Abstract
This chapter presents an overview of computational modeling as a tool for multilevel cancer care and intervention research. Model-based analyses have been conducted at various “beneath the skin” or biological scales as well as at various “above the skin” or socioecological levels of cancer care delivery. We review the basic elements of computational modeling and illustrate its applications in four cancer control intervention areas: tobacco use, colorectal cancer screening, cervical cancer screening, and racial disparities in access to breast cancer care. Most of these models have examined cancer processes and outcomes at only one or two levels. We suggest ways these models can be expanded to consider interactions involving three or more levels. Looking forward, a number of methodological, structural, and communication barriers must be overcome to create useful computational models of multilevel cancer interventions and population health.
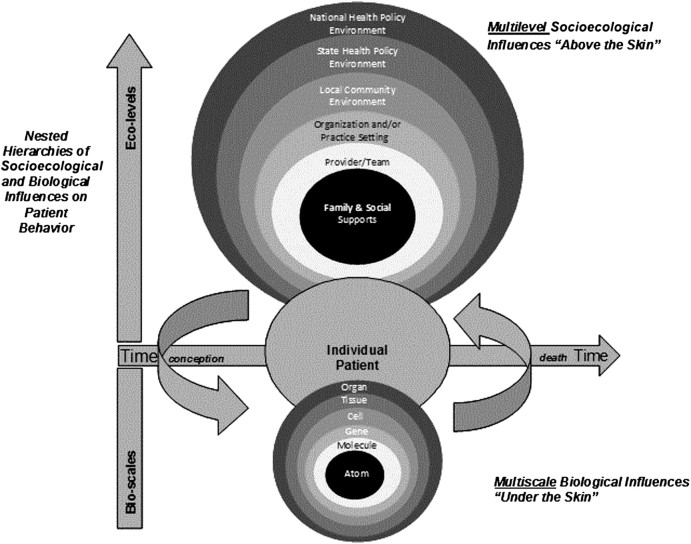
Cancer control research spans “basic and applied research in the behavioral, social, and population sciences that, independently, or in combination with biomedical approaches, reduces cancer risk, incidence, morbidity, and mortality and improves quality of life” (1). This dynamic and complex field includes genetic, behavioral and environmental risk assessments, primary and secondary prevention strategies, and interventions to improve diagnosis, treatment, surveillance for recurrence, and end-of-life care (2). Outcomes at each of these diverse phases of the cancer care continuum are affected by influences at multiple socioecological and biological levels (Figure 1). Biological levels refer to “below the skin” genetic and cellular processes such as mutations that increase risk of disease, damage to DNA repair mechanisms arising from environmental exposures that predispose to cancer, or biomarkers of tumors that affect response to therapy and outcomes (4).
Figure 1.
Multiscale and multilevel influences in cancer prevention and control (3).
Interventions to improve cancer-related health outcomes may include such varied and complex efforts as national, state, and community-wide policies; interventions directed at the behavior of health-care organizations and providers, individual patients, or the general public; or efforts to disseminate molecularly-targeted diagnostic and therapeutic advances (eg, oncotype DX use, BRCA 1 and 2 genetic testing for breast and ovarian cancer susceptibility or herceptin therapy). Most of these cancer control interventions estimate the impact of outcomes of a specific intervention on a narrow outcome in a well-defined population. However, from a policy and planning perspective, we often need to estimate the effects of interventions, or combinations of multiple interventions on overall population cancer incidence or mortality, given the effects of interventions at multiple levels.
In these situations, computational modeling is a valuable tool for integrating complex data on multiple interventions targeted at different biological and socioecological levels while estimating net effects on the population. Computational models (5–7) are computer-based representations of real-world systems that use mathematics, rules, and logic in combination with observed data to portray cancer and the dynamic multifaceted influences of cancer processes over the lifetime of the organism or system. Cancer is an optimal disease for applying computational modeling because multilevel influences affect the onset, progression, and outcomes of the disease and result in the need for complex intervention strategies, especially those that bridge “above the skin” and “below the skin” influences (3,8,9).
Models are useful in cancer control research to explicitly describe interrelationships between factors across levels, estimate the impact of uncertainty, identify high-yield intervention points, extend the time horizon of clinical trials, project results from an experimental setting to the whole population, and compare alternative “what if” multilevel strategies in a “virtual” laboratory (10–14). Modeling is especially valuable when real-world experiments are impractical for pragmatic, theoretical, or ethical reasons.
The purpose of this chapter is to provide an overview of computational modeling and illustrate its applications in four areas of cancer control: tobacco use, colorectal cancer screening, cervical cancer screening, and racial disparities in access to breast cancer care. We then suggest ways these models can be broadened to encompass more multilevel variables and identify some of the challenges to future progress in this area.
Overview of Computational Modeling
Computational models vary along several independent dimensions. Briefly, decision tree models (15) represent chance events and decisions over time with each path through the tree representing one possible sequence of events, each with its associated probability and outcome, such as life expectancy. State-transition models allocate and reallocate members or segments of a population into one of several categories or health states over time (eg, cancer stages, recurrences) and are more efficient in capturing recurring events than are decision trees. These models may be based on “microsimulations” (16), which use the individual (eg, cells, persons, or families) as the unit to simulate large aggregates or populations of these individuals or “macrosimulations” (17). The macro approach uses explanatory variables that are measured at the population level, such as national smoking quit rates for the United States (18).
Transitions between states represented in the models occur with specified probabilities and can depend on age, race, risk group, or other population characteristics. Mathematical computation is used to estimate the numbers of individuals in a given state at any point in time. Markov models (19,20) are one type of state-transition model that require a simplifying assumption that the probability of being in one state does not depend on the prior state, a situation that does not often occur in biological systems. Other assumptions can of course be made about transition probabilities over time.
All models include individuals, subgroups, or populations that are relevant to the research question. Computational models then project future outcomes for these categories. Referent groups can be constituted using two different methods, each providing a different answer to a given question (21). The first method, known as longitudinal modeling, calculates outcomes for hypothetical typical individuals or cohorts (eg, women aged 40 years) and follows them over time to evaluate what the outcomes would be under two or more competing scenarios. This approach is most typically used in decision trees where a single cohort is followed to assess very specific clinical outcomes. The second method, known as cross-sectional modeling, calculates the health outcomes of an entire cross section of the population (representing all relevant birth cohorts) for an observed time horizon, typically until death.
