Abstract
We conducted literature searches and analyses to describe the current state of multilevel intervention (MLI) research and to identify opportunities to advance cancer control and prevention. We found single-level studies that considered other contextually important levels, and multilevel health-care systems research and community-wide studies. This literature is characterized by limited reporting of theoretical, contextual, temporal, and implementation factors. Most MLIs focus on prevention and screening, rather than diagnosis, treatment, or survivorship. Opportunities relate to 1) dynamic, adaptive emergent interventions and research designs that evolve over time by attending to contextual factors and interactions across levels; 2) analyses that include simulation modeling, or multimethod approaches that integrate quantitative and qualitative methods; and 3) translation and intervention approaches that locally reinvent MLIs in different contexts. MLIs have great potential to reduce cancer burden by using theory and integrating quantitative, qualitative, participatory, and transdisciplinary methods that continually seek alignment across intervention levels, pay attention to context, and adapt over time.
The impact of cancer on the quantity and quality of life can be addressed at multiple levels (1–3), including public policy, the environment, communities, health-care organizations and teams, clinicians, families, and individuals (4). Strategically intervening at the interfaces across levels has potential to yield stronger effects on population health (5) than working at only one level.
In this chapter, we provide an overview of what is known about the effects of multilevel interventions (MLIs), drawing from the cancer control, chronic disease care, and prevention literature. We examine how the idea of multilevel influences across the cancer control continuum (6) has been applied and reported in the empiric literature and identify opportunities for MLIs to improve health care and health in a rapidly changing environment (7).
Methods
We developed a transdisciplinary team (8,9) representing training and experience in cancer control, public health, clinical practice, behavioral science, and health services research. We designed an initial literature search strategy to sample the broad range of MLIs across the fields of cancer control, health, and health-care studies. Based on searches conducted by a consulting research librarian and staff member with experience in identifying relevant scientific literature, we identified the most relevant articles and ascertained topics that were missing. The librarian staff and the authors then conducted additional searches using snowball techniques to identify the range of scientific articles relevant to the topic. The search strategy is shown in Supplemental Appendix A (available online).
We developed a matrix of article types (cancer/noncancer; intervention/observation; theory/empiric/methodological) and identified enlightening examples across the domains of the matrix that pointed to helpful examples upon which the field can build. We characterized how multilevel research has been conceptualized and implemented, and sampled additional articles to fill gaps that emerged from our review and analysis of themes. In response to peer reviewers, we added examples to more fully represent health-care interventions at the practice- and system-level. In individual and group analyses, we identified current controversies and potential missed opportunities and developed recommendations to more fully realize the potential of multilevel research for improving cancer control.
Characterization of the Literature
We found a mix of empiric observational and intervention studies, as well as thought and theory pieces that address multiple levels affecting health, health care, and cancer prevention and control. We identified the following threads of shared thinking and research:
Contextualized single-level studies that intervene at the level of the person/patient or provider/practice but consider other contextually important levels [eg, (10,11)];
Health-care systems research that includes practice- and individual-level interventions, and sometimes consider community or policy factors [eg, (12–16)];
Table 1 outlines some of our global observations. This literature recognizes that intervening at a single-level does not match the complexity of influences on cancer control. Fleetingly, the literature glimpses and moves toward solutions through theory, observation, and intervention, but then typically falls short of its promise due to the limitations of current ways of conceptualizing, funding, performing, and reporting the findings of research.
Table 1.
Observations from the literature
| Multilevel interventions are contextual, but context is reported inadequately in most reports. |
| Typically, fewer than three levels are reported. |
| Theory, models, and interventions are not well-integrated in reports/studies. |
| Across the cancer control continuum, most multilevel interventions have focused on prevention, screening, and end-of-life, but seldom on diagnosis, treatment, and surveillance. |
| Temporal issues may affect the various levels in different ways at different times. The rate of change is different between levels. |
| There are few detailed reports of how multilevel interventions have been implemented and have become successful or unsuccessful. |
| Likely due to funding and publication bias, the majority of empiric reports have been randomized controlled trials. |
Much of the multilevel literature relates to cancer prevention, cancer screening, and, to a lesser extent, end-of-life care, but few studies address cancer survivorship, diagnosis, treatment, or surveillance, and even fewer address the underresearched but increasingly important problem of interrelated risk factors and multimorbid illnesses (30–37). We also found that the field of cancer care across the continuum from prevention to survivorship could learn from multilevel studies in other fields.
One of the most serious limitations in this literature is that context—the surrounding, interrelated factors that influence complex phenomenon—is scantily reported, even though understanding context is vital for effective MLIs (38). Although factors affecting internal validity frequently are reported, information usually is absent about the history, circumstances, and settings that would allow others to make informed decisions about modifications needed to apply findings in other settings (39,40). In addition, details often are lacking on temporal factors and resource and cost requirements that affect interactions between levels.
We found references to underlying theories, intervention frameworks, and models, but limited use in implementing the study, measuring hypothesized mediators, guiding analysis or informing others who might be trying to replicate the study or make modifications appropriate for their own context.
Not all informative studies were randomized controlled trials. The challenges and limitations of conducting randomized controlled trials in complex applied settings can constrain interventions to the tyranny of what fits within highly specified designs, rather than studying the emergent potential of multilevel effects. The potential of quasi-experimental, rapid cycle, multiple learning, and multiple baseline designs, and simulation modeling have been recognized, particularly for community and multilevel organizational research (41–43), but were used infrequently.
Below, we further characterize the theory/models and methods in this literature and depict its potential by describing an illustrative study (and three other examples in Supplementary Appendix B, available online) that demonstrate the potential of MLI research. We close by identifying opportunities suggested by this literature.
Theory and Models
Models in the MLI literature are published to inform interventions, policies, and intervention research. Most of these models implicitly or explicitly are based on social ecological (44–46), systems (47–49), or complexity (50–53) theories. Despite the complex evolving relationships implied by these underlying theories, the models that apply them often struggle to depict the interplay between determinants in nonlinear ways that reflect the multifaceted coevolving nature of the multilevel phenomena being described.
The models guiding MLI studies, when they are specified, often represent attempts to depict the contextual and cultural environments for interventions. There appear to be two types. Personal models start with the individual and work outward. Community models start with a population, society, health-care system, or group and work inward. [Models for “personalized medicine” (54–60), that start with the genome and work outward toward potential pharmacological interventions, are covered in Khoury et al. (61).]
Personal Models.
Personal models that inform MLI depict individuals as nested within social, economic, and cultural context. Distinct populations such as racial/ethnic, immigrant, or low-income groups have differing systems of belief about health and illness that affect how they think, feel, and seek health care or respond to prevention interventions (62). Consequently, applying personal models across multiple levels can elucidate how and where these various groups intersect within the wider society, which is essential to understanding behavior across multiple levels and how MLI affects health disparities (63,64).
Community and Society Models.
These frameworks often include health-care system, practice, and individual interventions nested within this larger context (65). For example, Best et al. (1) consider individual, organizational, and system levels in a “knowledge integration” framework for translating cancer control knowledge into practice and policy. Similarly, the E2D2 model by Peterman and Petz (66) accounts for multilevel influences on individual and population health, quality of life, and cancer risk. This model focuses on four pillars of health promotion intervention practice (evidence, evaluation, development, and delivery) and moves through three phases: identification of risk factors and sensitizing concepts mediating mechanisms and modifiable contexts; and program development, delivery, and evaluation. E2D2 is designed to be applied both sequentially and with feedback to allow for the emergence of new evidence, revisions, and knowledge exchange at any phase (66).
Approaches to social change intended to help underserved populations often are based loosely on concepts from Diffusion of Innovation Theory (67). Community-based programs based on application of this theory identify participants (the adopters) who are asked to consider problematic aspects of the innovation and steps to be taken to ameliorate problems before implementation (68).
Among community models, health-care system models currently are prominent, spurred by the increasing recognition and use of the Chronic Care Model of Wagner et al. (69–73). In its application, it has been extended to include preventive service delivery (74) and community and policy issues (75). Although originally developed to understand and guide health-care system improvement, many of the Chronic Care Model elements, such as self-management support, decision support, and community resources, imply linkages at different, multiple, and, optimally coordinated levels. The Chronic Care Model suggests that success may not be due as much to the number of components addressed, as to the extent to which modifications at multiple levels reinforce each other (76). The Expanded Chronic Care Model (77) includes a population health perspective that can be applied to broadly based prevention efforts that include the social determinants of health and enhanced community participation—all of which may be particularly relevant to MLI on complex cancer control phenomena.
Application of Theory.
