Abstract
Health care in the United States is notoriously expensive while often failing to deliver the care recommended in published guidelines. There is, therefore, a need to consider our approach to health-care delivery. Cancer care is a good example for consideration because it spans the continuum of health-care issues from primary prevention through long-term survival and end-of-life care. In this monograph, we emphasize that health-care delivery occurs in a multilevel system that includes organizations, teams, and individuals. To achieve health-care delivery consistent with the Institute of Medicine's six quality aims (safety, effectiveness, timeliness, efficiency, patient-centeredness, and equity), we must influence multiple levels of that multilevel system. The notion that multiple levels of contextual influence affect behaviors through interdependent interactions is a well-established ecological view. This view has been used to analyze health-care delivery and health disparities. However, experience considering multilevel interventions in health care is much less robust. This monograph includes 13 chapters relevant to expanding the foundation of research for multilevel interventions in health-care delivery. Subjects include clinical cases of multilevel thinking in health-care delivery, the state of knowledge regarding multilevel interventions, study design and measurement considerations, methods for combining interventions, time as a consideration in the evaluation of effects, measurement of effects, simulations, application of multilevel thinking to health-care systems and disparities, and implementation of the Affordable Care Act of 2010. Our goal is to outline an agenda to proceed with multilevel intervention research, not because it guarantees improvement in our current approach to health care, but because ignoring the complexity of the multilevel environment in which care occurs has not achieved the desired improvements in care quality outlined by the Institute of Medicine at the turn of the millennium.
Introduction: Contextual Levels of Influence on Health Behavior, Health Care, and Health Outcomes
Health-care delivery in the United States is notoriously expensive while often failing to deliver the care recommended in published guidelines (1,2). Practitioners do not apply all that is known about quality practice, concerns about safety abound, and patients are confused by the choices they face (3–5). It is therefore not surprising that the United States is 19th among developed countries in reducing avoidable mortality (1). Calls for improvement have focused on the gap between research-tested interventions and implementation in medical practice (6–8). The need to close that gap is reason enough to rethink our approach to care in general, and cancer care in particular, but leading the world in health-care spending while lagging behind in achievement should provide additional motivation.
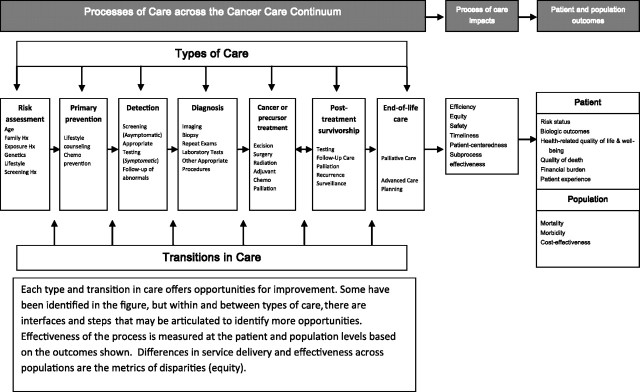
Cancer care is a good example for considering the process of health-care delivery because it spans the continuum of health-care issues from primary prevention through long-term survival and end-of-life care (Figure 1). Movement across this continuum involves several types of care, as well as transitions between them (Figure 1) (9). We also recognize that each type of care is made up of multiple small steps and the interfaces between them (Table 1) (10). The process of care is defined as the set of activities that occur within and between types of care across the cancer continuum. These activities include communication, testing, analysis, and interaction among health-care practitioners, patients, and their families. Although Figure 1 portrays the continuum as a linear process, we recognize that individuals enter and leave at different points along the continuum and may pass repeatedly through some types of care while not proceeding to others. We also recognize that the interactions occur within and between health-care settings.
Figure 1.
Opportunities to influence the process of care across the cancer continuum. Adapted from Zapka JG, Taplin SH, Solberg LI, Manos MM (9) with permission from the American Association for Cancer Research.
Table 1.
Definitions of key terms*
| Term | Definition |
| Level | The various ways that humans aggregate, such as the nation, organization, team, family, and individual, which directly or indirectly influence a range of care outcomes. |
| Intervention | A specified strategy or set of strategies designed to change the knowledge, perceptions, skills, and/or behavior of individuals, groups or organizations with the goal of improving patients’ health outcomes. |
| Multilevel intervention | An intervention is considered to be multilevel if it addresses the individual patient, as well as at least two levels of contextual influence, such as organizations and providers, thereby targeting at least three different sources of influence. |
| Cancer care continuum | The set of health-care activities that cover the spectrum of risk and disease in the population from being at risk of cancer through being treated, becoming a long-term survivor, and in some cases needing palliative and/or end-of-life care (9). |
| Process of care | The set of activities that go on within and between health-care organizations and practitioners and patients across the cancer continuum. |
| Type of care | The care delivered to accomplish a specific goal of care across the cancer continuum, such as detection, diagnosis, or treatment. |
| Steps of care | Each type of care involves multiple specific activities such as performing the screening test or delivering a dose of chemotherapy (10). |
| Transition | The set of interactions necessary to go from one type of care to another, such as the transition from detection to diagnosis. |
| Interface of care | A finer grade of transition where information and responsibility are transferred, such as communicating test results, calling to schedule an appointment, or contact between physicians to communicate details of a referral (10). |
| Health behavior | Activities that are presumed to affect health over time (eg, shared decision making, cancer screening, follow-up to abnormal tests, use of recommended treatments, and engagement in care). |
| Process of care impacts | Five of the IOM's six quality aims (including safety, effectiveness, patient-centeredness, timeliness, and efficiency) that can be measured for the process as a whole or subprocesses within a type of care (eg, screening). Consistent with AHRQ's Health Care Quality measurement, equities (disparities) are a separate metric of quality that can be measured across populations for all the indicators. |
| Patient and population outcomes | Intermediate and long-term outcomes of health-care quality on patient. Intermediate outcomes include changes in risk status, stage at diagnosis, quality of life, quality of death, and financial burden. Long-term outcomes are changes in morbidity and mortality for the population. The aggregate of patient outcomes represents population outcomes. |
AHRQ = Agency for Healthcare Research and Quality; IOM = Institute of Medicine
One approach to improving care has been to focus on one of the many individual steps in the process. This reductionist approach has led to improvements in specific technical aspects of care, such as new imaging or new diagnostic or treatment technology (3,11,12). However, these advances are slowly incorporated into care, and there is evidence for cancer care that the result may not be coordinated or supportive of patients (5,8,13–15). The reductionist approach to improving care has brought many new advances, but the sum of these advances is less than the integrated care that is desired (16).