These models are well suited for estimating the impact of changes in the population along multiple levels, including changes in risk factors (such as hormone replacement therapy use), behavior, policies, or new technology on trends in incidence and mortality (21–24). Such models are used by the Cancer Intervention Surveillance Network (CISNET). CISNET is a National Cancer Institute (NCI)–funded research collaborative consisting of investigator groups working on computational models for lung, prostate, colorectal, and breast cancer that have been used extensively to evaluate the impact of policy guidelines on population outcomes. Other research teams also have been building models to capture the interactions of different diseases with common risk factors (25,26) or impacts on mortality (27,28).
Computational modeling has two predominant analytic approaches: deterministic and stochastic. Deterministic models (29) calculate the probabilities as an average number of observed health events and are most suited to relatively simple models involving limited combinations of events and decisions. In stochastic models (30,31), such as discrete event models or Monte Carlo simulation, probabilities for each individual in the cohort over time are simulated using computer-generated random numbers to represent chance events or uncertain model parameters, allowing investigators to generate a representative sample of possible combinations based on observed population distributions of a given parameter. This stochastic process yields a confidence interval around the point estimate, capturing uncertainty in model parameters. Bayesian approaches also can be used to estimate distributions of parameters in a population using the prior distributions of known events. The BRCA-pro model for estimating risk of having a genetic mutation that places individuals at risk of cancer is one example of a Bayesian approach (32–34).
Thus, models can differ along several dimensions in their approach to a specific question, with each approach providing answers to different questions about varying levels of influence on cancer outcomes. The appropriate model depends upon the research question and intended uses of the results. In the next sections, we illustrate how four groups of investigators using validated well-established computational models have begun to address multilevel influences in cancer control.
Illustrative Cancer Control Models
We illustrate four models that employ stochastic simulations (Table 1). Each model has both unique and shared features. The SimSmoke model, for example, tries to model a large number of policies, explicitly considers their dynamic effects, and examines the synergies between policies on tobacco control, whereas the colorectal model orders the cost-effectiveness of different screening policies as their effects vary by age and gender. Table 2 provides a summary of the shared or generic parameters often used in these four models, as well as examples of factors from various socioecological and biological levels likely to affect each parameter.
Table 1.
Illustrative simulation models addressing multilevel challenges in cancer care research*
| Multilevel challenge in cancer care research | Illustrative simulation model(s) | Objective | Intervention levels/scales under study | Potential for multilevel/multiscale adaptation |
| Effective cancer control requires interventions at multiple ecological levels | SimSmoke | To inform tobacco-control policy decision making in the United States and internationally | National and state policies Community provider reimbursement Patient education |
Addresses >2 ecological levels Needs to adapt to include cellular and genetic levels (eg, genotypes for nicotine addiction) |
| New technologies | CISNET Colorectal Cancer Screening | To assess the effectiveness and cost-effectiveness of screening for colorectal cancer with a variety of screening tests | Patient adherence to screening Cellular growth of adenomas |
Extend to examine smaller scales, such as genetic basis of adenoma risk and growth Extend to encompass other ecological levels affecting patient adherence to screening at the provider, practice, or policy level |
| For each cancer site, disease is heterogeneous | Goldie et al. Cervical Cancer Screening Strategies | To examine alternative cervical cancer prevention strategies in the context of HIV | Patient adherence to screening and HIV treatment regimens Cellular processes of HPV infection |
Extend to include other ecological levels, including state policies on HIV prevention, community effects on individual sexual behavior, practice-level availability of HIV medicines |
| Disparities in cancer mortality | CISNET Breast Cancer Disparities | To ascertain how much of the black–white mortality gap in breast cancer is attributable to mutable factors | Patient adherence to screening Biomarkers of cancer natural history (ER/HER2) |
Extend to include federal or state policies that affect access to screening or emerging therapies |
CISNET = Cancer Intervention Surveillance Network; ER = estrogen receptor; HPV = human papillomavirus.
Table 2.
Examples of simulation model input parameters and multilevel influences*
| Parameter | Description | Conditional on | Levels that influence parameter |
| Incidence from age–period–cohort model | Incidence | Age, race, breast density, birth cohort | Ecological forces that affect risk behavior (eg, reproductive forces, trends in smoking post-WWII) Ecological factors that affect hormonal exposures Individual-level factors affecting risk such as family history and diet |
| Population birth distribution | Probability distribution of birth-years in US population | Race | Ecological factors affecting diffusion of contraception; policies about use of contraception |
| Non-breast cancer mortality | All-cause mortality exclusive of deaths from breast cancer | Race, age, birth cohort | Ecological factors affecting mortality such as occupations, insurance Individual factors related to social class, health habits |
| Unscreened stage (or tumor size) distribution | Distribution of stages (or sizes) of tumors diagnosed in the absence of screening | Race, age | Biological scale factors related to cellular and molecular aspects of cancer progression |
| Dwell-time distributions | Mean in stage and in preclinical state (sojourn time) | Age | Unobservable biological level of cellular and molecular events Interactions of biological levels with individual-level health behaviors and exposures that may modify biological processes |
| Screened stage distribution | Distribution of stages of tumors that are screen-diagnosed by each test | Race, age, and first-vs-later screen | Ecological factors affecting structure of care, policies regarding insurance coverage, and access to screening and diagnostic services Individual factors related to adherence to screening use and diagnostic follow-up Biological level related to ability of technology to detect tissue changes related to cancer |
| Screening dissemination | Distribution by cohort, age, time period | Cohort, age, calendar year | Ecological factors affecting structure of care, policies regarding insurance coverage, and access to screening and diagnostic services Individual factors related to adherence to screening use and diagnostic follow-up |
| Operating characteristics | Sensitivity and specificity, initial and later screens | Race, age, tumor size, density | Ecological-level factors that affect the quality of screening facilities Population level related to training and skill of radiologists Biological level related to ability of technology to detect tissue changes related to cancer |
| Screening-induced care | Biopsies and other diagnostic tests | Age | Ecological level in access to care Individual level in adherence and health seeking behaviors |
| HR/HER2 distribution | Probabilities of tumors exhibiting ER and HER2 positivity | Race, age, stage/size at diagnosis | Individual level in health behaviors increasing risk of particular tumors (largely unknown at present) Biological level of cellular and genetic processes that lead to different types of tumors |
| Treatment dissemination | Probability distribution of treatment regimens | Race, age, year, stage, ER/HER2 | Ecological level in access to care Individual level in adherence and health-seeking behaviors |
| Natural history survival | Survival functions before use of adjuvant Rx | Race, age, stage | Biological level of unobservable natural history of disease in the absence of intervention |
| Treatment effectiveness | Hazard ratios for regimens; modifies survival without Rx | Race, age, Rx, stage, ER/HER2 | Ecological level in access to treatment and quality of treatment Individual level in adherence to treatment regimen Biological levels of effects of treatments on cellular and molecular processes of carcinogenesis and metastasis |
| Quality of life | Utility for each state | Age, stage | Ecological level of societal perspectives Individual-level preferences |
ER = estrogen receptor; HR = hormone receptor; WWII = World War II.