MLIs often are grounded in core assumptions and principles that integrate psychological, cultural, community planning, organizational, and regulatory perspectives, as well as “partnership approaches that build on the principles of community organization theory which promotes the planned involvement and contribution(s) of community citizens, leaders, and organizations” (78). Interventions such as the large Community Intervention Trial for Smoking Cessation (COMMIT) focus on health outcomes and social problems (28,29). Although COMMIT incorporated several intervention levels (eg, school, media, worksite, medical office, faith organizations), the study was constrained from conducting a number of community-focused activities such as lobbying, political action and advocacy, or providing feedback to communities on their progress in reducing smoking.
Theoretical origins of the subsequent American Stop Smoking Intervention Study for Cancer Prevention Trial (ASSIST) (23) personify sociological theory, which includes social activism principles and policy advocacy as well as social ecological assumptions (79) to understand the interrelations among diverse personal and environmental factors in human behavior (8).
In several of the large community cardiovascular risk factor reduction studies, such as the Pawtucket (80) and Stanford (20) Heart Health Programs (22,81–83), interventions are based on a blend of social-learning theory, community organization models, community psychology tenets, and diffusion research. This approach allows for multifaceted programs that target individuals, groups, health-care settings, organizations, and the entire community to alter cardiovascular risk factors. The community-activation and volunteer-based delivery system approach targets the development of an optimal community environment for heart health behaviors, or what has been referred to as “systems-level or competence-enhancement” (80). In contrast, the North Karelia Project, which has produced stronger and more long-lasting improvements in population heart health, took a more policy-based environmental change approach, with actions at other levels congruent with policy changes (19,21,22,82,84–88).
Methodological Findings
Published empiric multilevel studies use a variety of experimental, quasi-experimental, and observational designs. A few studies use ethnographic and other qualitative methods, and fewer still integrate qualitative and quantitative methods, despite the complementary nature of these two approaches (89–93). The use of multilevel modeling has been rare, but increasing in more recent studies (94).
Most studies report outcomes at only one level, typically the patient or individual community member. Other levels (such as health-care practices, health-care systems, community organizations, or public policies) tend to be characterized with process measures or anecdotally. Interactions across levels, and how interventions relate, are rarely reported, even though the importance of these interactions often is implied in the design of the studies.
Computer simulations (17,95), agent-based dynamic models (96,97), or ethnographic methods (98) rarely are used to represent levels deemed to be important but for which collection of sufficient amounts of high-quality quantitative data is not feasible.
Another important methodological issue relates to assessing the pace of change across different levels. In general, it is much easier to produce rapid short-term improvements at the individual or small group level than at broader health-care system, community, regional or national, and policy levels (8). The paradoxical countervailing finding is that although it typically takes longer to produce change at “higher or outer” layers of the multilevel “onion” (4), such as the built environment or policy actions, such changes often have broader reach or community-wide impact across the population and may be much more sustainable (99). Thus, studies that evaluate outcomes or compare interventions at different ecological levels need to report outcomes at different points in time to get an accurate picture of the impact and contributions of distinct factors.
Few studies have produced sufficient variation in community or policy levels to adequately evaluate their effects. Interventions within the same state or region tend to have many similarities at these larger cultural, historical, policy levels, and may not have adequate variation across such factors to be able to effectively evaluate their impact on outcomes (100,101). Trends at the levels of policy, community, or society can sometimes overpower intervention effects, particularly in the situations of large clinical trials, in which the evidence necessary to justify the trial also is in the process of becoming common knowledge that influences behavior in comparison groups. Thus, a number of studies including COMMIT (28,29) and Cancer Action in Rural Towns [CART (25–27)] found improvements in outcomes in both intervention and comparison communities associated with societal and policy changes.
The most successful and sustainable MLIs appear to be those that have built sufficient capacity, resources, and scanning/health communication components; are prepared to take advantage of emerging external events; and have the flexibility to be locally relevant and actionable (19,21,82,84,87).
Few MLI reports compare a multilevel to either a single- or dual-level intervention or compared different types of MLIs. Comparative effectiveness research (CER) would seem to provide an appropriate context within which to test such comparisons and would represent an appropriate advance in both fields (102–106).
Instructive Empiric Examples
Table 2 provides a brief summary of the design, focus, and person/family, practice, health-care system, and community levels addressed by four example studies. Together, these studies show that it is possible to simultaneously intervene at multiple levels to produce sustainable change. They also demonstrate the benefits of a flexible contextually sensitive intervention strategy that is allowed to evolve based on input from participants at multiple levels and from varying partnership approaches. These studies hint at the potential for enhanced effects within and across levels (64), with upper levels often being permissive, motivational, or reinforcing, and intermediate evels being instrumentally helpful in fostering change at the levels of families or individuals.
Table 2.
Overview of example studies*
| Level | Margolis (107) | Dietrich (24) | Dietrich (108,109) | Feldstein (110–112) |
| Design | Observational intervention study | Randomized controlled trial† | Randomized controlled trial | Randomized controlled trials |
| Focus | Preventive services and health behavior | Sun protection | Screening for breast, colorectal, and cervical cancer | Mammography, colonoscopy |
| Person/Family | Low-income pregnant mothers, and their children <2 years | Children 2–9 years in day care and school | Patients in MMC | Only through patient barriers |
| Practice | Primary care and home visits | Primary care | Six primary care practices where patients received care were introduced to project and USPSTF cancer screening guidelines | Removing burden and responsibility from provider, but providing feedback to teams |
| Health-care system | Multilevel advisory committee included Medicaid representatives | MMC organization provided trained bilingual staff to conduct telephone outreach and claims data to select patients and to evaluate the intervention | Researchers work with operations teams; increased availability of mammograms | |
| Community | Community organization and town and state government | Town beaches |
MMC = Medicaid Managed Care; USPSTF = US Preventive Services Task Force.
Cancer Control P.L.A.N.E.T. sponsored by Agency for Healthcare Research and Quality, American Cancer Society, Centers for Disease Control and Prevention, the Commission on Cancer, National Cancer Institute, and Substance Abuse and Mental Health Services Administration. Research-tested Intervention Programs (RTIPs). Sun Safe http://rtips.cancer.gov/rtips/programDetails.do?programId=180266.
Three of these studies are described in Supplementary Appendix B (available online), and one is described below.
An MLI on Preventive Services for Children.
The Community-Wide Intervention to Improve the Delivery of Preventive Services to Children was an observational/intervention study in a North Carolina county with 182 000 residents (107). The intervention objective was to change the delivery of preventive care to all children, especially for low-income mothers and infants. Interventions were directed toward fundamental determinants of health, such as poverty and ineffective care systems for preventive care in office practices. Multiple interventions were carried out at the level of families, primary care practices, state and county government, and community organizations.
To achieve policy change at the community level, the intervention convened an advisory board with participants from Medicaid, community agencies, primary care practices, and county government. The advisory board provided advice on how the project would fit among existing community health improvement projects with the implementation of a home visiting program and provided leadership that would increase communication about problems surrounding immunization care delivery. In addition, the health department assisted with developing strategies and processes to screen, recruit, and enroll families.
To overcome barriers to care delivery, a multipronged practice-level intervention provided assistance for staff hiring, training, ongoing supervision, and consultation. Within primary care practices, the intervention focused on the office delivery system, use of teamwork, and use of office systems data. The “office systems” approach begins with developing a practice policy, auditing medical records for baseline performance, developing and implementing a plan for efficient delivery of preventive care, involving office staff and then monitoring progress (118).
The family-level component of the intervention used public health nurses and early childhood educators to reach poor pregnant women (n = 274) and their infants through home visits. These visits addressed risk factors for adverse health outcomes and educated the mothers on fetal and infant health and development. The visits also provided links to health and human services and information about support systems, injury prevention, and ways to get help in obtaining care from the primary care office.
Two evaluation designs were used: an interrupted time series to assess preventive service delivery in office practices and a historical cohort design to evaluate infant and maternal outcomes for women enrolled in the home visitation program. Outcomes were assessed at three levels of the intervention.
Community-level impact was evaluated by changes in participation, financing, and organization of services delivered by participating organizations. Data were abstracted from social services reports for reported child abuse and neglect, and hospital medical records were searched to identify emergency room visits or hospital admissions for injuries or harmful ingestions. Immunization records were identified through a registry, and data on the number of prenatal visits, pregnancy weight gain, gestational age, and pregnancy complication were obtained from hospital records.
Practice-level interventions were assessed by measuring rates of preventive services before and after the interventions were delivered in each setting. Data were obtained by abstracting a random sample of charts from each practice.
Using a historical cohort design, the family-level interventions compared two groups of women: women enrolled in the intensive home visiting program and women who had sought prenatal care during the 9 months before the program's initiation. Trained interviewers assessed women at the time of enrollment and when the child reached 12 months of age. Direct observation was used to determine the safety of the home environment. A baseline survey assessed parent knowledge and skills, satisfaction with parenting, social support, and the 12-month interview inquired about postnatal work, educational history, type of child care arrangement, and number of hours per week the child used the services.