To achieve that integrated care, we need to think about it as a process with multiple steps and interfaces that need to proceed smoothly. Furthermore, we need to begin thinking about how multiple levels of influence interact with the steps and interfaces of care in an interdependent way that creates a system (17,18). Consistent with the theory of complex adaptive systems, there are an infinite number of small steps to be taken by the people seeking care and those delivering it. Each step is connected to multiple other steps through interfaces where information and responsibility are transferred back and forth (10). These interactions are affected by the levels of contextual influence that change starting conditions and the pace of progression of an individual through an episode of care. Considering all the variations makes health care appear chaotic, highly variable, and subject to occurring in starts and stops (19). The challenge for those interested in improving the quality of cancer care becomes how to influence this system and avoid the usually faulty assumption that the addition of one improved step will result in quality improvement overall. Ignoring the complexity of the multilevel environment in which care occurs has not achieved the desired improvements in care quality outlined by the Institute of Medicine (IOM) at the turn of the millennium (7). The time has come to step back from this reductionist approach and begin considering care as a process in a dynamic system (18).
The notion that multiple levels of contextual influence affect behaviors through interdependent interactions is an ecological view that has a long tradition (18–24). Characterization of the levels that affect the behavior of people, however, varies. Some investigators have identified the following levels as key: intrapersonal (biological, psychological), interpersonal/cultural, organizational, physical environmental (built, natural), and policy (25). Others argue that there are three fundamental levels: individuals and groups, organizations, and economic and social systems (26,27). Still others emphasize a hierarchical set of levels: micro (eg, family), mezzo (eg, community, worksite), macro (eg, nation, state), and global (eg, geopolitical, economic) levels (24). This hierarchical view is consistent with the biopsychosocial model of health proposed by George Engel in the 1980s, which identifies multiple levels of influence, from molecules to the biosphere (22). For example, tobacco addiction may be impacted by an individual's physiological response to nicotine, but also by family attitudes and beliefs, or the community's culture and public policies that affect the price of cigarettes. Each of these factors is a potential level of influence upon smoking behavior (22). Despite the different operational definitions, all conceptualizations consistently suggest that individuals live and seek care in a complex environment (22,25).
The theory of complex adaptive systems provides one way to think about the system as a whole (17,19). This theory suggests that interactions between people and levels travel in multiple directions and that individuals and layers within a system are constantly adapting. For example, provider teams must live within the rules of their organizations on a daily basis, but over time providers and their teams may interact with their organizations to change those rules. Furthermore, the influences between contextual levels may not be completely hierarchical. For example, a change in national policy may directly influence the structures and processes of health-care organizations, without being “filtered” by intermediate levels of influence such as state health policies or local community environments. Similarly, a change in health-care organizational structure may directly influence patient outcomes, without intermediate effects on family or other social support systems.
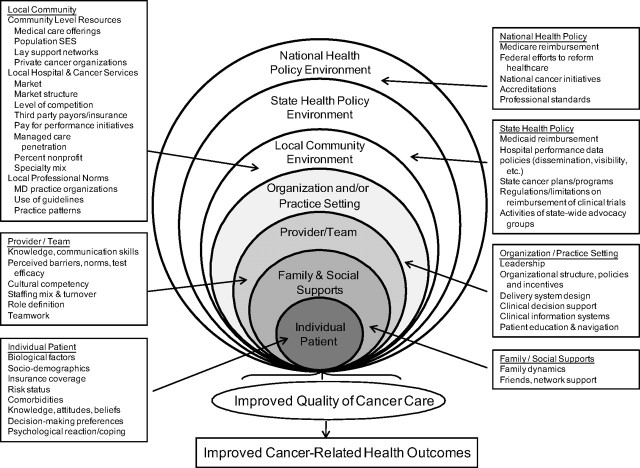
Because our interest is to understand and intervene in cancer care delivery, we adopt a conceptualization that uses levels of human aggregation. The conceptualization adapts Engel's ecological model of influences upon health (22) and identifies a hierarchy of potential intervention targets that are influential in health-care delivery (Figure 2) (28). These targets are the individual patient, including biological factors, beliefs and attitudes, sociodemographic characteristics, and risk factors; the provider/team, including skills and attitudes of providers, and the functioning of the provider team; family and social supports, including social networks; the organization or practice setting, including human and capital resources and processes designed to improve care; the local community environment, including local health-care markets, and social and professional norms; the state environment, including state reimbursement policies, taxation, or cancer programs; and the national environment, including such factors as national health reform, reimbursement policies, or cancer programs. Though the model identifies potential levels of intervention, it does not specify the mechanism of effect of the levels on each other or the behavior of providers and people seeking care.