Model 1: SimSmoke
SimSmoke is a computational model developed within CISNET to evaluate competing policies and the feasibility of meeting Healthy People 2010 tobacco use targets (35–39). The model simulates population-level tobacco use in the base case (status quo), under five different policy scenarios where interventions are implemented individually and under a variety of combination scenarios (Table 3). Simulated scenarios span multiple socioecological levels, including: patient/provider, organization/practice setting, local community environment, state health policy environment, and national policy environment. Specific policy interventions considered include increased tobacco taxes, smoke-free indoor air laws, mass media/educational initiatives, and enhanced cessation treatment support.
Table 3.
SimSmoke simulation: the effects of public policies on adult (aged ≥18 years) smoking prevalence, 2008–2020 (% unless otherwise indicated)*
| Policy | 2008 | 2009 | 2010† | Δ vs 2010 SQ | 2015† | 2020† | Δ vs 2020 SQ | Upper bound 2020‡ | Δ vs 2020 SQ | Lower bound 2020§ | Δ vs 2020 SQ |
| Status quo | 20.1 | 19.9 | 19.6 | NA | 18.6 | 17.5 | NA | 17.5 | NA | 17.5 | NA |
| 50% tax increase | 20.1 | 19.3 | 18.5 | −5.6 | 16.3 | 15.0 | −14.3 | 13.8 | −21.4 | 15.9 | −9.4 |
| Smoke-free indoor air laws | 20.1 | 19.6 | 19.1 | −2.7 | 17.5 | 16.3 | −7.2 | 15.6 | −11.0 | 16.7 | −4.7 |
| Mass media/educational policies | 20.1 | 19.7 | 19.3 | −1.6 | 17.9 | 16.8 | −4.1 | 16.3 | −6.7 | 17.0 | −2.9 |
| Evidence-based cessation treatment policies‖ | 20.1 | 19.2 | 18.4 | −6.2 | 16.1 | 14.7 | −15.5 | 13.4 | −23.4 | 15.7 | −10.5 |
| With promising cessation treatment policies¶ | 20.1 | 18.6 | 17.3 | −11.7 | 14.2 | 12.8 | −26.9 | 10.7 | −39.1 | 14.5 | −17.4 |
| Evidence-based cessation treatment policies and tobacco control policies | |||||||||||
| Without promising cessation treatment policies | 20.1 | 18.4 | 17.0 | −13.4 | 13.6 | 12.2 | −30.6 | 9.9 | −43.4 | 14.0 | −20.4 |
| With promising cessation treatment policies¶ | 20.1 | 17.4 | 15.3 | −22.1 | 11.1 | 9.7 | −44.6 | 7.1 | −59.5 | 12.2 | −30.6 |
% Change (Δ) is measured relative to the status quo in the same year (ie, [SRp,t − SRStatus quo,t]/SRStatus quo,t, where SRp,t is the smoking rate in year t with policy p and SRStatus quo,t is the smoking rate in year t under the status quo). NA = not applicable; SQ = status quo. Reprinted from Levy et al. (38) with permission from Elsevier.
Policy effects decay at a rate of 10% per year.
Policy effects decay at a rate of 0% per year.
Policy effects decay at a rate of 25% per year.
Includes treatment coverage, quitlines with no-cost nicotine replacement therapy, and health-care provider interventions.
Includes above and changes that increase treatment effectiveness of evidence-based treatments by 100%.
What Does the Model Look Like?
SimSmoke is a state-transition model. The population is split into a set of mutually exclusive states based on age and smoking status (never smokers, smokers, and former smokers). Individuals can transition from one smoking state to another over time, for example, from former smokers to current smokers after relapsing. Movement through the simulated population occurs through birth or immigration, aging, smoking initiation, smoking cessation, relapse, or death (due to smoking or not). The model tracks—or counts—these events over time, and interventions are simulated by specifying their impact on these events.
To illustrate how interventions are operationalized, consider “enhanced cessation support” (Table 3), which includes three specific policies: expanded coverage of cessation treatment and provider reimbursement, mandates of adequately-funded telephone quitlines, and support for health-care system-level change to “prompt, guide, and incentivize tobacco treatment.” The authors quantify the impact of realistic implementation of these three policies together on the rate of quit attempts, cessation treatment use, and treatment.
What Are the Results?
For each scenario, adult smoking prevalence is estimated for the next 20 years and compared with the base case. Based on the results reproduced in Table 3, the authors conclude that a comprehensive intervention package, including both enhanced cessation support for individuals and considered tobacco control strategies, could decrease smoking prevalence to 12.2% in 2020. Furthermore, if new and promising interventions are added (including the provision of publicly available Internet-based cessation support and personally tailored and long-term support for evidence-based cessation), smoking prevalence could be reduced further to 9.7%. These results shed light not only on feasible goals in reducing smoking prevalence but also on the relative contribution of various intervention scenarios. Interestingly, the model shows how some policies tend to influence quit attempts while other influence quit success, and the combination of policies leads to mutually reinforcing synergies.
Although this application simulates what the authors consider to be the most realistic impacts of each intervention, it is possible to use the model to explore likely variation in effect due to variation in implementation fidelity. Or, as often happens when these models are used in workshops with system stakeholders and policy makers, discussion of the modeling team’s translation of interventions into effects can lead to richer discussion about what it would really take to achieve this effect and thus the more “real-world” tradeoffs between simulated scenarios. In these model-supported workshops, participants can shape what they perceive to be more feasible alternate implementation scenarios to compare with model-based results.