High rates of participation were found across all levels, and modest improvement in rates of delivery of preventive services, health behaviors, and health outcomes. The study led to sustained policy changes at the state and community levels, changes in the organization and financing of care, and expansion to other communities across the state. These modifications enabled change to occur in preventive health service delivery.
At the practice-level, seven out of eight practices implemented at least one new office system. Practices cooperated with each other in joint contracting, staff training, defining program eligibility, and improving office system elements, which resulted in reduced duplication and improved coordination of care.
At the family-level, 89% of women agreed to participate in the program. Compared with the comparison group, women in the intervention group were more likely to use contraceptives (69% vs 47%), not smoke tobacco (27% vs 54%), and have a safe and stimulating home environment for the children. Children in the intervention group were more likely to have had an appropriate number of well-care visits (57% vs 37%) and less likely to be injured (2% vs 7%). In addition, families in the intervention group received public aid for fewer months (7.7 vs 11.3 months).
Changes were achieved over a relatively brief 3-year study period, and many have been sustained since the project was completed. They suggest that system-level interventions hold promise to improve the effectiveness and outcomes of care for children.
Opportunities
As summarized in Table 3, we identified a number of interrelated strategies to advance the impact of MLI research. These opportunities relate to design, analyses, and translation.
Table 3.
Opportunities to advance the potential of multilevel interventions
| Design |
| Greater attention to identifying, conceptualizing, and reporting important contextual factors across levels and over time |
| More use of dynamic, adaptive, emergent rapid learning designs that evolve and learn over time, rather than static designs |
| Greater attention to the interfaces across and among levels |
| Being explicit in all research about the potential effects of which levels are being studied and which levels might influence the phenomenon under study, even if they are not the focus of the study |
| More focus on effectiveness trials in real-world practical (typical) settings to inform practice and policy, and on more transparent reporting of adjustments |
| Conceptually based strategic interventions using pragmatic designs that inform practice and policy |
| Analysis |
| Reconceptualize reliability, sustainability, and fidelity to allow for intervention evolution and local and temporal adaptation |
| Capture, but also move beyond, only measures of central tendency and study of subgroups including biologic, economic, and environmental factors |
| Use of multimethod approaches that integrate quantitative modeling across multiple levels where relevant qualitative data can be generated and qualitative methods to evaluate levels with small numbers and to identify specific interlevel processes that are important for the outcomes of interest |
| Complex systems and dynamic simulation modeling may provide additional insights where data are sparse |
| Reporting on unintended consequences, factors emergent during studies, and how they were addressed |
| More thorough and transparent reporting of resources and costs expended, including program promotion and supervision, and sensitivity analyses to estimate the impact of variations in setting, staff, patients, etc. |
| Translation |
| More transparent reporting of setting, site, and clinician selection and representativeness; context and range of application are needed |
| Moving beyond fidelity to interventions that are locally adapted, evolutionary, and participatory, and publication of implementation lessons learned and guidelines |
| Study of emergent properties, as well as identification of how multiple levels interact in context rather than in isolation; we need to move beyond conceptualizations of “maintenance” to evolvable and capacity enhancing interventions |
| Funders, review groups, and training programs need to expand beyond the currently dominant reductionistic approaches; instead, we must value and support transdisciplinary methods, theories, and empiric approaches needed to conduct research across the multiple levels affecting health care and health |
Design
Interventions can be improved by explicitly considering how theory is operationalized in models that focus interventions that work together to enhance impact across multiple levels. Deeper understanding of highly context-dependent interactions between levels will require multimethod research designs that integrate quantitative and qualitative methods (92,93,119,120). Qualitative methods can be particularly helpful for assessing higher levels in which sample sizes are too small for statistical analyses, and where contextualized understanding is important, such as in understanding or intervening across levels. Quantitative methods are good for testing a priori hypotheses about factors that can be measured and that have sufficient sample sizes to support statistical tests.
Designs are needed that reflect the dynamic nature of the phenomena affected by multilevel interactions and that can help to conceptualize and provide direction on promoting and responding to emergent factors (121). The classic randomized clinical trial designs, especially those focused more on efficacy than effectiveness, have produced only modest, incomplete, and relatively slow progress (41,122). MLI designs that give greater attention to external validity and narrative unity than to traditional efficacy trials offer great promise (39,40,123,124).
Particularly promising are pragmatic trials and study designs (125) that involve multiple representative settings, diverse patient samples, feasible interventions that can be implemented in many settings and by a variety of types of staff, outcomes that have practical importance for key stakeholders, sustainability that is supported by information technology and relationship development, and inclusion of cost and economic measures. Another key feature, compatible with comparative effectiveness studies (104,126), is that pragmatic trials use comparison conditions that are real-world alternatives or current state-of-the-art practices, rather than using no-treatment or attention placebo controls.
Analysis
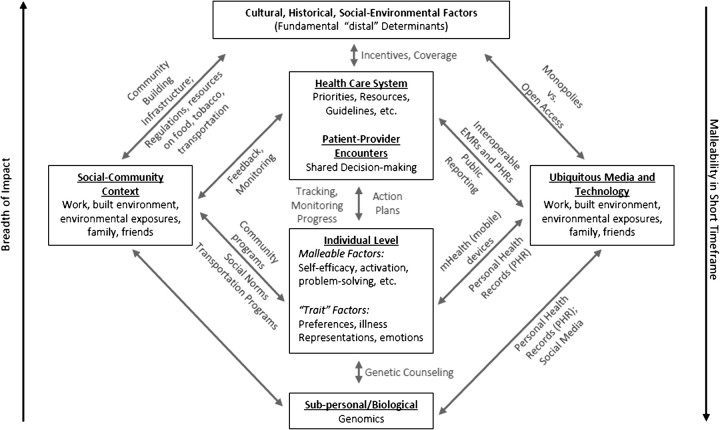
Greater analytic attention to cross-level relationships, such as those shown in Figures 1 and 2, is needed. It is plausible (128,129), but not assured (130), that simple but complementary interventions at different levels may have greater effect than intensive interventions at a single level. The boxes in Figure 1 indicate levels and the arrows depicting interactions between levels are labeled with some of the elements that impart complexity to the interactions. Some elements in Figure 1, such as social media and ongoing and reciprocal goal setting and feedback, are emerging features of the health behavior and health-care settings. As illustrated, numerous opportunities for greater collaboration among entities such as workplaces, health-care settings, and public health organizations exist. In particular, the way that care is delivered will likely be transformed by current ubiquitous media and technology environment, and rapid developments within eHealth. This includes personal health records, community health indicators, and the overwhelming amount of health information and social connections among patients, clinicians, and health systems. These also will likely promote disruptive innovations (131–134) that may fundamentally change health care.
Figure 1.
Opportunities for integrating across levels.
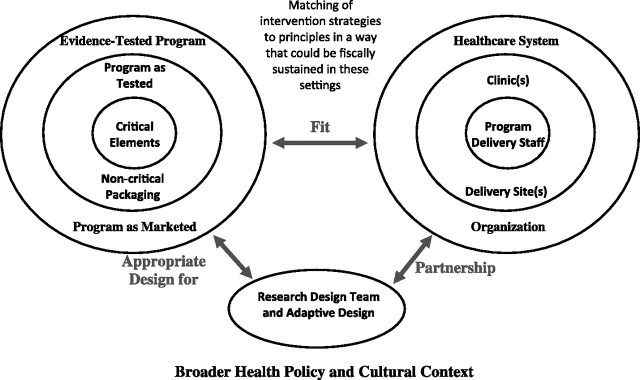
Figure 2.
Interplay and potential partnerships among research design and teams, intervention settings and staff in community settings, and the evidence-based interventions and principles used in a given project. Adapted from Eastabrooks and Glasgow (127) with permission from Elsevier.
Figure 2 illustrates the dynamic interplay and potential partnerships among research design and teams, intervention settings and staff in community settings, and the evidence-based interventions and principles used in a given project (127). As indicated in the figure, all of these interactions are influenced in often nonobvious ways by the broader policy, regulatory, cultural, and economic environments in which health-care transactions take place. Both figures emphasize the importance of fit or integration among different components of MLIs. Figure 2, however, uses the analogy of the multilevel onion (4) to visually illustrate and emphasize key features of action or partnership research (135–137). What is important is that investigators and implementers consider the possible cross-level interactions and their potential to be either additive or dampening, assess and pay attention to emergent learning about these interactions, and adapt their interventions, assessment, and dissemination strategies accordingly. As Sallis et al. (138) point out, at present, most ecological models of MLIs do not specify how to select intervention activities from among the myriad of possibilities so that they complement and enhance activities at other levels.
Less obvious but important points embedded in the figures and tables are that 1) research designs, interventions, and analyses often need to evolve over time to be maximally effective (121); 2) there are subtle reasons why one application of an evidence-based intervention is successful in some settings, but not in others (139); and 3) successful MLIs conduct a “balancing act” evolving over time to fit the unique and changing implementation context while attempting to retain the evidenced-based principles or key components underlying effective interventions strategies (64,121,140,141).