Figure 2.
Multilevel influences on the cancer care continuum. Reprinted from Taplin SH and Rodgers A (10) and adapted from Zapka J (28) with permission from Wolters Kluwer Health. SES = socioeconomic status; MD = physician.
To consider the mechanisms of effect, there are many theories that vary with the level being considered. For example, institutional theory describes how organizational performance is constrained or enhanced by actors, institutional logics, and governance arrangements in an organizational field (29). The organizational transformation model offers practical steps to changing an organization, but expectations for that change may need to be tempered by an understanding of complex adaptive systems (30). Network theory describes webs of linkages between organizations, with linkages across organizations of similar forms (such as hospitals linked to other hospitals), or linkages between organizations at different levels of the environment (18). Social cognitive theory helps to explain how individuals, their social and built environment, and behaviors interact (31). Intra- and interpersonal theories describe mediators, moderators, and drivers of health-related behaviors at the individual patient level (32). All of these theories provide valuable perspectives on specific aspects of the multilevel context, but they do not provide a single unifying framework that can explain this complex context. While there is a need for unifying theory or model, interventionists can in the meantime address and measure a variety of mechanisms such as education, communication, physical environment, and policy.
We define an intervention as a specified strategy or set of strategies designed to change the knowledge, perceptions, skills, and/or behavior of individuals, groups, or organizations, with the goal of improving patients’ health outcomes (Table 1). We define a multilevel intervention as one that addresses at least three levels of the multilayer system (eg, the individual, the team of health-care providers, the health-care organization, or the community where the organization is located). Such interventions thereby target at least three sources of influence upon health behavior that may ultimately result in improved patient and population outcomes (Figure 2). We focus on individuals providing, seeking, and receiving cancer care (referring to them as providers and patients). We recognize that cellular mechanisms also are regulated by contextual influences and that there is considerable interest in the potential for genomic and other biologic information to personalize care. But our primary focus in this supplement is on the individual, group, organizational, and societal contexts that influence health-care delivery. We choose to focus on factors that affect health-care delivery because of the large per capita expenditure on health care and the need to provide coordinated care that improves the experience for patients while reducing the costs. The right columns and the bottom panel of Figure 1 summarize the effects of multilevel interventions: improved quality of the cancer care process, resulting in improved cancer-related health outcomes.
Despite the lack of a unifying framework, efforts to analyze and understand the effects of contextual influences upon health behavior and health care have made some progress. Application of qualitative and quantitative methods has resulted in a substantial increase in the volume and scope of studies of contextual effects on provider behavior, individual health behaviors, and health outcomes (33). Quantification of the relative contributions of different levels of influence is made possible through multilevel statistical methods and models (34,35). These techniques help researchers examine multilevel determinants of health disparities (36), factors affecting cancer screening (37–39), and strategies to improve adherence to clinical practice guidelines (40,41), among other examples.
Methods of intervening to affect contextual influences simultaneously in measurable ways that improve health remain largely unexamined (7). In the 1970s, there were some key projects demonstrating the benefits of community interventions to affect cardiovascular risk (40,41). We conducted a PubMed search in March 2010 using terms such as “multilevel,” “ecological,” and “interventions” to identify examples of multilevel intervention trials in health care. We identified a few examples that met our specific definition of multilevel intervention trials in health care (Box 1), but most of the literature we identified described multilevel statistical analyses of uni- or bilevel interventions. Furthermore, the intervention trials tested effects of multilevel interventions on individual health behaviors, such as smoking and physical activity. We were not able to identify trials in health-care delivery that included both an intervention and relevant measurements at three levels represented in our multilevel model. The multilevel trials we found focused on community interventions, and only one had links to health-care delivery.
Box 1. Examples of multilevel intervention trials.
COMMIT (Community Intervention Trial for Smoking Cessation): This trial randomly assigned one of each of 11 matched pairs of cities to either intervention or control status (60,61). The intervention communities received a set of interventions over 4 years using a community mobilization model. Creation of a Community Planning Group with representatives who could facilitate access to public media, worksites, and health-care settings was the first intervention. They, in turn, promoted the use of four broad interventions with multiple activities in each: 1) public education through media, 2) development and promotion of cessation resources in the community, 3) encouragement of organizational interventions, such as smoking cessation policy change at worksites, and 4) health-care provider education. These interventions targeted three levels of influence: community (media, planning group), organizations (worksite, provider teams, religious groups), and individuals (heavy smokers). Measurement occurred by surveys of individuals in the community, health-care organizations, religious groups, and worksites. The outcome of interest was smoking cessation among heavy smokers, but the significant effect was shown among light smokers. Quit rates among light to moderate smokers were 0.306 vs 0.275 (P = .004) in intervention vs control communities (60,61).
