Abstract
The complex environmental context must be considered as we move forward to improve cancer care and, ultimately, patient and population outcomes. The cancer care continuum represents several care types, each of which includes multiple technical and communication steps and interfaces among patients, providers, and organizations. We use two case scenarios to 1) illustrate the variability, diversity, and interaction of factors from multiple levels that affect care quality and 2) discuss research implications and provide hypothetical examples of multilevel interventions. Each scenario includes a targeted literature review to illustrate contextual influences upon care and sets the stage for theory-informed interventions. The screening case highlights access issues in older women, and the survivorship case illustrates the multiple transition challenges faced by patients, families, and organizations. Example interventions show the potential gains of implementing intervention strategies that work synergistically at multiple levels. While research examining multilevel intervention is a priority, it presents numerous study design, measurement, and analytic challenges.
The aging of the population and the increased prevalence of cancer, as a leading cause of death and morbidity in the United States (1), will present many challenges for the health-care system in the years ahead. The stakes are high, given the millions of people who will require care along the cancer continuum, which includes risk assessment, prevention advice, screening, diagnosis, treatment, survivorship support, surveillance, and end-of-life care (2). Although exciting scientific and technological advances continue to be made, ensuring translation to effective, efficient, and equitable service delivery remains a national concern (3). The types of care along the cancer care continuum each include multiple technical steps and interfaces among providers and organizations, which can affect care outcomes (4,5). The complexity is magnified by the more than 100 types of cancer, challenging patients, families, providers, and medical care organizations which must coordinate care between health-care sectors and across the cancer continuum.
The broader environmental context (including scientific, policy, and financing trends) has implications for cancer care to the extent that it affects the processes of care (6). New trends in technology and service delivery—such as biotechnology advances, including personalized medicine (7); health informatics, such as electronic medical records (EMRs) and decision support tools (8); identification of a patient-centered medical home (9); and comparative effectiveness research (10)—can influence processes of care by affecting providers’ and patients’ ability to traverse the cancer care continuum efficiently and effectively. Policy-related initiatives, such as financial reform (including entitlement limitations and expansions and provider reimbursement), also may affect the processes of care as they influence the quality, equity, and efficiency of care. Understanding the broader environmental contextual factors is essential to improving care. A sole focus on providers, patients, or organizations will not improve care in an enduring way. The ecological perspective recognizes that the interaction of factors at multiple levels could significantly influence and sustain the quality of the process and ultimately improve health outcomes (11,12).
The purpose of this chapter is to use case scenarios to 1) illustrate the variability, diversity, and interaction of factors from multiple levels that affect the quality of care across the cancer continuum and 2) discuss implications for research and provide examples of multilevel interventions. The overall goal of such interventions is to have a positive impact on quality by addressing the key care characteristics (safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity), and, ultimately, to affect morbidity and mortality (11,13).
Methods
We use two case scenarios—screening and transition to survivorship care—to carry out the two major purposes of this paper. We recognize that not everyone traverses the continuum and that people enter, leave, and reenter it at different points. We also recognize that other types of care exist, but we have chosen these two to illustrate the concepts of multilevel influences of care. Each scenario includes a targeted review of literature to illustrate contextual influences upon care and raises questions about the potential effects of these influences on outcomes.
The Scenarios From an Ecological Perspective
Case Scenario 1: Screening in the Elderly.
Ms Smith is a 66-year-old African American woman who worked for 40 years in automotive assembly before retiring at age 64, when her plant management downsized its workforce during an economic downturn. She was offered her full retirement package, including some health benefits, though she was not yet 65. Her benefits covered catastrophic illness but not routine preventive care. This had been her only insurance, as her husband's coverage ended with his death 10 years earlier. Though Ms Smith worked for decades on the assembly line, she had not suffered any injuries except a gradual decrement in her hearing. She felt so good that she used her free time to pursue an active social life. After retirement, she had more time for dancing, dating, regular walking with women from her church, and traveling to visit her two daughters’ families. She did not routinely see a physician because of the expense and, additionally, she was distrustful of doctors. She has never been screened for breast or colorectal cancer and has not had cervical cancer screening in 25 years.
This example of screening in older women raises numerous issues that can be analyzed from a multilevel ecological perspective. At the individual level, this is a healthy woman who does not see physicians often. She might benefit from screening but does not have a regular physician to cue her to action and, because she believes that she is healthy, screening may not seem important to her. This represents a common phenomenon in the United States with its healthy aging baby boomer generation. Data from the National Health Interview Survey showed that among women aged 65 years and older, 79.3% had fewer than three medical conditions and could be classified by the Charlson comorbidity index as being in average or above-average health, and 10% had not seen a physician within the past year. These investigators estimated that 75% of the women aged 70 years and older will live an average of 12–18 additional years (14). Now that Ms Smith is Medicare-eligible, screening services are more financially accessible. Her individual perceptions of risk in view of her health status as well as her mistrust of physicians may contribute to her pattern of not accessing care (15,16). Additionally, her active dating life may warrant rethinking her risk of cervical cancer (17).
At the family and social level, Ms Smith’ s family characteristics and interpersonal interactions potentially may influence her screening behavior. Her daughters live in another town, and how they interact with her could affect whether she seeks care. We know little about her social network, but she is exercising with friends from church, dating, and dancing. These friends, and her daughters, may play a role in encouraging screening (15,18). Studies exploring the mechanisms underlying these social influences are needed so that interventions to capitalize on them can be developed (19).