Analysis approaches are needed that are dynamic, capable of handling adaptations in MLIs over time, and that can capture and make sense of emergent factors (121). MLI designs that give greater attention to external validity issues than traditional efficacy trials offer great promise (38–40,129). Similarly, more use of simulation modeling, and evolving approaches to simulations of outcomes (17,95), as a result of different potential MLIs [see Cleary et al. in this issue (142)] would greatly advance the field. Some might even argue that before expensive multisite collaborative trials are begun, case studies and simulation modeling of expected-, worst-, and best-case scenarios should be conducted to produce more informed intervention programs and design selections.
Translation
Transparent reporting of contextual factors (17,82,143–149) such as settings, historical and cultural issues (38–40), and narrative reports, would create a fuller understanding of why and how a MLI works or does not work. It would also substantially increase the ability to transport and adapt findings from one study to other locations and situations. MLI studies need to consider the possibilities for transportability within their design and evaluation, in addition to a smaller number of studies evaluating the dissemination process (122,150–153). We suspect that many studies conducting multilevel research have information about these factors, but they are seldom analyzed or reported (93).
Many of the changes needed to advance MLI research represent deviations from the type of reductionistic science that is currently proposed, funded by scientific review committees, and published by leading journals (40,43). Such changes are needed to speed the currently slow and incomplete translation of research to practice and policy. To produce change in these areas, additional training is needed at multiple levels (154). Such training should start with medical and graduate training but extend to professional organizations, institutional review boards, continuing medical education, summer institutes, and grant and journal reviewers.
Two other translational issues that could benefit from more comprehensive conceptualizations are those of fidelity adaptation and sustainability (121,155,156). Implementation “fidelity” typically has been thought to be one of the key canons of methodological rigor (121). However, for complex interventions that cross levels of individuals, systems and community, flexibility may be more important in gaining participation in and acting on potential and opportunistic interactions across levels (64,121,157,158) to produce intervention success. Especially needed are participatory or action research approaches because they work at levels having different temporal speeds (135,137,159–161). Simultaneously, local adaptations of interventions need to be careful about sacrificing conceptually and empirically based effective ingredients and theoretical principles.
Similar issues arise with the concept of sustainability (162,163), a term that implicitly suggests that long-term interventions are best if they remain exactly the same. A more dynamic conceptualization of sustainability is needed, along with transparent reporting that describes trajectories of problem solving and capacity over time, how new challenges and opportunities are addressed, and how communities and settings become learning organizations (164–167) and persons whose capacity is enhanced (164–166,168).
Finally, to accomplish many of the above recommendations, both training and research partnerships need to be much more transdisciplinary, inclusive, and participatory (154). Key stakeholders and anticipated target audiences and disciplines not usually involved need to be integrated (68,169–171). Documenting the quality of such interactions and teamwork (9,107,172–179) rather than just paying lip service to these concepts is greatly needed. This is difficult work to do, and more examples of both successes and failure in settings such as Clinical and Translational Science Awards, Practice-Based Research Networks, Comprehensive Cancer Centers, Health Maintenance and Accountable Care Organizations, Veteran's Administration, and Community Health Center networks, community engagement programs, and other collaborations are needed (2,9,113–117,176,180,181) to achieve their considerable potential of MLI to address some of the most important problems in cancer prevention and control.
Footnotes
The authors are grateful to Michaela Hancock and David Tran of Scientific Consulting Group, Inc, who were very helpful with the literature searches. K. C. Stange’s time is supported in part by the Intergovernmental Personnel Act Mobility Program through the Division of Cancer Control and Population Sciences at the National Cancer Institute, and by a Clinical Research Professorship from the American Cancer Society. The first authors who were not federal employees received a small stipend from the National Cancer Institute to prepare the manuscript for publication and travel expenses to participate in the meeting where this paper was discussed. The opinions expressed do not necessarily reflect the perspective of the National Cancer Institute or the American Cancer Society.
References
- 1.Best A, Hiatt RA, Norman CD. Knowledge integration: conceptualizing communications in cancer control systems. Patient Educ Couns. 2008;71(3):319–327. doi: 10.1016/j.pec.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hiatt RA, Breen N. The social determinants of cancer: a challenge for transdisciplinary science. Am J Prev Med. 2008;35(2 suppl):S141–S150. doi: 10.1016/j.amepre.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;44:2–10. doi: 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorensen G, Emmons K, Hunt MK, Johnston D. Implications of the results of community intervention trials. Annu Rev Public Health. 1998;19:379–416. doi: 10.1146/annurev.publhealth.19.1.379. [DOI] [PubMed] [Google Scholar]
- 6. The cancer control continuum. National Cancer Institute Web site. http://cancercontrol.cancer.gov/od/continuum.html. Accessed June 12, 2010.
- 7.Bowden DE, Smits SJ. Understanding the multifaceted nature of change in the healthcare system. In: Wolf JA, Hanson H, Moin MJ, editors. Organization Development in Health Care: High Impact Practices for a Complex and Changing Environment. Charlotte, NC: Information Age Publishing Inc; 2011. pp. 3–24. [Google Scholar]
- 8.Stokols D. Toward a science of transdisciplinary action research. Am J Community Psychol. 2006;38(1–2):63–77. doi: 10.1007/s10464-006-9060-5. [DOI] [PubMed] [Google Scholar]
- 9.Stokols D, Misra S, Moser RP, Hall KL, Taylor BK. The ecology of team science: understanding contextual influences on transdisciplinary collaboration. Am J Prev Med. 2008;35(2 suppl):S96–S115. doi: 10.1016/j.amepre.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich AJ, Oxman TE, Williams JW, Jr, et al. Re-engineering systems for the treatment of depression in primary care: cluster randomised controlled trial. BMJ. 2004;329(7466):602. doi: 10.1136/bmj.38219.481250.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel CC, Oxman T, Yamamoto C, et al. RESPECT-Mil: feasibility of a systems-level collaborative care approach to depression and post-traumatic stress disorder in military primary care. Mil Med. 2008;173(10):935–940. doi: 10.7205/milmed.173.10.935. [DOI] [PubMed] [Google Scholar]
- 12.Taplin SH, Haggstrom D, Jacobs T, et al. Implementing colorectal cancer screening in community health centers: addressing cancer health disparities through a regional cancer collaborative. Med Care. 2008;46(9) suppl 1:S74–S83. doi: 10.1097/MLR.0b013e31817fdf68. [DOI] [PubMed] [Google Scholar]
- 13.Thompson RS, Taplin SH, McAfee TA, Mandelson MT, Smith AE. Primary and secondary prevention services in clinical practice. Twenty years’ experience in development, implementation and evaluation. JAMA. 1995;273(14):1130–1135. [PubMed] [Google Scholar]
- 14.Yano EM. The role of organizational research in implementing evidence-based practice: QUERI Series. Implement Sci. 2008;3:29. doi: 10.1186/1748-5908-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lasser KE, Murillo J, Medlin E, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: results of a pilot study. BMC Fam Pract. 2009;10:37. doi: 10.1186/1471-2296-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emery J, Morris H, Goodchild R, et al. The GRAIDS Trial: a cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care. Br J Cancer. 2007;97(4):486–493. doi: 10.1038/sj.bjc.6603897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen G, Emmons K, Hunt MK, et al. Model for incorporating social context in health behavior interventions: applications for cancer prevention for working-class, multiethnic populations. Prev Med. 2003;37(3):188–197. doi: 10.1016/s0091-7435(03)00111-7. [DOI] [PubMed] [Google Scholar]