ASSIST (A Stop Smoking in Schools Trial): This randomized trial was conducted in seven intervention and seven control communities in Minnesota to test a community organizing approach to reducing smoking among school-age children who were in grades 8 through 10 at the beginning of the study (42). The intervention communities were mobilized through the active leadership of a half-time community organizer who established local teams of activists as the first intervention. The local teams were then trained and encouraged to undertake the following three interventions: 1) change ordinances regarding smoking, 2) educate community members about youth tobacco access, and 3) enforce ordinances against tobacco sales to minors. The trial used three interventions at three levels (policy creation, policy enforcement, education) of an ecological model: community, activist teams, and individuals. The study interventions did not directly address individuals, but they measured smoking at the individual level. The investigators focused measurement on smoking rates among minors by surveying samples of youth in the communities, but they also observed whether cities adopted and enforced ordinances against sales to minors. Although daily smoking among adolescents continued to increase in intervention communities, increases were smaller than in control communities.
CATCH (Child and Adolescent Trial for Cardiovascular Health): This randomized trial in four communities tested interventions at three levels (schools, families, and students) (43). They encouraged schools to 1) reduce the fat content of food served, 2) increase the amount of time devoted to physical exercise, and 3) implement classroom curricula addressing healthy eating. A random subset of intervention schools included packets of home activities that complemented the school curricula. Measurement included fat analysis of school menus, random visits to schools to assess physical activity, and surveys of students including psychosocial metrics, dietary recall, a physical activity checklist, individual serum cholesterol levels, and other physiological measures. The intervention was associated with reduction in fats served in schools and increase in the time devoted to physical exercise. Intervention families were more likely to complete home study curricula. Individuals in the intervention schools reported more exercise, but body mass index and cholesterol levels did not differ significantly.
The trials described in Box 1 showed modest effects for many potential reasons, including weak or poorly implemented interventions or measurement of effects before they could manifest. The challenge these examples present is how to develop strong interventions focused in health care whose effects can be measured at all the relevant levels. The challenge of developing strong interventions at multiple levels is compounded in the current environment that seeks “evidence-based” incremental progress. It is much easier to propose single-level interventions based on the current evidence base. However, addressing the quality chasm by implementing small evidence-based improvements has been the approach for at least a decade, and there is little to show that the fundamental character of expensive care divorced from evidence-based guidelines has been altered significantly since the publication of the IOM's report on the quality chasm at the turn of the century. Multilevel intervention research is therefore not based on evidence for the efficacy of such an approach, but upon the recognition that care occurs in a multilevel context that must be accounted for and influenced. The hope is that will increase the likelihood of sustainable improvements in the quality of health care (22).
Our interest in multilevel intervention strategies also includes a concomitant interest in articulating the intermediate processes and impacts of care that drive improvement in patient and population health outcomes. To develop interventions, we need to consider how each level affects the others, when to expect effects to manifest themselves in the care process, and how that process affects the health outcomes of interest. Effects may occur through policy, organizational, and team structure or through interpersonal interactions, but all the effects ultimately facilitate or restrict behavior to achieve a desired health-related outcome.
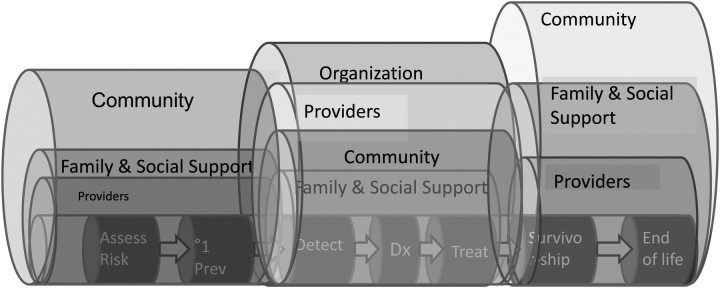
We also need to recognize that the interactions and effects of levels may differ across the cancer continuum (Figure 3). At the beginning of the continuum of care, the connection between primary care and the community may play a prominent role (44). In the middle of the continuum, when people are being treated for a cancer, the oncology specialist provider teams and organizations become a predominant influence (14). But this influence wanes as individuals complete their oncology therapy and become long-term cancer survivors (45). The types of care (eg, screening, diagnosis, treatment) aggregate collections of specific steps in the care process and involve interfaces among individuals and organizations (10). Interventions must begin to consider how multilevel contextual influences affect steps and interfaces, how policy affects who can move through the steps of care, and how communication can be improved.
Figure 3.
Contextual influences occur across the cancer care continuum and are likely to vary in importance.
Health-Care Quality Aims and Intermediate Impacts on Health Outcomes
Our hope is that in designing interventions that acknowledge and address the individual, group, organizational, and/or societal contexts that affect the processes of care, we will better influence the steps and interfaces that make up those processes. The ultimate purpose of multilevel interventions is to improve the quality and outcomes of health-care delivery. Health-care quality is “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge” (46). In its blueprint for national health-care quality improvement, Crossing the Quality Chasm, the IOM defined six national quality aims: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity (7). These aims guided the Agency for Healthcare Research and Quality (AHRQ) in developing quality measures in 2002. One focus for new research is to establish whether efforts at optimizing care consistent with these quality aims will have a positive impact on patient outcomes. Individually, the quality-of-care aims represent indicators for the processes of care across the entire cancer care continuum. We borrow from the AHRQ and the IOM definition of quality to propose desired measures of success for multilevel interventions in cancer care: increased quality of care across the cancer care continuum, resulting in improved cancer-related long-term patient health outcomes (eg, reduced morbidity and mortality from cancer, reduced financial burden to patients, and improved health-related quality of life) (see Figure 1 and Box 2).