How Can the Model Incorporate Biological Levels?
Interventions targeting biological events could be included in SimSmoke as our understanding of the science of “below the skin” influences on tobacco use and control grows and additional interventions become available. For instance, as evidence evolves on genotypes for nicotine addiction (40,41), SimSmoke could scale down to the cellular and genetic level and portray benefits only in the subset of the population likely to be affected. Similarly, genotypes related to lung cancer risk (42) or other factors that promote tumor growth could be added to the model to reflect the mortality impact of new interventions.
Model 2: Colorectal Cancer Screening
Three independently developed and validated CISNET models have been used to address the relative effectiveness and cost-effectiveness of multiple screening tests for colorectal cancer, each with different test characteristics. Computed tomography (CT) colonography, the newest and most expensive of the tests, is compared with screening with older recommended tests, including the fecal occult blood test, flexible sigmoidoscopy, and colonoscopy (in various combinations) following recommended clinical guidelines (43–46).
What Do the Models Look Like?
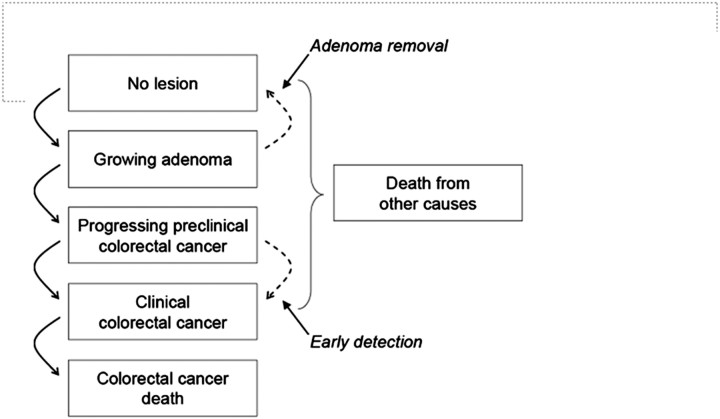
The models calculate the costs and health outcomes from birth to death for multiple birth cohorts of individuals in the US population under different screening scenarios (46). The natural history of disease is characterized as the progression of underlying disease in the absence of screening. As simulated individuals age, they face differing probabilities of developing an adenoma and of those adenomas growing, progressing into preclinical colorectal cancer, cancer, and/or resulting in cancer death (Figure 2). In any given screening year, the chance that the simulated individual's adenoma or preclinical cancer is detected depends on the sensitivity of the screening test for that lesion.
Figure 2.
Schematic for the Cancer Intervention Surveillance Network (CISNET) Colorectal Cancer Models. Graphical representation of natural history of colorectal cancer as modeled by MISCAN, SimCRC, and CRC-SPIN models. The opportunity to intervene in the natural history through screening (adenoma detection and removal and early detection) is noted by the dotted lines. Screening can either remove a precancerous lesion (ie, adenoma), thus moving a person to the “No lesion” state, or through early detection, which makes an undiagnosed cancer clinically detected at a potentially earlier stage of disease where it is more amenable to treatment. Reprinted from Zauber et al. (46).
What Are the Results?
In all three models, CT colonography was found to be more expensive with fewer life-years gained than colonoscopy (46). However, CT colonography was determined to be an efficient screening strategy—even at much higher cost—if patients were assumed to be 10% or 25% more adherent to CT colonography than to other screening tests (46).
How Can the Models Include Additional Biological or Socioecological Levels?
These models include patient level risk of disease and compliance with screening and one “below the skin” scale component for the growth of adenomas. The models could be extended to examine smaller biological scales such as the genetic basis of adenoma risk and growth. The analyses also could be extended to encompass other ecological levels that influence individual opportunities, constraints, and behaviors, including the provider level [eg, specifying different rates of recommended screening procedures (47)], practice level [eg, varying reminders and other office systems to improve screening adherence (48)], and policy level [eg, specifying different adoption rates for clinical guidelines (49) or insurance type (50,51)].
Model 3: Cervical Cancer Screening
The state-transition models developed by Goldie et al. to assess optimal cervical cancer control strategies in the face of multiple interactions with underlying health conditions for women infected with HIV are an excellent example of modeling across biological and socioecological levels (25,26).
What Do the Models Look Like?
First, the models portray the cellular process of human papillomavirus (HPV) infection, the putative etiological agent in this disease. Immune responses by T-cells can clear the infection. If the infection is not cleared, the HPV DNA infection persists and has a known probability of causing malignant transformations. Next, the models consider the natural history of HIV infection in terms of T-cell counts. As HIV viral loads increase and T-cell counts decrease over time in the course of untreated HIV infection, this biological process interacts with the probability of persistent HPV infection and increases malignant transformation of cervical cells. The models overlay ecological factors, such as use of antiretroviral agents to treat HIV and compliance with screening, on these interactive “below the skin” cellular mechanisms.
What Are the Results?
The results indicate that frequent Pap smear screening is more cost-effective than colposcopy, but that screening for HPV and using the results to determine Pap smear screening intervals also is cost-effective (25,26). Interestingly, results were most sensitive to assumptions about the “below the skin” events, such as the progression rates of HPV infection, demonstrating the importance of considering the biological aspects of disease in multilevel policy modeling.
How Can the Models Include Additional Socioecological Levels?
This type of model could be expanded readily to consider additional multilevel socioecological influences on high-risk populations, such as individual sexual behavior, social networks, access to HIV treatment, interventions to enhance compliance with HIV therapy, and state or federal government policies on HIV prevention (52,53). It could also be extended to evaluate the costs of promoting increased screening (25,54) and/or the costs of establishing different types of screening. Such applications would be of special interest to governments in developing countries where cervical cancer is still a common killer (55).
