of Radiation Oncologists. The palpable breast lump: information and recommendations to assist decision-making when a breast lump is detected. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. CMAJ. 1998;158(suppl 3):S3–S8. [PubMed] [Google Scholar]
- 41.Referral Guidelines for Suspected Cancer. London, UK: 2000. Department of Health. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4008746. Accessed September 9, 2009. [Google Scholar]
- 42.Debnath D, Dielehner N, Gunning KA. Guidelines, compliance, and effectiveness: a 12 months’ audit in an acute district general healthcare trust on the two week rule for suspected colorectal cancer. Postgrad Med J. 2002;78(926):748–751. doi: 10.1136/pmj.78.926.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts DL, Anstey AV, Barlow RJ, et al. U.K. guidelines for the management of cutaneous melanoma. Br J Dermatol. 2002;146(1):7–17. doi: 10.1046/j.1365-2133.2001.04614.x. [DOI] [PubMed] [Google Scholar]
- 44.BRIDGE Study Group. Responses of primary health care professionals to UK national guidelines on the management and referral of women with breast conditions. J Eval Clin Pract. 2002;8(3):319–325. doi: 10.1046/j.1365-2753.2002.00335.x. [DOI] [PubMed] [Google Scholar]
- 45.Browman GP, Levine MN, Mohide EA, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13(2):502–512. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 46.Browman GP, Newman TE, Mohide EA, et al. Progress of clinical oncology guidelines development using the Practice Guidelines Development Cycle: the role of practitioner feedback. J Clin Oncol. 1998;16(3):1226–1231. doi: 10.1200/JCO.1998.16.3.1226. [DOI] [PubMed] [Google Scholar]
- 47.Graham ID, Harrison MB, Lorimer K, et al. Adapting national and international leg ulcer practice guidelines for local use: the Ontario Leg Ulcer Community Care Protocol. Adv Skin Wound Care. 2005;18(6):307–318. doi: 10.1097/00129334-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Lugtenberg M, Burgers JS, Westert GP. Effects of evidence-based clinical practice guidelines on quality of care: a systematic review. Qual Saf Health Care. 2009;18(5):385–392. doi: 10.1136/qshc.2008.028043. [DOI] [PubMed] [Google Scholar]
- 49.Thomas L, Cullum N, McColl E, Rousseau N, Soutter J, Steen N. Guidelines in professions allied to medicine. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000349. (2):CD000349. [DOI] [PubMed] [Google Scholar]
- 50.Uff CE, Lawson DD, Giles G, Bavetta S. The two-week waiting time standard for cancer: a neurosurgical perspective. Br J Neurosurg. 2005;19(4):334–337. doi: 10.1080/02688690500305365. [DOI] [PubMed] [Google Scholar]
- 51.Hawary AM, Warburton HE, Brough RJ, et al. The ‘2-week wait’ rule for referrals for suspected urological cancers—urgent need for refinement of criteria. Ann R Coll Surg Engl. 2008;90(6):517–522. doi: 10.1308/003588408X301082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bjerregaard NC, Tottrup A, Sorensen HT, Laurberg S. Evaluation of the Danish national strategy for selective use of colonoscopy in symptomatic outpatients without known risk factors for colorectal cancer. Scand J Gastroenterol. 2007;42(2):228–236. doi: 10.1080/00365520600815662. [DOI] [PubMed] [Google Scholar]
- 53.Panter SJ, Bramble MG, O'Flanagan H, Hungin AP. Urgent cancer referral guidelines: a retrospective cohort study of referrals for upper gastrointestinal adenocarcinoma. Br J Gen Pract. 2004;54(505):611–613. [PMC free article] [PubMed] [Google Scholar]
- 54.Chan TH, Goh KL. Appropriateness of colonoscopy using the ASGE guidelines: experience in a large Asian hospital. Chin J Dig Dis. 2006;7(1):24–32. doi: 10.1111/j.1443-9573.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 55.Hemingway DM, Jameson J, Kelly MJ. Straight to test: introduction of a city-wide protocol driven investigation of suspected colorectal cancer. Colorectal Dis. 2006;8(4):289–295. doi: 10.1111/j.1463-1318.2005.00935.x. [DOI] [PubMed] [Google Scholar]
- 56.van der Kam WJ, Branger PJ, van Bemmel JH, Meyboom-de Jong B. Communication between physicians and with patients suffering from breast cancer. Fam Pract. 1998;15(5):415–419. doi: 10.1093/fampra/15.5.415. [DOI] [PubMed] [Google Scholar]
- 57.McConnell D, Butow PN, Tattersall MH. Improving the letters we write: an exploration of doctor-doctor communication in cancer care. Br J Cancer. 1999;80(3-4):427–437. doi: 10.1038/sj.bjc.6690374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dworkind M, Towers A, Murnaghan D, Guibert R, Iverson D. Communication between family physicians and oncologists: qualitative results of an exploratory study. Cancer Prev Control. 1999;3(2):137–144. [PubMed] [Google Scholar]
- 59.Akbari A, Mayhew A, Al Alawi MA, et al. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005471.pub2. (4):CD005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farmer AP, Legare F, Turcot L, et al. Printed educational materials: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD004398.pub2. (3):CD004398. [DOI] [PubMed] [Google Scholar]
- 61.Gagliardi A. Use of referral reply letters for continuing medical education: a review. J Contin Educ Health Prof. 2002;22(4):222–229. doi: 10.1002/chp.1340220406. [DOI] [PubMed] [Google Scholar]