Box 2. Quality aims from the Institute of Medicine (62).
Safety—Avoiding injuries to patients from the care that is supposed to help them; may include reductions in complications of care or inappropriate medication prescription, for example.
Effectiveness—Providing services based on scientific knowledge to all who could benefit, and refraining from providing services to those not likely to benefit (often classified as underuse, overuse, and misuse of care).
Patient-centeredness—Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.
Timeliness—Reducing waits and sometimes harmful delays for both those who receive and those who give care; may include time to initiation of treatment for patients with acute conditions and patients’ perceptions of the timeliness of appointments, for example.
Efficiency—Reducing waste and administrative cost; may include reduction in overuse of health-care services.
Equity—Providing equal opportunity to access care that does not vary in quality by personal characteristics, such as gender, ethnicity, geographic location, and socioeconomic status.
Quality of care process measures for cancer interventions can be identified for each type of care and transition. Health-care processes differ across each type and transition of care on the continuum, and therefore metrics must also change. Box 3 offers examples of quality aims across the types and transitions on the cancer care continuum.
Box 3. Examples of health-care quality aims across the cancer care continuum.
Risk assessment—Safety may be measured by the use of counseling to explain risks to patients in a way that minimizes emotional and psychological harms. Patient-centeredness may focus on ensuring patients are informed of their risk status and engaged to participate in prevention decisions and behaviors.
Primary prevention—Effectiveness and equity may take the form of multilevel promotion efforts and availability of primary care services—as opposed to emergency medical care—for all individuals.
Detection—An intervention aimed at equity could promote screening for low-income individuals and those who face geographic and financial obstacles to screening. Timeliness might minimize the time between when patients inform providers of a symptom and when diagnostic tests are performed.
Diagnosis—Timeliness may be increased with the use of electronic health records to coordinate care quickly across medical specialties. Efficiency may focus on the most cost-efficient use of diagnostic tools for the patient and health system as a whole.
Treatment—Safety may be considered when the physician uses a genetic test to estimate tumor recurrence risk in effort to avoid unnecessary and potentially harmful treatments. Patient-centeredness may involve providers explaining the diagnosis, efficacy and potential side effects of treatments, and encouraging patient participation in treatment decisions.
Survivorship—Safety measures may include the application of tested interventions for reducing long-term side effects of treatment, while a patient-centered aim may be to provide psychosocial support for the patient and family.
End-of-life care—A patient-centered approach would take into consideration the patient's quality-of-life preferences in decision making, whereas efficiency and effectiveness aims might provide access to palliative care and hospice resources which in turn reduce use of futile treatments.
Transitions—Patient-centeredness, timeliness, and efficiency may be addressed by using patient navigators or designing information systems to help individuals negotiate the transitions between steps of care.
Cancer Care as a Case Study for Examining Multilevel Influences and Interventions
Cancer is a good model for evaluating multilevel influences on health-care quality, as its development and progression is amenable to interventions at several levels of influence across multiple types of care, from prevention through the end of life. In fact, consideration and analysis of multilevel interventions has been proposed as a primary means of advancing the field of intervention research in cancer screening, among other types of cancer care (47).
Multilevel interventions focused upon improving processes in accordance with the six health-care quality aims face a daunting task, however. Interventionists must choose the place in the continuum where they want to intervene, the levels that must be influenced to achieve an impact, and the potentially optimal strategies. The relative importance of each contextual influence, however, may expand or diminish in size and importance depending on the cancer care type or transition being addressed, and the individual circumstances of a patient. For example, underuse of cervical or breast cancer screening among uninsured low-income women is influenced by the availability of federally-funded screening programs, whereas screening participation among insured women may be improved by reminder systems at the practice level or increased recommendation of appropriate screening at the provider level. Quality of life for those undergoing treatment for invasive cancers may be improved by the presence of familial and social supports, whereas individual patient characteristics such as belief systems may predispose patients to adhere to or to dismiss follow-up recommendations.
Purpose and Organization of the Monograph
This monograph contributes to the further development and evaluation of multilevel interventions in health care. In June 2009, a multidisciplinary group of experts met at the National Cancer Institute to consider what was needed to expand the foundation for multilevel approaches. They considered theories, models, and methods from health services, behavioral sciences, and several disciplines to explore potential multilevel influences across the cancer care continuum. They sought priorities for research, considered research designs, and explored measurement issues. The meeting participants were behavioral scientists, economists, epidemiologists, health services researchers, physicians, sociologists, and statisticians from across the country. The discussion exposed the need for a standardized vocabulary to facilitate communication across disciplines (Table 1). In addition, a number of questions arose that formed the basis for the content of this monograph:
Although the idea of multilevel interventions has intuitive heuristic appeal, how can it be made operational and meaningful?