- 18.Yano EM, Washington DL, Goldzweig C, Caffrey C, Turner C. The organization and delivery of women's health care in Department of Veterans Affairs Medical Center. Womens Health Issues. 2003;13(2):55–61. doi: 10.1016/s1049-3867(02)00198-6. [DOI] [PubMed] [Google Scholar]
- 19.Papadakis S, Moroz I. Population-level interventions for coronary heart disease prevention: what have we learned since the North Karelia project? Curr Opin Cardiol. 2008;23(5):452–461. doi: 10.1097/HCO.0b013e32830c217e. [DOI] [PubMed] [Google Scholar]
- 20.Farquhar JW, Behnke KS, Detels MP, Albright CL. Short- and long-term outcomes of a health promotion program in a small rural community. Am J Health Promot. 1997;11(6):411–414. doi: 10.4278/0890-1171-11.6.411. [DOI] [PubMed] [Google Scholar]
- 21.Puska P. Successful prevention on noncommunicable disease: 25 year experiences with North Karelia Project in Finland. Public Health Med. 2002;4:5–7. [Google Scholar]
- 22.Shea S, Basch CE. A review of five major community-based cardiovascular disease prevention programs. Part II: intervention strategies, evaluation methods, and results. Am J Health Promot. 1990;4(4):279–287. doi: 10.4278/0890-1171-4.4.279. [DOI] [PubMed] [Google Scholar]
- 23.Stillman FA, Hartman AM, Graubard BI, Gilpin EA, Murray DM, Gibson JT. Evaluation of the American Stop Smoking Intervention Study (ASSIST): a report of outcomes. J. Natl. Cancer Inst. 2003;95(22):1681–1691. doi: 10.1093/jnci/djg098. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich AJ, Olson AL, Sox CH, et al. A community-based randomized trial encouraging sun protection for children. Pediatrics. 1998;102(6):E64. doi: 10.1542/peds.102.6.e64. [DOI] [PubMed] [Google Scholar]
- 25.Hancock L, Sanson-Fisher R, Perkins J, Girgis A, Howley P, Schofield M. The effect of a community action intervention on adolescent smoking rates in rural Australian towns: the CART project. Prev Med. 2001;32(4):332–340. doi: 10.1006/pmed.2000.0823. [DOI] [PubMed] [Google Scholar]
- 26.Hancock L, Sanson-Fisher R, Perkins J, McClintock A, Howley P, Gibberd R. Effect of a community action program on adult quit smoking rates in rural Australian towns: the CART project. Prev Med. 2001;32(2):118–127. doi: 10.1006/pmed.2000.0798. [DOI] [PubMed] [Google Scholar]
- 27.Hancock L, Sanson-Fisher R, Perkins J, Corkrey R, Burton R, Reid S. Effect of a community action intervention on cervical cancer screening rates in rural Australian towns: the CART project. Prev Med. 2001;32(2):109–117. doi: 10.1006/pmed.2000.0776. [DOI] [PubMed] [Google Scholar]
- 28.The COMMIT Research Group. Community intervention trial for smoking cessation (COMMIT): I. Cohort results from a four-year community intervention. Am J Public Health. 1995;85(2):183–192. doi: 10.2105/ajph.85.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The COMMIT Research Group. Community intervention trial for smoking cessation (COMMIT): II. Changes in adult cigarette smoking prevalence. Am J Public Health. 1995;85(2):193–200. doi: 10.2105/ajph.85.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eyre H, Kahn R, Robertson RM, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004;109(25):3244–3255. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 31.Fortin M, Lapointe L, Hudon C, Vanasse A. Multimorbidity is common to family practice: is it commonly researched? Can Fam Physician. 2005;51:244–245. [PMC free article] [PubMed] [Google Scholar]
- 32.Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223–228. doi: 10.1370/afm.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fortin M, Dionne J, Pinho G, Gignac J, Almirall J, Lapointe L. Randomized controlled trials: do they have external validity for patients with multiple comorbidities? Ann Fam Med. 2006;4(2):104–108. doi: 10.1370/afm.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortin M, Bravo G, Hudon C, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006;15(1):83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- 35.Fortin M, Soubhi H, Hudon C, Bayliss EA, van den Akker M. Multimorbidity's many challenges. Time to focus on the needs of this vulnerable and growing population. BMJ. 2007;334(7602):1016–1017. doi: 10.1136/bmj.39201.463819.2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer SW, Smith SM, Wyke S, O’Dowd T, Watt GCM. Multimorbidity in primary care: developing the research agenda. Fam Pract. 2009;26(1):79–80. doi: 10.1093/fampra/cmp020. [DOI] [PubMed] [Google Scholar]
- 37.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7(4):357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green LW, Glasgow RE, Atkins D, Stange K. Making evidence from research more relevant, useful, and actionable in policy, program planning, and practice: slips “twixt cup and lip”. Am J Prev Med. 2009;37(6) suppl 1:S187–S191. doi: 10.1016/j.amepre.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Glasgow RE, Green LW, Klesges LM, et al. External validity: we need to do more. Ann Behav Med. 2006;31(2):105–108. doi: 10.1207/s15324796abm3102_1. [DOI] [PubMed] [Google Scholar]
- 40.Green LW, Glasgow RE. Evaluating the relevance, generalization, and applicability of research: issues in external validation and translation methodology. Eval Health Prof. 2006;29(1):126–153. doi: 10.1177/0163278705284445. [DOI] [PubMed] [Google Scholar]
- 41.Sanson-Fisher RW, Bonevski B, Green LW, D’Este C. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med. 2007;33(2):155–161. doi: 10.1016/j.amepre.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Hawkins NG, Sanson-Fisher RW, Shakeshaft A, D’Este C, Green LW. The multiple baseline design for evaluating population-based research. Am J Prev Med. 2007;33(2):162–168. doi: 10.1016/j.amepre.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Kessler R, Glasgow RE. A proposal to speed translation of healthcare intervention research into practice: dramatic change is needed. Am J Prev Med. 2011;40(6):637–44. doi: 10.1016/j.amepre.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 44.Green LW, Kreuter MW, editors. Health Program Planning: An Educational and Ecological Approach. 4th ed. New York, NY: McGraw-Hill; 2005. [Google Scholar]
- 45.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1998;15(4):351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 46.Berkes F, Colding J, Folke C. Navigating Social-Ecological Systems: Building Resilience for Complexity and Change. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- 47.Midgley G, editor. Systems Thinking. Thousand Oaks, CA: Sage Publications; 2003. [Google Scholar]
- 48.Reid PP, Compton WD, Grossman JH, Fanjiang G, editors. Institute of Medicine. Building a Better Delivery System: A New Engineering/Health Care Partnership. Washington, DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- 49.Leischow SJ, Best A, Trochim WM, et al. Systems thinking to improve the public's health. Am J Prev Med. 2008;35(2 suppl):S196–S203. doi: 10.1016/j.amepre.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albrecht G, Freeman S, Higginbotham N. Complexity and human health: the case for a transdisciplinary paradigm. Cult Med Psychiatry. 1998;22(1):55–92. doi: 10.1023/a:1005328821675. [DOI] [PubMed] [Google Scholar]
- 51.Kernick D. Complexity and Healthcare Organization: A View from the Street. San Francisco, CA: Radcliffe Medical Press; 2004. [Google Scholar]
- 52.Litaker D, Tomolo A, Liberatore V, Stange KC, Aron DC. Using complexity theory to build interventions that improve health care delivery in primary care. J Gen Intern Med. 2006;21(suppl 2):S30–S34. doi: 10.1111/j.1525-1497.2006.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plsek PE, Greenhalgh T. Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628. doi: 10.1136/bmj.323.7313.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aspinall MG, Hamermesh RG. Realizing the promise of personalized medicine. Harv Bus Rev. 2007;85(10):108–117. , 165. [PubMed] [Google Scholar]
- 55.Carlson RJ. The disruptive nature of personalized medicine technologies: implications for the health care system. Public Health Genomics. 2009;12(3):180–184. doi: 10.1159/000189631. [DOI] [PubMed] [Google Scholar]
- 56.Collins F. Francis Collins interview. Departing U.S. genome institute director takes stock of personalized medicine. Interview by Jocelyn Kaiser. Science. 2008;320(5881):1272. doi: 10.1126/science.320.5881.1272. [DOI] [PubMed] [Google Scholar]