Model 4: Breast Cancer Disparities
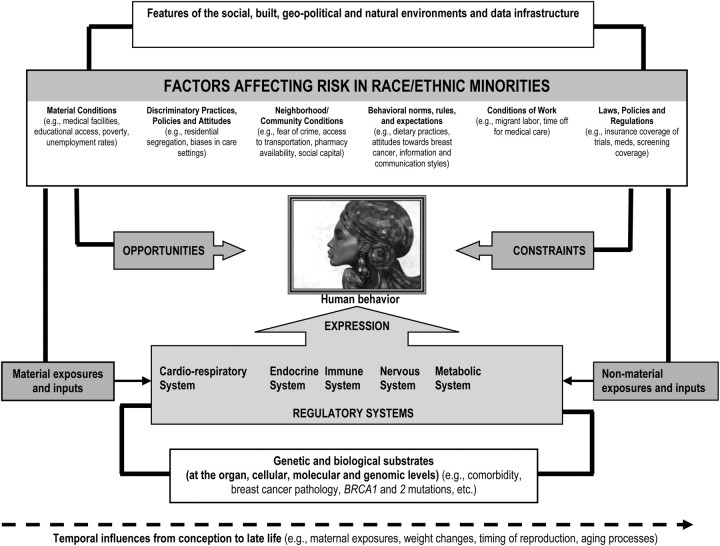
Despite progress in screening for breast cancer in the United States (56), black women continue to have persistently higher breast cancer mortality rates than white women despite lower incidence. The reasons for this disparity remain uncertain. Two established simulation models (57–59) have been developed within CISNET to illuminate reasons for observed population-level race-specific breast cancer mortality disparities. The goal of these two models is to ascertain how much of the black–white mortality gap can be attributed to differences in mutable factors along various points in the cancer control process (Figure 3).
Figure 3.
Schematic for racial disparities in breast cancer care. Reprinted from Mandelblatt et al. (59) with permission from Lippincott, Williams and Wilkins.
What Do the Models Look Like?
The models begin with estimates of what race-specific breast cancer incidence, and mortality trends would have been in the absence of screening and treatment and then overlays race-specific screening use and improvements in survival associated with treatment. Multiple birth cohorts of black and white women are followed for their lifetimes through simulations to depict the US population.
For both races, breast cancer is depicted as having a preclinical screen detectable period (sojourn time) and a clinical detection point. Based on mammography sensitivity (or thresholds of detection), screening finds disease in the preclinical screen-detection period, resulting in the identification of earlier-stage/smaller cancers than what might occur through clinical detection, and to breast cancer mortality reductions. Age and tumor size/stage-specific treatment have independent impacts on mortality for invasive cancers. The models include biological or “below the skin” biomarkers of cancer natural history (eg, estrogen receptor and HER2 status) to evaluate the relative contribution of biology to observed population outcomes.
What Are the Results?
The results of both models indicate that simulation of historical improvements in screening and treatment decreased mortality to a lesser extent in black than white women. The lower mortality impact of these cancer control interventions among black women was partly explained by differences in the “below the skin” natural history parameters (explaining 45% and 23% of variation in the first and second modeling projects, respectively), followed by treatment (11% and 21%) and screening use (7% and 11%), leaving between 36% and 46% of the difference in mortality impact unexplained (60). The investigators concluded that the greatest portion of mortality disparities appear to be due to differences in “below the skin” biological factors that affect natural history.
How Can the Models Incorporate Additional Biological and Socioecological Levels?
In future iterations, modelers could explicitly examine several multilevel socioecological factors that may account for the observed race difference in use of screening over time. These include federal or local policies or programs that affect screening access, individual compliance with screening, delays in follow-up after abnormal mammograms (46), cultural beliefs and attitudes that may lead to irregular use of screening, delayed presentation in response to symptoms (61), access to emerging therapies, provider behavior, or patient acceptance of prescribed adjuvant regimens.
Summary of Model Examples
It is clear from Table 1 and the examples presented here that a number of efforts are already underway using computational modeling to study multilevel cancer control issues that link “above the skin” with “below the skin” influences. These models already have been useful in formulating new policies and guidelines. The colorectal cancer models (model 2) were used to adjust Medicare coverage (46,62) and, along with the breast models (model 4), the screening guidelines issued by the US Preventive Services Task Force (63). The cervical cancer models (model 3) were used to support screening recommendations for HIV-infected women. The tobacco control models (model 1) have influenced tobacco access regulations in the United States and abroad.
Other recent efforts to advance multilevel modeling include the work of the NCI's Integrative Cancer Biology Program with CISNET modelers to bridge the biological and population levels (http://icbp.nci.nih.gov). Outside of the cancer field, the Archimedes model developed by Eddy et al. (27,28) is a broad ambitious modeling approach that considers several chronic diseases simultaneously to identify multilevel interventions at the biological, clinical, and administrative/policy levels that are likely to have the greatest benefits on overall population health.
Challenges and Future Directions
Despite the existence of numerous robust computational modeling efforts that have been successful in supporting cancer control policy decision-making, a number of methodological, structural, and communication obstacles stand in the way of future success for these and related multilevel computational modeling efforts.
Methodological Challenges
Cancer is different from other areas of modeling because, with rare exceptions (eg, watchful waiting for localized low grade prostate cancer), it must be treated. As a result, we are unable to study the counterfactual of what happens in the absence of treatment. In addition, most preclinical carcinogenetic processes are not directly observable. To obtain biomarkers, computational models may have to rely upon archival specimens from an era that preceded interventions, international datasets on unexposed populations, or basic science models to estimate population parameters. In these situations, integrative biology modeling of genetic and cellular scales may be informative for developing realistic inputs for population models of cancer.
Multiple levels of intervention involving patients, practitioners, and policies heighten the complexity of the modeling task itself. Integrating data and measuring interactions between levels also is problematic. An understanding of these interactions is important because it can change the questions as well as the answers in cancer care research. Most current computational modeling in cancer is based upon linear thinking. Off-the-shelf software based upon linear models will not work in considering multilevel interventions as it does not allow for interaction terms or the amounts of computer memory needed to solve complex equations. Multilevel computational modeling presents a substantial learning curve for the modeler for it is not as easy as running regression models in multivariable statistical packages such as SAS, SPSS, or STATA.
Representing uncertainty also is an issue in multilevel models as is finding suitable situations for external validation. Despite technical advances in high-speed computers, it may take weeks to run multiple iterations of complex models with dynamic feedback loops between variables at different levels. More efficient computational algorithms and distributed computer networks (64) are needed for this work, meaning that computer scientists are essential members of multilevel modeling teams.