How can theories and definitions of context from across disciplines be brought to bear on the multilevel framework?
How should time and timing be addressed to identify appropriate measures of contextual influences and their effects longitudinally?
What are the mechanisms by which interactions across levels occur, and how can these interactions be measured?
What is the potential for systems or simulation modeling to examine the effects of combinations of factors across levels?
How can knowledge about multilevel effects from other disease areas, such as heart disease and diabetes, be applied to cancer?
How can partnerships be developed to examine multilevel interventions from a larger research platform?
How can multilevel interventions and analyses inform health-care reform decisions?
How might multilevel interventions and analyses contribute to ongoing research and implementation of genomic and personalized medicine?
Overview of the Monograph
The articles in this monograph address these questions directly and indirectly. Section I describes multilevel influences and interventions across the cancer care continuum, highlights examples from the literature on chronic disease care and prevention, and explores important conceptual issues. Zapka et al. (48) illustrate core multilevel issues that emerge in the course of providing cancer care. Stange et al. (49) examine how the idea of multilevel influences across the cancer control continuum is actualized in the empiric literature. Section II addresses the challenges and opportunities for research on multilevel interventions. Innovative study designs and measurement techniques, application of systems modeling approaches, and development of research partnerships are discussed. Weiner et al. (50) describe strategies for combining interventions at different levels to produce complementary or synergistic effects. Alexander et al. (51) discuss how time and timing relate to conceptual issues of disease life course and treatment theory, the analysis of multilevel data in the context of cancer treatment and prevention, and approaches to research design in the context of cancer treatment and prevention studies. Charns et al. (52) address measurement issues in multilevel intervention research. Cleary et al. (53) review design and analytic approaches to multilevel interventions. Morrissey et al. (54) describe how simulation models can be used to examine intervention effects at multiple levels, and propose extensions of existing models of the natural history of cancer and cancer care.
Section III outlines future directions for multilevel interventions and research, with special emphasis on implementation, sustainability, and application of multilevel frameworks to current issues in health care. Flood et al. (55) consider how health-care reform in the United States is a massive intervention that will require longitudinal, multisite primary studies assessing its effects at different levels of the health-care context. Such an effort will require partnerships across institutes, agencies, foundations, public and private sources of support, as well as new data sources. Yano et al. (56) examine and discuss the application of evidence-based multilevel interventions in clinical practice settings, from small practices to large integrated health-care systems, such as those of the Department of Veterans Affairs. Sheinfeld Gorin et al. (57) review a body of work that has been growing recently and addresses whether multilevel interventions reduce health disparities. Khoury et al. (58) propose how multilevel interventions and analyses may contribute to ongoing research and implementation of genomic and personalized medicine.
We close the monograph by returning to the questions posed in June of 2009 and considering an agenda for future research. This was a complicated undertaking that benefited from the contributions of people who have been struggling with multilevel models for generations, the authors who contributed to the articles, but also by the 168 people who actively discussed early versions of these articles at a meeting in Las Vegas in March of 2011. Those people are listed in the conference summary by Edwards et al. (59). We thank them all and we look forward to a growing circle of investigators going beyond understanding the multilevel context of care to intervene successfully.
Funding
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
References
- 1.Nolte E, McKee CM. Measuring the health of nations: updating an earlier analysis. Health Aff (Millwood). 2008;27(1):58–71. doi: 10.1377/hlthaff.27.1.58. [DOI] [PubMed] [Google Scholar]
- 2.Asch SM, Kerr EA, Keesey J, et al. Who is at greatest risk for receiving poor-quality health care? N Engl J Med. 2006;354(11):1147–1156. doi: 10.1056/NEJMsa044464. [DOI] [PubMed] [Google Scholar]
- 3.Abernethy AP, Etheredge LM, Ganz PA, et al. Rapid-learning system for cancer care. J Clin Oncol. 2010;28(27):4268–4274. doi: 10.1200/JCO.2010.28.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leape L, Berwick D, Clancy C, et al. Transforming healthcare: a safety imperative. Qual Saf Health Care. 2009;18(6):424–428. doi: 10.1136/qshc.2009.036954. [DOI] [PubMed] [Google Scholar]
- 5.Wagner EH, Aiello Bowles EJ, Greene SM, et al. The quality of cancer patient experience: perspectives of patients, family members, providers and experts. Qual Saf Health Care. 2010;19(6):484–489. doi: 10.1136/qshc.2010.042374. [DOI] [PubMed] [Google Scholar]
- 6.Ginsburg JA, Doherty RB, Ralston JF, Jr, Senkeeto N. Achieving a high-performance health care system with universal access: what the United States can learn from other countries. Ann Intern Med. 2008;148(1):55–75. doi: 10.7326/0003-4819-148-1-200801010-00196. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 8.Hewitt M, Simone JV Institute of Medicine. Ensuring Quality Cancer Care. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 9.Zapka J, Taplin S, Solberg L, Manos M. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4–13. [PubMed] [Google Scholar]