- 57.Ferrara J. Personalized medicine: challenges in assessing and capturing value in the commercial environment. Expert Rev Mol Diagn. 2006;6(2):129–131. doi: 10.1586/14737159.6.2.129. [DOI] [PubMed] [Google Scholar]
- 58.Kalow W. Pharmacogenetics and pharmacogenomics: origin, status, and the hope for personalized medicine. Pharmacogenomics J. 2006;6(3):162–165. doi: 10.1038/sj.tpj.6500361. [DOI] [PubMed] [Google Scholar]
- 59.Lesko LJ. Personalized medicine: elusive dream or imminent reality? Clin Pharmacol Ther. 2007;81(6):807–816. doi: 10.1038/sj.clpt.6100204. [DOI] [PubMed] [Google Scholar]
- 60.Svinte M. The promise of personalized medicine: a conversation with Michael Svinte. Interview by Michael Millenson. Health Aff (Millwood). 2006;25(2):w54–w60. doi: 10.1377/hlthaff.25.w54. [DOI] [PubMed] [Google Scholar]
- 61.Khoury MJ, Coates RJ, Fennell ML, et al. Multilevel research and the challenges of implementing genomic medicine. J Natl Cancer Inst Monogr. 2012;44:112–120. doi: 10.1093/jncimonographs/lgs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasick RJ, Burke NJ. A critical review of theory in breast cancer screening promotion across cultures. Annu Rev Public Health. 2008;29:351–368. doi: 10.1146/annurev.publhealth.29.020907.143420. [DOI] [PubMed] [Google Scholar]
- 63.Schensul JJ. Community, culture and sustainability in multilevel dynamic systems intervention science. Am J Community Psychol. 2009;43(3–4):241–256. doi: 10.1007/s10464-009-9228-x. [DOI] [PubMed] [Google Scholar]
- 64.Gibbons DE. Interorganizational network structures and diffusion of information through a health system. Am J Public Health. 2007;97(9):1684–1692. doi: 10.2105/AJPH.2005.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abed J, Reilley B, Butler MO, Kean T, Wong F, Hohman K. Developing a framework for comprehensive cancer prevention and control in the United States: an initiative of the Centers for Disease Control and Prevention. J Public Health Manag Pract. 2000;6(2):67–78. doi: 10.1097/00124784-200006020-00011. [DOI] [PubMed] [Google Scholar]
- 66.Petermann L, Petz G. The E2D2 Model: a dynamic approach to cancer prevention interventions [published online ahead of print February 16, 2010] Health Promot Pract. 2011;12(4):561–568. doi: 10.1177/1524839909357317. [DOI] [PubMed] [Google Scholar]
- 67.Rogers EM. Diffusion of Innovations. 4th ed. New York, NY: The Free Press; 1995. [Google Scholar]
- 68.Abed J, Reilley B, Butler MO, Kean T, Wong F, Hohman K. Comprehensive cancer control initiative of the Centers for Disease Control and Prevention: an example of participatory innovation diffusion. J Public Health Manag Pract. 2000;6(2):79–92. doi: 10.1097/00124784-200006020-00012. [DOI] [PubMed] [Google Scholar]
- 69.Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q. 1996;4(2):12–25. [PubMed] [Google Scholar]
- 70.Blakely TJ, Dziadosz GM. The Chronic Care Model for behavioral health care. Popul Health Manag. 2008;11(6):341–346. doi: 10.1089/pop.2007.0022. [DOI] [PubMed] [Google Scholar]
- 71.Wagner EH, Glasgow RE, Davis C, et al. Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv. 2001;27(2):63–80. doi: 10.1016/s1070-3241(01)27007-2. [DOI] [PubMed] [Google Scholar]
- 72.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Milwood). 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 73.Wagner EH, Bennett SM, Austin BT, Greene SM, Schaefer JK, Vonkorff M. Finding common ground: patient-centeredness and evidence-based chronic illness care. J Altern Complement Med. 2005;11(suppl 1):S7–S15. doi: 10.1089/acm.2005.11.s-7. [DOI] [PubMed] [Google Scholar]
- 74.Glasgow RE, Orleans CT, Wagner EH. Does the Chronic Care Model serve also as a template for improving prevention? Milbank Q. 2001;79(4):579–612. doi: 10.1111/1468-0009.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp. Q. 2003;7(1):73–82. doi: 10.12927/hcq.2003.16763. . http://www.primaryhealthcarebc.ca/phc/pdf/eccm_article.pdf. [DOI] [PubMed] [Google Scholar]
- 76.Jenkins C, Pope C, Magwood G, et al. Expanding the chronic care framework to improve diabetes management: the REACH case study. Prog Community Health Partnersh. 2010;4(1):65–79. doi: 10.1353/cpr.0.0108. [DOI] [PubMed] [Google Scholar]
- 77.Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7(1):73–82. doi: 10.12927/hcq.2003.16763. [DOI] [PubMed] [Google Scholar]
- 78.Bracht NF. Community Partnership Strategies in Health Campaigns. Public Communication Campaigns. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 79.Forster JL, Murray DM, Wolfson M, Blaine TM, Wagenaar AC, Hennrikus DJ. The effects of community policies to reduce youth access to tobacco. Am J Public Health. 1998;88(8):1193–1198. doi: 10.2105/ajph.88.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lefebvre RC, Lasater TM, Carleton RA, Peterson G. Theory and delivery of health programming in the community: the Pawtucket Heart Health Program. Prev Med. 1987;16(1):80–95. doi: 10.1016/0091-7435(87)90008-9. [DOI] [PubMed] [Google Scholar]
- 81.Lefebvre RC, Harden EA, Rakowski W, Lasater TM, Carleton RA. Characteristics of participants in community health promotion programs: four-year results. Am J Public Health. 1987;77(10):1342–1344. doi: 10.2105/ajph.77.10.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McLaren L, Ghali LM, Lorenzetti D, Rock M. Out of context? Translating evidence from the North Karelia project over place and time. Health Educ Res. 2007;22(3):414–424. doi: 10.1093/her/cyl097. [DOI] [PubMed] [Google Scholar]
- 83.Mittelmark MB, Hunt MK, Heath GW, Schmid TL. Realistic outcomes: lessons from community-based research and demonstration programs for the prevention of cardiovascular diseases. J Public Health Policy. 1993;14(4):437–462. [PubMed] [Google Scholar]
- 84.Gyarfas I. Review of community intervention studies on cardiovascular risk factors. Clin Exp Hypertens A. 1992;14(1–2):223–237. doi: 10.3109/10641969209036184. [DOI] [PubMed] [Google Scholar]
- 85.Langeluddecke PM. The role of behavioral change procedures in multifactorial coronary heart disease prevention programs. Prog Behav Modif. 1986;20:199–225. doi: 10.1016/b978-0-12-535620-6.50010-x. [DOI] [PubMed] [Google Scholar]
- 86.MacLean DR. Theoretical rationale of community intervention for the prevention and control of cardiovascular disease. Health Rep. 1994;6(1):174–180. [PubMed] [Google Scholar]
- 87.Pietinen P, Lahti-Koski M, Vartiainen E, Puska P. Nutrition and cardiovascular disease in Finland since the early 1970s: a success story. J Nutr Health Aging. 2001;5(3):150–154. [PubMed] [Google Scholar]
- 88.Stamler J. Towards cardiovascular health. Ann Med. 1989;21(3):141–155. doi: 10.3109/07853898909149925. [DOI] [PubMed] [Google Scholar]
- 89.Crabtree BF, Miller WL, Addison RB, Gilchrist VJ, Kuzel A. Part III: the search for multimethod research. In: Crabtree BF, Miller WL, Addison RB, Gilchrist VJ, Kuzel A, editors. Exploring Collaborative Research in Primary Care. Thousand Oaks, CA: Sage Publications; 1994. pp. 177–179. [Google Scholar]
- 90.Feifer C, Ornstein SM, Jenkins RG, et al. The logic behind a multimethod intervention to improve adherence to clinical practice guidelines in a nationwide network of primary care practices. Eval Health Prof. 2006;29(1):65–88. doi: 10.1177/0163278705284443. [DOI] [PubMed] [Google Scholar]
- 91.Louis KS. Multisite/multimethod studies: an introduction. Am Behav Sci. 1982;26(1):6–22. [Google Scholar]
- 92.Stange KC, Miller WL, Crabtree BF, O’Connor PJ, Zyzanski SJ. Multimethod research: approaches for integrating qualitative and quantitative methods. J Gen Intern Med. 1994;9(5):278–282. doi: 10.1007/BF02599656. [DOI] [PubMed] [Google Scholar]
- 93.Stange KC, Crabtree BF, Miller WL. Publishing multimethod research. Ann. Fam. Med. 2006;4(4):292–294. doi: 10.1370/afm.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Publ Health. 2000;21:171–192. doi: 10.1146/annurev.publhealth.21.1.171. [DOI] [PubMed] [Google Scholar]
- 95.Mobley LR, Kuo TM, Driscoll D, Clayton L, Anselin L. Heterogeneity in mammography use across the nation: separating evidence of disparities from the disproportionate effects of geography. Int J Health Geogr. 2008;7:32. doi: 10.1186/1476-072X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hargrove JL. Dynamic Modeling in the Health Sciences. New York, NY: Springer; 1998. [Google Scholar]
- 97. Homer J, Milstein B. Optimal decision making in a dynamic model of poor community health. Paper presented at 37th Hawaii International Conference on Systems Science; January 5-8, 2004; Big Island, HI.