Structural Challenges
A key structural barrier in this field is that progress in multilevel modeling requires cross-disciplinary research wherein biologists, clinicians, social scientists, computer scientists, and health services researchers work together to address common problems. Yet, the super-specialization of science often has investigators on the same campus working in unconnected research programs. Such discipline-focused research creates a structural obstacle to the growth of multilevel modeling. Moreover, shortages of training programs that emphasize this approach are a continuing problem. The academic home of computational modeling is generally in industrial engineering schools, and few established programs focus on simulation applications for cancer or other health concerns.
Another structural barrier to successful modeling is the lack of grant review and funding infrastructure specific to modeling disciplines. With the exception of CISNET and the Integrative Cancer Biology Program at NCI, no dedicated funding mechanisms with ample set-aside dollars to support multilevel modeling in cancer or other health fields have been established.
Communication Challenges
Lastly, learning how to communicate models to patients, providers, and policy-makers in understandable and actionable ways remains a challenge. Many people believe models are “not real” or simply not right. How can we encourage/educate target audiences to trust model results and to use them in personal decision making, clinical practice, and the support of cancer control interventions? The use of multiple models employing common parameters to address the same research question is one approach to enhance credibility (24,63). Another approach is comparing model projections with real-world epidemiological data over time to show the potential for models to accurately project health outcomes. Making model structure and assumptions transparent can also increase credibility. For instance, all CISNET models are summarized on the model profiler website (http://cisnet.cancer.gov/modeling/comparative.html) so that users or interested parties can readily access model details. Another strategy is to work with communication scientists to improve the interpretability and messaging of multilevel models for diverse audiences.
Future Prospects
A number of recent developments augur well for overcoming the above obstacles and for advancing multilevel modeling in cancer and other health areas. The National Institutes of Health Office of Behavioral and Social Science Research (OBSSR) has taken a leadership role in promoting interdisciplinary research among behavioral, social, and biological scientists and fostering systems science approaches to public health problems (9). A new funding mechanism has been created to encourage R03 and R21 research applications using computational modeling, network analyses, and other systems science approaches to protect and improve population health (http://grants.nih.gov/grants/guide/pa-files/PAR-08-224.html). Annual weeklong training institutes sponsored by OBSSR are hosted by a different university each year to expose students and researchers to systems science tools. OBSSR also sponsored a special issue of the American Journal of Public Health in July 2010 to showcase system science approaches to tobacco control.
These developments are promising but are likely to be inadequate to nurture and sustain the vision for multilevel modeling as presented in the various articles in this supplement. Still needed are opportunities to create a learning community among multilevel modelers to promote the sharing of ideas, data, and common measurement and analysis strategies. Collaboration can help create a common language for describing and shaping multilevel computational modeling. Currently, a number of discipline-based traditions and modeling vocabularies associated with computer simulations use the same procedures and approaches but call them by different names and metaphors. This steepens the learning curve and impedes cross-discipline communication and collaboration.
The collaborative modeling approach underlying CISNET exemplifies how common strategies can be promoted, but we also need to allow for distributed modeling, where innovative approaches of individual investigators or local teams can push the envelope of convention and orthodoxy in cancer research. We also need to encourage modelers to link to people in practice or policy circles as well as patients who could help motivate the questions addressed by multilevel models.
One cautionary note is that we must be selective in pursuing a multilevel modeling agenda. Modeling must be driven by a focus on core problems identified by collaborations with policy makers and clinicians and grounded in strong evidence for effectiveness, rather than a serendipitous search for connections that build ever bigger and bigger models that become hopelessly complex, tenuous, and unproductive. A great strength of computational modeling is that numerous data sources and time periods can be combined to create realistic estimates to inform policy making as well as individual patient choice.
However, much of the data needed to measure causal relationships for complex multilevel modeling do not now exist. Current efforts to expand comparative effectiveness research in health care (65) may produce the types of data that can inform these modeling efforts in the next decade. More compelling data and variables, such as costs of interventions and improvements or institutional measures and pathways that resonate with clinicians and policy makers, will promote “actionable” findings. Furthermore, we need to develop even more advanced methods for conducting sensitivity analyses, given the increased level of complexity and uncertainty associated with multilevel simulation models. Finally, we need models of cancer that aid in design of new and better interventions to lessen its occurrence and mitigate the burden of disease when it does occur.
Funding
This work was supported by grants from the National Cancer Institute at the National Institutes of Health (UO1CA088283, KO5CA096940, U01CA086076-10S1, and RC2 CA148577 to JM), the Department of Defense Breast Cancer Center of Excellence Award (BC043120 to JM), and the National Center for Research Resources (KL2RR025746 to KHL). JPM, who is not a federal government employee, received a small stipend from the National Cancer Institute to prepare the manuscript for publication and for travel expenses to participate in the meeting where this paper was discussed.
Footnotes
The opinions in this article are those of the authors and should not be interpreted as official positions of the National Cancer Institute, the National Center for Research Resources (K. Hassmiller Lich), the Department of Health and Human Services, or the US government.