- 10.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;40:3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356(13):1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 12.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 13.Nekhlyudov L, Latosinsky S. The interface between primary oncology specialty care: from symptoms to diagnosis. J Natl Cancer Inst Monogr. 2010;40:11–17. doi: 10.1093/jncimonographs/lgq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sussman J, Baldwin LM. The interface between primary and oncology specialty care: from diagnosis through primary treatment. J Natl Cancer Inst Monogr. 2010;40:18–24. doi: 10.1093/jncimonographs/lgq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han PKJ, Rayson D. The coordination of primary and oncology specialty care at the end of life. J Natl Cancer Inst Monogr. 2010;40:31–37. doi: 10.1093/jncimonographs/lgq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessler R, Glasgow RE. A proposal to speed translation of healthcare research into practice: dramatic change is needed. Am J Prev Med. 2011;40(6):637–644. doi: 10.1016/j.amepre.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Scott WR, Davis GF. Organizations and Organizing: Rational, Natural and Open Systems Perspectives. 1st ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2007. [Google Scholar]
- 18.Reid PP, Compton WD, Grossman JH, Fanjiang G, editors. Building a Better Delivery System: A New Engineering/Health Care Partnership. Washington, DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- 19.Resnicow K, Page SE. Embracing chaos and complexity: a quantum change for public health. Am J Public Health. 2008;98(8):1382–1389. doi: 10.2105/AJPH.2007.129460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Susser M, Susser E. Choosing a future for epidemiology: II. From black box to Chinese boxes and eco-epidemiology. Am J Public Health. 1996;86(5):674–677. doi: 10.2105/ajph.86.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkman LF, Kawachi I. Social Epidemiology. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 22.Engel GL. The clinical application of the biopsychosocial approach. In: Frankel RM, Quill TE, McDaniel S, editors. The Biopsychosocial Approach: Past, Present, Future. Rochester, NY: University of Rochester Press; 2003. pp. 1–20. [Google Scholar]
- 23.Shortell SM, Kaluzny AD. Organization theory and health services management. In: Shortell SM, Kaluzny AD, editors. Health Care Management: Organization, Design, and Behavior. 5th ed. Clifton Park, NY: Thomson Delmar Learning; 2006. pp. 5–41. [Google Scholar]
- 24.Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62(7):1650–1671. doi: 10.1016/j.socscimed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 25.Sallis JF, Cervero RB, Ascher W, Henderson KA, Kraft MK, Kerr J. An ecological approach to creating active living communities. Annu Rev Public Health. 2006;27:297–322. doi: 10.1146/annurev.publhealth.27.021405.102100. [DOI] [PubMed] [Google Scholar]
- 26.Molloy JC, Ployhart RE, Wright PM. The myth of “the” micro–macro divide: bridging system-level and disciplinary divides. J Manage. 2011;37(2):581–609. [Google Scholar]
- 27.Klein KJ, Kozlowski SWJ. From micro to meso: critical steps in conceptualizing and conducting multilevel research. Organizational Research Methods. 2000;3(3):211–236. [Google Scholar]
- 28.Zapka J. Innovative provider- and health system-directed approaches to improving colorectal cancer screening delivery. Med Care. 2008;46(suppl 9):S62–S67. doi: 10.1097/MLR.0b013e31817fdf57. [DOI] [PubMed] [Google Scholar]
- 29.Ruef M, Mendel P, Scott WR. An organizational field approach to resource environments in healthcare: comparing entries of hospitals and home health agencies in the San Francisco Bay region. Health Serv Res. 1998;32(6):775–803. [PMC free article] [PubMed] [Google Scholar]
- 30.Weiner BJ, Helfrich CD, Hernandez SR. Organizational learning, innovation, and change. In: Shortell SM, Kaluzny AD, editors. Health Care Management: Organization Design, and Behavior. 5th ed. Clifton Park, NY: Thomson Delmar Learning; 2006. pp. 383–411. [Google Scholar]
- 31.Baranowski T, Perry CL, Parcel GS. How individuals, environments, and health behaviors interact: Social Cognitive Theory. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education: Theory, Research and Practice. 3rd ed. San Francisco, CA: Jossey-Bass; 2002. pp. 165–184. [Google Scholar]
- 32.Glanz K, Rimer BK, Lewis FM. Models of individual health behavior. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education: Theory, Research and Practice. 3rd ed. San Francisco, CA: Jossey-Bass; 2002. pp. 41–44. [Google Scholar]
- 33.Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health. 2000;21:171–192. doi: 10.1146/annurev.publhealth.21.1.171. [DOI] [PubMed] [Google Scholar]
- 34.Subramanian S, Jones K, Duncan C. Multilevel methods for public health research. In: Kawachi I, Berkman L, editors. Neighborhoods and Health. New York, NY: Oxford University Press; 2003. pp. 65–111. [Google Scholar]
- 35.Duncan C, Jones K, Moon G. Health-related behaviour in context: a multilevel modelling approach. Soc Sci Med. 1996;42(6):817–830. doi: 10.1016/0277-9536(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 36.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mobley L, Kuo TM, Urato M, Boos J, Lozano-Gracia N, Anselin L. Predictors of endoscopic colorectal cancer screening over time in 11 states. Cancer Causes Control. 2010;21(3):445–461. doi: 10.1007/s10552-009-9476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Datta GD, Colditz GA, Kawachi I, Subramanian SV, Palmer JR, Rosenberg L. Individual, neighborhood, and state-level socioeconomic predictors of cervical carcinoma screening among U.S. black women: a multilevel analysis. Cancer. 2006;106(3):664–669. doi: 10.1002/cncr.21660. [DOI] [PubMed] [Google Scholar]