- 98.Schensul SL, Saggurti N, Singh R, Verma RK, Nastasi BK, Mazumder PG. Multilevel perspectives on community intervention: an example from an Indo-US HIV prevention project in Mumbai, India. Am J Community Psychol. 2009;43(3–4):277–291. doi: 10.1007/s10464-009-9241-0. [DOI] [PubMed] [Google Scholar]
- 99.Krieger N. Proximal, distal, and the politics of causation: what's level got to do with it? Am J Public Health. 2008;98(2):221–230. doi: 10.2105/AJPH.2007.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525–533. doi: 10.1016/j.amepre.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schootman M, Lian M, Deshpande AD, et al. Temporal trends in geographic disparities in small-area breast cancer incidence and mortality, 1988-2005. Cancer Epidemiol Biomarkers Prev. 2010;19(4):1122–1131. doi: 10.1158/1055-9965.EPI-09-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Conway PH, Clancy C. Comparative-effectiveness research—implications of the Federal Coordinating Council's report. N Engl J Med. 2009;361(4):328–330. doi: 10.1056/NEJMp0905631. [DOI] [PubMed] [Google Scholar]
- 103.Gillick MR. Medicine as ecoculture. Ann Intern Med. 2009;151(8):577–580. doi: 10.7326/0003-4819-151-8-200910200-00012. [DOI] [PubMed] [Google Scholar]
- 104.Hoffman A, Pearson SD. ‘Marginal medicine’: targeting comparative effectiveness research to reduce waste. Health Aff (Millwood). 2009;28(4):w710–w718. doi: 10.1377/hlthaff.28.4.w710. [DOI] [PubMed] [Google Scholar]
- 105.Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations. N Engl J. Med. 2009;361(4):325–328. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 106.Volpp KG, Das A. Comparative effectiveness—thinking beyond medication A versus medication B. N Engl J Med. 2009;361(4):331–333. doi: 10.1056/NEJMp0903496. [DOI] [PubMed] [Google Scholar]
- 107.Margolis PA, Stevens R, Bordley WC, et al. From concept to application: the impact of a community-wide intervention to improve the delivery of preventive services to children. Pediatrics. 2001;108(3):E42. doi: 10.1542/peds.108.3.e42. [DOI] [PubMed] [Google Scholar]
- 108.Dietrich AJ, Tobin JN, Cassells A, et al. Translation of an efficacious cancer-screening intervention to women enrolled in a Medicaid managed care organization. Ann Fam Med. 2007;5(4):320–327. doi: 10.1370/afm.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dietrich AJ, Tobin JN, Cassells A, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann Intern Med. 2006;144(8):563–571. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mosen DM, Feldstein AC, Perrin N, et al. Automated telephone calls improved completion of fecal occult blood testing. Med Care. 2010;48(7):604–610. doi: 10.1097/MLR.0b013e3181dbdce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feldstein AC, Perrin N, Rosales AG, et al. Effect of a multimodal reminder program on repeat mammogram screening. Am J Prev Med. 2009;37(2):94–101. doi: 10.1016/j.amepre.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228–243. doi: 10.1016/s1553-7250(08)34030-6. [DOI] [PubMed] [Google Scholar]
- 113.Harper GW, Neubauer LC, Bangi AK, Francisco VT. Transdisciplinary research and evaluation for community health initiatives. Health Promot Pract. 2008;9(4):328–337. doi: 10.1177/1524839908325334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holmes JH, Lehman A, Hade E, et al. Challenges for multilevel health disparities research in a transdisciplinary environment. Am J. Prev Med. 2008;35(2 suppl):S182–S192. doi: 10.1016/j.amepre.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.King D, Miranda P, Gor B, et al. Addressing cancer health disparities using a global biopsychosocial approach. Cancer. 2010;116(2):264–269. doi: 10.1002/cncr.24765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nash JM. Transdisciplinary training: key components and prerequisites for success. Am J Prev Med. 2008;35(2 suppl):S133–S140. doi: 10.1016/j.amepre.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 117.Provan KG, Clark PI, Huerta T. Transdisciplinarity among tobacco harm-reduction researchers: a network analytic approach. Am J Prev Med. 2008;35(2 suppl):S173–S181. doi: 10.1016/j.amepre.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 118.Leininger LS, Finn L, Dickey L, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med. 1996;5(2):108–115. doi: 10.1001/archfami.5.2.108. [DOI] [PubMed] [Google Scholar]
- 119.Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. 2nd ed. Thousand Oaks, CA: Sage Publications; 2003. [Google Scholar]
- 120.Tashakkori A, Teddlie C, editors. Handbook of Mixed Methods. London, UK: Sage Publications; 2002. [Google Scholar]
- 121.Cohen DJ, Crabtree BF, Etz RS, et al. Fidelity versus flexibility: translating evidence-based research into practice. Am J Prev Med. 2008;35:S381–S389. doi: 10.1016/j.amepre.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 122.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ. 2009;180(10):E47–E57. doi: 10.1503/cmaj.090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Glasgow RE, Bull SS, Gillette C, Klesges LM, Dzewaltowski DA. Behavior change intervention research in healthcare settings: a review of recent reports with emphasis on external validity. Am J Prev Med. 2002;23(1):62–69. doi: 10.1016/s0749-3797(02)00437-3. [DOI] [PubMed] [Google Scholar]
- 124.Patrick K, Scutchfield FD, Woolf SH. External validity reporting in prevention research. Am J Prev Med. 2008;34(3):260–262. doi: 10.1016/j.amepre.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 125.Glasgow RE, Magid DJ, Beck A, Ritzwoller D, Estabrooks PA. Practical clinical trials for translating research to practice: design and measurement recommendations. Med Care. 2005;43(6):551–557. doi: 10.1097/01.mlr.0000163645.41407.09. [DOI] [PubMed] [Google Scholar]
- 126.Glasgow RE, Steiner JS. Comparative effectiveness research that translates. In: Brownson RC, Colditz GA, Proctor E, editors. Dissemination and Implementation Research. Oxford, UK: Oxford University Press; 2011. [Google Scholar]
- 127.Estabrooks PA, Glasgow RE. Translating effective clinic-based physical activity interventions into practice. Am J Prev Med. 2006;31(4 suppl):S45–S56. doi: 10.1016/j.amepre.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 128.Stetler CB, McQueen L, Demakis J, Mittman BS. An organizational framework and strategic implementation for system-level change to enhance research-based practice: QUERI Series. Implement Sci. 2008;3:30. doi: 10.1186/1748-5908-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. 2007;28(1):413–433. doi: 10.1146/annurev.publhealth.28.021406.144145. [DOI] [PubMed] [Google Scholar]
- 130.Verheijden MW, Kok FJ. Public health impact of community-based nutrition and lifestyle interventions. Eur J Clin Nutr. 2005;59(suppl 1):S66–S75. doi: 10.1038/sj.ejcn.1602176. [DOI] [PubMed] [Google Scholar]
- 131.Christensen CM, Bohmer R, Kenagy J. Will disruptive innovations cure health care? Harv Bus Rev. 2000;78(5):102–112. [PubMed] [Google Scholar]
- 132.Christensen CM, Overdorf M. Meeting the Challenge of Disruptive Change. Boston, MA: Harvard Business School Publishing; 2000. [Google Scholar]
- 133.Christensen CM, Baumann H, Ruggles R, Sadtler TM. Disruptive innovation for social change. Harv Bus Rev. 2006;84(12):94–101. , 163. [PubMed] [Google Scholar]
- 134.Christensen CM. Disruptive innovation: can health care learn from other industries? A conversation with Clayton M. Christensen. Interview by Mark D. Smith. Health Aff (Millwood). 2007;26(3):w288–w295. doi: 10.1377/hlthaff.26.3.w288. [DOI] [PubMed] [Google Scholar]
- 135.Minkler M. Using participatory action research to build healthy communities. Public Health Rep. 2000;115(2–3):191–197. doi: 10.1093/phr/115.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Minkler M, Wallerstein N, editors. Community-Based Participatory Research for Health. San Francisco, CA: Jossey-Bass; 2003. [Google Scholar]
- 137.Mercer SL, DeVinney BJ, Fine LJ, Green LW, Dougherty D. Study designs for effectiveness and translation research: identifying trade-offs. Am J Prev Med. 2007;33(2):139–154. doi: 10.1016/j.amepre.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 138.Sallis J, Owen N, Fisher EB. Ecological models of health behavior. In: Glanz K, Rimer B, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. 4th ed. New York, NY: Jossey-Bass; 2008. pp. 465–482. [Google Scholar]
- 139.Miller WL, McDaniel RR, Jr, Crabtree BF, Stange KC. Practice jazz: understanding variation in family practices using complexity science. J Fam Pract. 2001;50(10):872–878. [PubMed] [Google Scholar]
- 140.Checkland K, Harrison S, Marshall M. Is the metaphor of ‘barriers to change’ useful in understanding implementation? Evidence from general medical practice. J Health Serv Res Policy. 2007;12(2):95–100. doi: 10.1258/135581907780279657. [DOI] [PubMed] [Google Scholar]