References
- 1.Hiatt RA, Rimer BK. A new strategy for cancer control research. Cancer Epidemiol Biomarkers Prev. 1999;8(11):957–964. [PubMed] [Google Scholar]
- 2.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4–13. [PubMed] [Google Scholar]
- 3.Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62(7):1650–1671. doi: 10.1016/j.socscimed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 4.Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;(44):2–10. doi: 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bower JM, Bolouri H, editors. Computational Modeling of Genetic and Biochemical Networks. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- 6.Banks J. Handbook of Simulation: Principles, Methodology, Advances, Applications, and Practice. New York, NY: John Wiley & Sons, Inc.; 1998. [Google Scholar]
- 7.Perros H. Computer simulation techniques: the definitive introduction. North Carolina State University Web site. http://www4.ncsu.edu/∼hp/simulation.pdf. Published 2009. Accessed March 9, 2012. [Google Scholar]
- 8.Diez Roux AV. Integrating social and biologic factors in health research: a systems view. Ann Epidemiol. 2007;17(7):569–574. doi: 10.1016/j.annepidem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Mabry PL, Marcus SE, Clark PI, Leischow SJ, Méndez D. Systems science: a revolution in public health policy research. Am J Public Health. 2010;100(7):1161–1163. doi: 10.2105/AJPH.2010.198176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann S. The world as a process: simulations in the natural and social sciences. In: Hegselmann R, Müller U, Troitzsch K, editors. Modeling and Simulation in the Social Sciences from the Philosophy of Science Point of View. Dordrecht, The Netherlands: Kluwer; 1996. pp. 77–100. [Google Scholar]
- 11.Axelrod R. Advancing the art of simulation in the social sciences. Japan J Manag Inform Syst. 2003;12(3):1–19. [Google Scholar]
- 12.Mandelblatt J, Andrews H, Kao R, Wallace R, Kerner J. The late-stage diagnosis of colorectal cancer: demographic and socioeconomic factors. Am J Public Health. 1996;86(1):1794–1797. doi: 10.2105/ajph.86.12.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang S, Scott JC, et al. Environmental determinants of infectious disease: a framework for tracking causal links and guiding public health research. Environ Health Perspect. 2007;115(8):1216–1223. doi: 10.1289/ehp.9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg JN, Soller JA, Scott J, et al. A dynamic model to assess microbial health risks associated with beneficial uses of biosolids. Risk Anal. 2004;24(1):221–236. doi: 10.1111/j.0272-4332.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 15.Beck JR, Sonnenberg FA. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 16.Duffy SW, Day NE, Tabár L, Chen HH, Smith TC. Markov models of breast tumor progression: some age-specific results. J Natl Cancer Inst Monogr. 1997;(22):93–97. doi: 10.1093/jncimono/1997.22.93. [DOI] [PubMed] [Google Scholar]
- 17.Dewilde S, Anderson R. The cost-effectiveness of screening programs using single and multiple birth cohort simulations: a comparison using a model of cervical cancer. Med Decis Making. 2004;24(5):486–492. doi: 10.1177/0272989X04268953. [DOI] [PubMed] [Google Scholar]
- 18.Zauber AG, Lansdorp-Vogelaar I. Changes in risk factors and increases in screening contribute to the decline in colorectal cancer mortality, 1975 to 2000. Gastroenterology. 2010;139(2):698. doi: 10.1053/j.gastro.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Zauber A, Lansdorp-Vogelaar I, Knudsen A, Wilschut J, van Ballegooijen M, Kuntz K. Evidence Synthesis No. 65, Part 2. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Evaluating test strategies for colorectal cancer screening—age to begin, age to stop, and timing of screening intervals: a decision analysis of colorectal cancer screening for the U.S. Preventive Services Task Force from the Cancer Intervention and Surveillance Modeling Network (CISNET) AHRQ Publication No. 08-05124-EF-2. http://www.uspreventiveservicestaskforce.org/uspstf08/colocancer/colcanes2.pdf. Accessed March 9, 2012. [PubMed] [Google Scholar]
- 20.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 21.Goldie SJ, Weinstein MC, Kuntz KM, Freedberg KA. The costs, clinical benefits, and cost-effectiveness of screening for cervical cancer in HIV-infected women. Ann Intern Med. 1999;130(2):97–107. doi: 10.7326/0003-4819-130-2-199901190-00003. [DOI] [PubMed] [Google Scholar]
- 22.Goldie SJ, Freedberg KA, Weinstein MC, Wright TC, Kuntz KM. Cost effectiveness of human papillomavirus testing to augment cervical cancer screening in women infected with the human immunodeficiency virus. Am J Med. 2001;111(2):140–149. doi: 10.1016/s0002-9343(01)00780-x. [DOI] [PubMed] [Google Scholar]
- 23.Schlessinger L, Eddy DM. Archimedes: a new model for simulating health care systems—the mathematical formulation. J Biomed Inform. 2002;35(1):37–50. doi: 10.1016/s1532-0464(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 24.Stern M, Williams K, Eddy D, Kahn R. Validation of prediction of diabetes by the Archimedes model and comparison with other predicting models. Diabetes Care. 2008;31(8):1670–1671. doi: 10.2337/dc08-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delen D, Walker G, Kadam A. Predicting breast cancer survivability: a comparison of three data mining methods. Artif Intell Med. 2005;34(2):113–127. doi: 10.1016/j.artmed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and over diagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killewo J, Heggenhougen HK, Quah SR, editors. Epidemiology and Demography in Public Health. San Diego, CA: Academic Press; 2010. [Google Scholar]
- 28.Levy DT, Chaloupka F, Gitchell J. The effects of tobacco control policies on smoking rates: a tobacco control scorecard. J Public Health Manag Pract. 2004;10(4):338–353. doi: 10.1097/00124784-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava R, You L, Summers J, Yin J. Stochastic vs. deterministic modeling of intracellular viral kinetics. J Theor Biol. 2002;218(3):309–321. doi: 10.1006/jtbi.2002.3078. [DOI] [PubMed] [Google Scholar]
- 30.Tijms H. A First Course in Stochastic Models. Chichester, UK: Wiley; 2003. [Google Scholar]
- 31.Tan W-Y. Stochastic Models of Carcinogenesis. New York, NY: Marcel Dekker; 1991. [Google Scholar]
- 32.Berry DA, Parmigiani G. Assessing the benefits of testing for breast cancer susceptibility genes: a decision analysis. Breast Dis. 1998;10(1–2):115–125. doi: 10.3233/bd-1998-101-213. [DOI] [PubMed] [Google Scholar]
- 33.Berry DA, Iversen ES, Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20(11):2701–2712. doi: 10.1200/JCO.2002.05.121. [DOI] [PubMed] [Google Scholar]