- 62.Braun TC, Hagen NA, Smith C, Summers N. Oncologists and family physicians. Using a standardized letter to improve communication. Can Fam Physician. 2003;49(July):882–886. [PMC free article] [PubMed] [Google Scholar]
- 63.Davis TC, Williams MV, Marin E, Parker RM, Glass J. Health literacy and cancer communication. CA Cancer J Clin. 2002;52(3):134–149. doi: 10.3322/canjclin.52.3.134. [DOI] [PubMed] [Google Scholar]
- 64.Martin S, Ulrich C, Munsell M, Taylor S, Lange G, Bleyer A. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist. 2007;12(7):816–824. doi: 10.1634/theoncologist.12-7-816. [DOI] [PubMed] [Google Scholar]
- 65.Khan MA, Mangold LA, Epstein JI, Boitnott JK, Walsh PC, Partin AW. Impact of surgical delay on long-term cancer control for clinically localized prostate cancer. J Urol. 2004;172(5, pt 1):1835–1839. doi: 10.1097/01.ju.0000140277.08623.13. [DOI] [PubMed] [Google Scholar]
- 66.Gui GP, Allum WH, Perry NM, et al. Clinical audit of a specialist symptomatic breast clinic. J R Soc Med. 1995;88(6):330–333. [PMC free article] [PubMed] [Google Scholar]
- 67.Decker KM, Harrison M, Chateau D. Influence of direct referrals on time to diagnosis after an abnormal breast screening result. Cancer Detect Prev. 2004;28(5):361–367. doi: 10.1016/j.cdp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Taylor M, Turner D, Latosinsky S, Choptain N. Toward Canadian Benchmarks for Health Services Wait Times: Determining Acceptable Waiting Times for the Surgical Treatment of Solid Organ Malignancies—A Systematic Review. CancerCare Manitoba. http://www.cancercare.mb.ca/home/cancer_research/epidemiology_and_cancer_registry/reports/. Accessed September 9, 2009. [Google Scholar]
- 69.Leydon GM, Bynoe-Sutherland J, Coleman MP. The journey towards a cancer diagnosis: the experiences of people with cancer, their family and carers. Eur J Cancer Care (Engl) 2003;12(4):317–326. doi: 10.1046/j.1365-2354.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 70.Thorne SE, Harris SR, Hislop TG, Vestrup JA. The experience of waiting for diagnosis after an abnormal mammogram. Breast J. 1999;5(1):42–51. doi: 10.1046/j.1524-4741.1999.005001042.x. [DOI] [PubMed] [Google Scholar]
- 71.Risberg T, Sorbye SW, Norum J, Wist EA. Diagnostic delay causes more psychological distress in female than in male cancer patients. Anticancer Res. 1996;16(2):995–999. [PubMed] [Google Scholar]
- 72.Porter GA, Inglis KM, Wood LA, Veugelers PJ. Access to care and satisfaction in colorectal cancer patients. World J Surg. 2005;29(11):1444–1451. doi: 10.1007/s00268-005-7955-1. [DOI] [PubMed] [Google Scholar]
- 73.Potter S, Govindarajulu S, Shere M, et al. Referral patterns, cancer diagnoses, and waiting times after introduction of two week wait rule for breast cancer: prospective cohort study. BMJ. 2007;335(7614):288. doi: 10.1136/bmj.39258.688553.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorne K, Hutchings HA, Elwyn G. The effects of the Two-Week Rule on NHS colorectal cancer diagnostic services: a systematic literature review. BMC Health Serv Res. 2006;6(April 3):43. doi: 10.1186/1472-6963-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fransen GA, Janssen MJ, Muris JW, Laheij RJ, Jansen JB. Meta-analysis: the diagnostic value of alarm symptoms for upper gastrointestinal malignancy. Aliment Pharmacol Ther. 2004;20(10):1045–1052. doi: 10.1111/j.1365-2036.2004.02251.x. [DOI] [PubMed] [Google Scholar]
- 76.Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology. 2006;131(2):390–401. doi: 10.1053/j.gastro.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 77.Hamilton W, Sharp D. Diagnosis of lung cancer in primary care: a structured review. Fam Pract. 2004;21(6):605–611. doi: 10.1093/fampra/cmh605. [DOI] [PubMed] [Google Scholar]
- 78.Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291(22):2705–2712. doi: 10.1001/jama.291.22.2705. [DOI] [PubMed] [Google Scholar]
- 79.Richard MA, Grob JJ, Avril MF, et al. Delays in diagnosis and melanoma prognosis (II): the role of doctors. Int J Cancer. 2000;89(3):280–285. doi: 10.1002/1097-0215(20000520)89:3<280::aid-ijc11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 80.Summerton N, Mann S, Rigby AS, Ashley J, Palmer S, Hetherington JW. Patients with new onset haematuria: assessing the discriminant value of clinical information in relation to urological malignancies. Br J Gen Pract. 2002;52(477):284–289. [PMC free article] [PubMed] [Google Scholar]
- 81.Mainz J, Hjulsager M, Og MT, Burgaard J. National benchmarking between the Nordic countries on the quality of care. J Surg Oncol. 2009;99(8):505–507. doi: 10.1002/jso.21204. [DOI] [PubMed] [Google Scholar]
- 82.Kelley ET, Arispe I, Holmes J. Beyond the initial indicators: lessons from the OECD Health Care Quality Indicators Project and the US National Healthcare Quality Report. Int J Qual Health Care. 2006;18(suppl 1):45–51. doi: 10.1093/intqhc/mzl027. [DOI] [PubMed] [Google Scholar]
- 83.Institute of Health Improvement. How to Improve. http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/HowToImprove/. Accessed September 1, 2009. [Google Scholar]