- 141.Harrison MI, Koppel R, Bar-Lev S. Unintended consequences of information technologies in health care—an interactive sociotechnical analysis. J Am Med Inform Assoc. 2007 doi: 10.1197/jamia.M2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cleary PD, Gross CP, Zaslavsky AM, Taplin SH. Multilevel interventions: study design and analysis issues. J Natl Cancer Inst Mongr. 2012;44:49–55. doi: 10.1093/jncimonographs/lgs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mendel P, Meredith LS, Schoenbaum M, Sherbourne CD, Wells KB. Interventions in organizational and community context: a framework for building evidence on dissemination and implementation in health services research. Adm Policy Ment Health. 2008;35(1–2):21–37. doi: 10.1007/s10488-007-0144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McCormack B, Kitson A, Harvey G, Rycroft-Malone J, Titchen A, Seers K. Getting evidence into practice: the meaning of ‘context’. J Adv Nurs. 2002;38:94–104. doi: 10.1046/j.1365-2648.2002.02150.x. [DOI] [PubMed] [Google Scholar]
- 145.Kochevar LK, Yano EM. Understanding health care organization needs and context. Beyond performance gaps. J Gen Intern Med. 2006;21(suppl 2):S25–S29. doi: 10.1111/j.1525-1497.2006.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mandelblatt J, Andrews H, Kao R, Wallace R, Kerner J. Impact of access and social context on breast cancer stage at diagnosis. J Health Care Poor Underserved. 1995;6(3):342–351. doi: 10.1353/hpu.2010.0449. [DOI] [PubMed] [Google Scholar]
- 147.Dobrow MJ, Goel V, Lemieux-Charles L, Black NA. The impact of context on evidence utilization: a framework for expert groups developing health policy recommendations. Soc Sci Med. 2006;63:1811–1824. doi: 10.1016/j.socscimed.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 148.French B, Thomas LH, Baker P, Burton CR, Pennington L, Roddam H. What can management theories offer evidence-based practice? A comparative analysis of measurement tools for organisational context. Implement Sci. 2009;4:28. doi: 10.1186/1748-5908-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoff TJ. How work context shapes physician approach to safety and error. Qual Manag Health Care. 2008;17(2):140–153. doi: 10.1097/01.QMH.0000316992.94415.34. [DOI] [PubMed] [Google Scholar]
- 150.Osterling KL, Austin MJ. The dissemination and utilization of research for promoting evidence-based practice. J Evid Based Soc Work. 2008;5(1–2):295–319. doi: 10.1300/J394v05n01_11. [DOI] [PubMed] [Google Scholar]
- 151.Jbilou J, Amara N, Landry R. Research-based-decision-making in Canadian health organizations: a behavioural approach. J Med Syst. 2007;31(3):185–196. doi: 10.1007/s10916-007-9054-3. [DOI] [PubMed] [Google Scholar]
- 152.Kerner JF, Guirguis-Blake J, Hennessy KD, et al. Translating research into improved outcomes in comprehensive cancer control. Cancer Causes Control. 2005;16(suppl 1):27–40. doi: 10.1007/s10552-005-0488-y. [DOI] [PubMed] [Google Scholar]
- 153.Kilbourne AM, Neumann MS, Pincus HA, Bauer MS, Stall R. Implementing evidence-based interventions in health care: application of the replicating effective programs framework. Implement Sci. 2007;2:42. doi: 10.1186/1748-5908-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Candib LM, Stange KC, Levinson W. Qualitative research: perspectives on the future. In: Crabtree BF, Miller WL, editors. Doing Qualitative Research. 2nd ed. Thousand Oaks, CA: Sage Publications; 1999. pp. 347–362. [Google Scholar]
- 155.Woolf SH, Johnson RE. The break-even point: when medical advances are less important than improving the fidelity with which they are delivered. Ann Fam Med. 2005;3:545–552. doi: 10.1370/afm.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Woolf SH, Johnson RE. Inattention to the fidelity of health care delivery is costing lives. Am J Public Health. 2007;97(10):1732–1733. doi: 10.2105/AJPH.2007.116707. ; author reply 1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ruhe MC, Weyer SM, Zronek S, Wilkinson A, Wilkinson PS, Stange KC. Facilitating practice change: lessons from the STEP-UP clinical trial. Prev Med. 2005;40(6):729–734. doi: 10.1016/j.ypmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 158.Ruhe MC, Carter C, Litaker D, Stange KC. A systematic approach to practice assessment and quality improvement intervention tailoring. Qual Manag Health Care. 2009;18(4):268–277. doi: 10.1097/QMH.0b013e3181bee268. [DOI] [PubMed] [Google Scholar]
- 159.Wright DS, Anderson LA, Brownson RC, et al. Engaging partners to initiate evaluation efforts: tactics used and lessons learned from the prevention research centers program. Prev Chronic Dis. 2008;5(1):A21. [PMC free article] [PubMed] [Google Scholar]
- 160.Nastasi BK, Hitchcock J. Challenges of evaluating multilevel interventions. Am J Community Psychol. 2009;43(3–4):360–376. doi: 10.1007/s10464-009-9239-7. [DOI] [PubMed] [Google Scholar]
- 161.Macaulay AC, Commanda LE, Freeman WL, et al. Participatory research maximises community and lay involvement. North American Primary Care Research Group. BMJ. 1999;319(7212):774–778. doi: 10.1136/bmj.319.7212.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bopp M, Fallon E. Community-based interventions to promote increased physical activity: a primer. Appl Health Econ Health Policy. 2008;6(4):173–187. doi: 10.1007/BF03256132. [DOI] [PubMed] [Google Scholar]
- 163.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chawla S, Renesch J, editors. Learning Organizations. Portland, OR: Productivity Press; 1995. [Google Scholar]
- 165.Senge PM. The Fifth Discipline: The Art and Practice of the Learning Organization. New York, NY: Doubleday/Currency; 1990. [Google Scholar]
- 166.Senge PM, Kleiner A, Roberts C, Ross R, Roth G, Smith B. The Dance of Change: The Challenges of Sustaining Momentum in Learning Organizations. 1st ed. New York, NY: Currency/Doubleday; 1999. [Google Scholar]
- 167.Watkins KE, Marsick VJ. Sculpting the Learning Organization: Lessons in the Art and Science of Systemic Change. 1st ed. San Franscisco, CA: Jossey-Bass; 1993. [Google Scholar]
- 168.Kofman F, Senge P. Communities of commitment: the heart of learning organizations. In: Chawla S, Renesch J, editors. Learning Organizations: Developing Cultures for Tomorrow's Workplace. Portland, OR: Productivity Press; 1995. pp. 15–44. [Google Scholar]
- 169.Cargo M, Mercer SL. The value and challenges of participatory research: strengthening its practice. Annu Rev Public Health. 2008;29:325–350. doi: 10.1146/annurev.publhealth.29.091307.083824. [DOI] [PubMed] [Google Scholar]
- 170.Israel BA, Eng E, Schulz AJ, Parker EA, editors. Methods in Community-Based Participatory Research for Health. San Francisco, CA: Jossey-Bass; 2005. [Google Scholar]
- 171.Minkler M, Blackwell AG, Thompson M, Tamir H. Community-based participatory research: implications for public health funding. Am J Public Health. 2003;93(8):1210–1213. doi: 10.2105/ajph.93.8.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bosch M, Dijkstra R, Wensing M, van der Weijden T, Grol R. Organizational culture, team climate and diabetes care in small office-based practices. BMC Health Serv Res. 2008;8:180. doi: 10.1186/1472-6963-8-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hudson SV, Ohman-Strickland P, Cunningham R, Ferrante JM, Hahn K, Crabtree BF. The effects of teamwork and system support on colorectal cancer screening in primary care practices. Cancer Detect Prev. 2007;31(5):417–423. doi: 10.1016/j.cdp.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thomas P, Graver LD. The Liverpool intervention to promote teamwork in general practice: an action research approach. In: Pearson P, Spencer J, editors. Promoting Teamwork in Primary Care: A Research-based Approach. London, UK: Arnold; 1997. [Google Scholar]
- 175.Hall KL, Feng AX, Moser RP, Stokols D, Taylor BK. Moving the science of team science forward: collaboration and creativity. Am J Prev Med. 2008;35(2 suppl):S243–S249. doi: 10.1016/j.amepre.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stokols D, Hall KL, Taylor BK, Moser RP. The science of team science: overview of the field and introduction to the supplement. Am J Prev Med. 2008;35(2 suppl):S77–S89. doi: 10.1016/j.amepre.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 177.Margolius D, Bodenheimer T. Transforming primary care: from past practice to the practice of the future. Health Aff (Milwood). 2010;29(5):779–784. doi: 10.1377/hlthaff.2010.0045. [DOI] [PubMed] [Google Scholar]
- 178.Chesluk BJ, Holmboe ES. How teams work—or don’t—in primary care: a field study on internal medicine practices. Health Aff (Milwood). 2010;29(5):874–879. doi: 10.1377/hlthaff.2009.1093. [DOI] [PubMed] [Google Scholar]
- 179.Bohmer RM. Managing the new primary care: the new skills that will be needed. Health Aff (Milwood). 2010;29(5):1010–1014. doi: 10.1377/hlthaff.2010.0197. [DOI] [PubMed] [Google Scholar]
- 180.Croyle RT. The National Cancer Institute's transdisciplinary centers initiatives and the need for building a science of team science. Am J Prev Med. 2008;35(2 suppl):S90–S93. doi: 10.1016/j.amepre.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 181.Gray B. Enhancing transdisciplinary research through collaborative leadership. Am J Prev Med. 2008;35(2 suppl):S124–S132. doi: 10.1016/j.amepre.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]