- 34.Berry DA, Inoue L, Shen Y, et al. Modeling the impact of treatment and screening on U.S. breast cancer mortality: a Bayesian approach. J Natl Cancer Inst Monogr. 2006;(36):30–36. doi: 10.1093/jncimonographs/lgj006. [DOI] [PubMed] [Google Scholar]
- 35.Levy DT, Chaloupka F, Gitchell J, Mendez D, Warner KE. The use of simulation models for the surveillance, justification and understanding of tobacco control policies. Health Care Manag Sci. 2002;5(2):113–120. doi: 10.1023/a:1014476916361. [DOI] [PubMed] [Google Scholar]
- 36.Zaloshnja E, Ross H, Levy DT. The impact of tobacco control policies in Albania. Tob Control. 2010;19(6):463–468. doi: 10.1136/tc.2009.034652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy DT, Cho SI, Kim YM, Park S, Suh MK, Kam S. SimSmoke model evaluation of the effect of tobacco control policies in Korea: the unknown success story. Am J Public Health. 2010;100(7):1267–1273. doi: 10.2105/AJPH.2009.166900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy DT, Mabry PL, Graham AL, Orleans CT, Abrams DB. Reaching healthy people 2010 by 2013: a SimSmoke simulation. Am J Prev Med. 2010;38(3 suppl):S373–S381. doi: 10.1016/j.amepre.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy DT, Graham AL, Mabry PL, Abrams DB, Orleans CT. Modeling the impact of smoking-cessation treatment policies on quit rates. Am J Prev Med. 2010;38(3 suppl):S364–S372. doi: 10.1016/j.amepre.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munafò MR, Shields AE, Berrettini WH, Patterson F, Lerman C. Pharmacogenetics and nicotine addiction treatment. Pharmacogenomics. 2005;6(3):211–223. doi: 10.1517/14622416.6.3.211. [DOI] [PubMed] [Google Scholar]
- 41.Weiss RB, Baker TB, Cannon DS, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4(7):e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hung RJ, Hall J, Brennan P, Boffetta P. Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. Am J Epidemiol. 2005;162(10):925–942. doi: 10.1093/aje/kwi318. [DOI] [PubMed] [Google Scholar]
- 43.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284(15):1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 44.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JD. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32(1):13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 45.Knudsen A. Explaining Secular Trends in Colorectal Cancer Incidence and Mortality With an Empirically-Calibrated Microsimulation Model [dissertation] Cambridge, MA: Harvard University; 2005. [Google Scholar]
- 46.Zauber AG, Knudsen AB, Rutter CM, et al. Cost-effectiveness of CT colonography to screen for colorectal cancer. http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads//id58TA.pdf. Agency for Healthcare Research and Quality technology assessment report project ID: CTCC0608. Published January 22, 2009. Accessed March 9, 2012. [PubMed] [Google Scholar]
- 47.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006-2007. Am J Prev Med. 2009;37(1):8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med. 2002;136(9):641–651. doi: 10.7326/0003-4819-136-9-200205070-00006. [DOI] [PubMed] [Google Scholar]
- 49.Sarfaty M, Myers RE. The effect of HEDIS measurement of colorectal cancer screening on insurance plans in Pennsylvania. Am J Manag Care. 2008;14(5):277–282. [PubMed] [Google Scholar]
- 50.O’Malley AS, Forrest CB, Feng S, Mandelblatt J. Disparities despite coverage: gaps in colorectal cancer screening among Medicare beneficiaries. Arch Intern Med. 2005;165(18):2129–2135. doi: 10.1001/archinte.165.18.2129. [DOI] [PubMed] [Google Scholar]
- 51.Schneider EC, Rosenthal M, Gatsonis CG, Zheng J, Epstein AM. Is the type of Medicare insurance associated with colorectal cancer screening prevalence and selection of screening strategy? Med Care. 2008;46(9 suppl 1):S84–S90. doi: 10.1097/MLR.0b013e31817fdf80. [DOI] [PubMed] [Google Scholar]
- 52.Hagen MD, Garber AM, Goldie SJ, et al. Does cost-effectiveness analysis make a difference? Lessons from Pap smears. Med Decis Making. 2001;21(4):307–323. doi: 10.1177/0272989X0102100406. [DOI] [PubMed] [Google Scholar]
- 53.Goldie SJ, Paltiel AD, Weinstein MC, et al. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection. Am J Med. 2003;115(8):632–641. doi: 10.1016/j.amjmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Mandelblatt JS, Schechter CB, Yabroff KR, et al. Benefits and costs of interventions to improve breast cancer outcomes in African American women. J Clin Oncol. 2004;22(13):2554–2566. doi: 10.1200/JCO.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Katz IT, Wright AA. Preventing cervical cancer in the developing world. N Engl J Med. 2006;354(11):1110. doi: 10.1056/NEJMp068031. [DOI] [PubMed] [Google Scholar]
- 56.Berry D. Commentary: screening mammography: a decision analysis. Int J Epidemiol. 2004;33(1):68. doi: 10.1093/ije/dyh034. [DOI] [PubMed] [Google Scholar]
- 57.Mandelblatt J, Schechter CB, Lawrence W, Yi B, Cullen J. Chapter 8: the SPECTRUM population model of the impact of screening and treatment on U.S. breast cancer trends from 1975 to 2000: principles and practice of the model methods. J Natl Cancer Inst Monogr. 2006;(36):47–55. doi: 10.1093/jncimonographs/lgj008. [DOI] [PubMed] [Google Scholar]
- 58.Tan SY, van Oortmarssen GJ, de Koning HJ, Boer R, Habbema JD. The MISCAN-Fadia continuous tumor growth model for breast cancer. J Natl Cancer Inst Monogr. 2006;(36):56–65. doi: 10.1093/jncimonographs/lgj009. [DOI] [PubMed] [Google Scholar]
- 59.Mandelblatt J, Liang W, Sheppard VB, Wang J, Isaacs C. Breast cancer in minority women. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 4th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2010. pp. 1083–1093. [Google Scholar]
- 60.van Ravesteyn NT, Schechter CB, Near AM, et al. Race-specific impact of natural history, mammography screening and adjuvant treatment on breast cancer mortality rates in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(1):112–122. doi: 10.1158/1055-9965.EPI-10-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gluecker TM, Johnson CD, Harmsen WS, et al. Colorectal cancer screening with CT colonography, colonoscopy, and double-contrast barium enema examination: Prospective assessment of patient perceptions and preferences. Radiology. 2003;227(2):378–384. doi: 10.1148/radiol.2272020293. [DOI] [PubMed] [Google Scholar]
- 62.Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, et al. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the Medicare population. J Natl Cancer Inst. 2010;102(16):1238–1252. doi: 10.1093/jnci/djq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baum KG, Helguera M. Execution of the SimSET Monte Carlo PET/SPECT simulator in the condor distributed computing environment. J Digit Imaging. 2007;20(suppl 1):72–82. doi: 10.1007/s10278-007-9058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Int Med. 2009;151(3):203–205. doi: 10.7326/0003-4819-151-3-200908040-00125. [DOI] [PubMed] [Google Scholar]