- 39.Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc Sci Med. 2008;66(2):260–275. doi: 10.1016/j.socscimed.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Farquhar JW, Fortmann SP, Flora JA, et al. Effects of communitywide education on cardiovascular disease risk factors. The Stanford Five-City Project. JAMA. 1990;264(3):359–365. [PubMed] [Google Scholar]
- 41.Puska P, Vartiainen E, Laatikainen T, Jousilahti P, Paavola M. The North Karelia Project: From North Karelia to National Action. Helsinki, Finland: Helsinki University Printing House; 2009. [Google Scholar]
- 42.Forster JL, Murray DM, Wolfson M, Blaine TM, Wagenaar AC, Hennrikus DJ. The effects of community policies to reduce youth access to tobacco. Am J Public Health. 1998;88(8):1193–1198. doi: 10.2105/ajph.88.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lytle LA, Stone EJ, Nichaman MZ, et al. Changes in nutrient intakes of elementary school children following a school-based intervention: results from the CATCH Study. Prev Med. 1996;25(4):465–477. doi: 10.1006/pmed.1996.0078. [DOI] [PubMed] [Google Scholar]
- 44.Pasick RJ, Burke NJ, Barker JC, et al. Behavioral theory in a diverse society: like a compass on Mars. Health Educ Behav. 2009;36(suppl 5):11S–35S. doi: 10.1177/1090198109338917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grunfeld E, Earle C. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;40:25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohr KN. Medicare: A Strategy for Quality Assurance. Washington, DC: National Academies Press; 1990. [PubMed] [Google Scholar]
- 47.Meissner HI, Vernon SW, Rimer BK, et al. The future of research that promotes cancer screening. Cancer. 2004;101(5 suppl):1251–1259. doi: 10.1002/cncr.20510. [DOI] [PubMed] [Google Scholar]
- 48.Zapka J, Taplin SH, Ganz P, Grunfeld E, Sterba K. Multilevel factors affecting quality: examples from the cancer care continuum. J Natl Cancer Inst Monogr. 2012;44:11–19. doi: 10.1093/jncimonographs/lgs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stange KC, Breslau Es, Dietrich AJ, Glasgow RE. State-of-the-art and future directions in multilevel interventions across the cancer control continuum. J Natl Cancer Inst Monogr. 2012;44:20–31. doi: 10.1093/jncimonographs/lgs006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner BJ, Lewis MA, Clauser SB, Stitzenberg KB. In search of synergy: strategies for combining interventions at multiple levels. J Natl Cancer Inst Monogr. 2012;44:31–41. doi: 10.1093/jncimonographs/lgs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander J, Prabhu Das I, Johnson TP. Time issues in multilevel interventions for cancer treatment and prevention. J Natl Cancer Inst Monogr. 2012;44:42–48. doi: 10.1093/jncimonographs/lgs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charns MP, Foster MK, Alligood EC, et al. Multilevel interventions: measurement and measures. J Natl Cancer Inst Monogr. 2012;44:67–77. doi: 10.1093/jncimonographs/lgs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cleary PD, Gross CP, Zaslavsky AM, Taplin SH. Multilevel interventions: study design and analysis issues. J Natl Cancer Inst Monogr. 2012;44:49–55. doi: 10.1093/jncimonographs/lgs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrissey JP, Hassmiller Lich K, Anhang Price R, Mandelblatt J. Computational modeling and multilevel cancer control interventions. J Natl Cancer Inst Monogr. 2012;44:56–66. doi: 10.1093/jncimonographs/lgs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flood AB, Fennell ML, Devers KJ. Health reforms as examples of multilevel interventions in cancer care. J Natl Cancer Inst Monogr. 2012;44:80–85. doi: 10.1093/jncimonographs/lgs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yano EM, Green LW, Glanz K, et al. Implementation and spread of interventions into the multilevel context of routine practice and policy: implications for the cancer care continuum. J Natl Cancer Inst Monogr. 2012;44:86–99. doi: 10.1093/jncimonographs/lgs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheinfeld Gorin S, Badr H, Krebs P, Prabhu Das I. Multilevel interventions and racial/ethnic health disparities. J Natl Cancer Inst Monogr. 2012;44:100–111. doi: 10.1093/jncimonographs/lgs015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khoury MJ, Coates RJ, Fennell ML, et al. Multilevel research and the challenges of implementing genomic medicine. J Natl Cancer Inst Monogr. 2012;44:112–120. doi: 10.1093/jncimonographs/lgs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards HM, Taplin SH, Chollette V, Clauser SB, Prabhu Das I, Kaluzny AD. Summary of the multilevel interventions in health care conference. J Natl Cancer Inst Monogr. 2012;44:123–126. doi: 10.1093/jncimonographs/lgs018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.The COMMIT Research Group. Community intervention trial for smoking cessation (COMMIT): II. Changes in adult cigarette smoking prevalence. Am J Public Health. 1995;85(2):193–200. doi: 10.2105/ajph.85.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The COMMIT Research Group. Community Intervention Trial for Smoking Cessation (COMMIT): I. Cohort results from a four-year community intervention. Am J Public Health. 1995;85(2):183–192. doi: 10.2105/ajph.85.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280(11):1000–1005. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]