At the health provider/team level, opportunities to influence screening behavior are missed because Ms Smith seldom seeks care. No one identifies Ms Smith as his or her patient, and she lacks a medical home. In the United States, it is assumed that individuals are responsible for their own care, and neither patient nor provider necessarily identifies each other as the responsible party. In contrast, in many European countries, capitation systems and electronic databases enable physician teams to identify the populations they serve and use strategies such as outreach reminders to enhance screening rates (20,21). Although reminders for mammography and colorectal screening are increasing in the United States with the implementation of EMRs, the never screened still may not be reached. Should Ms Smith initiate regular visits to a physician, the implementation of EMRs and staff organization offers an opportunity to increase outreach reminders to her from a primary care provider (PCP) (22).
Given that Ms Smith is not connected with the medical care system, community efforts may be particularly important. Mass media campaigns have shown a consistent impact on breast and cervical cancer screening (23), as have news stories about public figures (24). Churches also have been successful intervention sites in communities (25–27), and lay health workers have been successful, (28,29), though additional rigorous work is needed to test their effects in multiple settings and circumstances (30,31).
Finally, state and national health policy–level factors could influence Ms Smith's use of screening. In years past, people without health insurance were least likely to be screened (32,33), but effective September 2010 the Affordable Care Act (34) mandates insurance coverage of screening recommended by the US Preventive Services Task Force (35). The growth of the primary care home and the establishment of health-care reforms may foster incentives to implement outreach reminders and other supportive strategies (36). Although providing insurance may reduce disparities in screening (37), eliminating financial barriers alone is not sufficient. For example, evidence from managed care organizations indicates that low-income populations are least likely to be screened, despite coverage (15,38,39). Potential reasons might include social and cultural factors such as mistrust of medical care, perceived susceptibility, or difficulty in navigating the screening process (40,41).
In summary, this case demonstrates one set of factors that could influence cancer screening (Table 1, italicized factors). Variation in race, age, gender, and usual care-seeking behaviors, among other characteristics, would reflect other potential individual-level factors and strategies for intervention. A community intervention to encourage Ms Smith to establish a medical home, and a health-care organization intervention to implement outreach, however, could work together to introduce her to screening. The challenge is to choose theory-informed synergistic strategies that will have the most significant impact on safe, effective, patient-centered, timely, and efficient screening.
Table 1.
Potential factors by level associated with outcome measures*
| Level of influence and examples of factors affecting outcome of interest |
||||||||||
| Outcome of interest | Individual patient | Family/social supports | Provider/team | Organization/practice setting | Local community | State health policy | National health policy | |||
| Case scenario 1: screening in the elderly | ||||||||||
| Increasing cancer screening rates Reduced cancer morbidity and mortality |
Health and functional status
Risk perceptions Cultural factors Knowledge about cancer and screening options Comorbidity Patterns of health-care utilization Access (eg, insurance, transportation) Personal demographics |
Proximity of family members
Interaction with family Family attitudes about patient's health status and behaviors Family and friend prior experiences with screening and cancer Screening behavior of people in social network |
Number of clinician encounters in last year
Clinician knowledge and communication about recommended screenings Physician incentives Performance reporting Time Team resources |
Standard practice concerning patient contact Outreach practices (eg, reminders) by organization/practice Opportunities for in-reach during routine visits Systematic links between providers (eg, primary and specialty) Medical record system type and quality Patient education resources Patient navigation to improve adherence |
Community screening promotion efforts (mass media, church, lay advisors) Specialist capacity |
Insurance coverage, policies and reimbursement
Special programs, for example, CDC-DPH collaborations |
Medicare benefits
National insurance mandates and policies |
|||
| Case scenario 2: cancer treatment and transition to survivorship | ||||||||||
| Improve coordinated/shared follow-up care Reduced morbidity and mortality and improved quality of life |
Life stage
Health and functional status Treatment toxicity Post-Tx symptoms Fear of recurrence Lack of control and uncertainty Potential for late effects Lack of information on surveillance needs Work and family roles and responsibilities Cultural factors Personal demographics Access (eg, insurance) |
Family understanding, communication and coping Problems with sexuality and intimacy Employer and coworker reactions and expectations |
Coordination between oncology treatment team and PCP
Follow-up plan Opportunities for health promotion and prevention Patient–provider and provider–provider communication Knowledge of clinical guidelines Time |
Extent of integrated care delivery
Access to quality EHR Incentives for care coordination Availability of reminder systems Standards for reporting, and surveillance plans |
Resources for cancer survivors | Insurance coverage, policies and reimbursement | National policy; Medicare coverage; Professional society standards |
|||
Case-specific issues are in italics. CDC = Centers for Disease Control and Prevention; DPH = department of public health; EHR = electronic health records; PCP = primary care provider.
Case Scenario 2: Cancer Treatment and Transition to Survivorship.
Zoe Adler is a 42-year-old woman who was diagnosed with breast cancer on her first screening mammogram 18 months previously and was found to have stage II disease with axillary node involvement. After breast-conserving surgery, she received 6 months of chemotherapy, followed by radiation. She currently has fatigue, problems concentrating, and complains of difficulty sleeping due to hot flashes and night sweats. She has gained about 13.6 kg, reports pervasive anxiety and fear of recurrence, and has not resumed sexual relations with her husband. She perceives her marriage as strong but is distressed that everyone in her family thinks that she should be getting on with her life after treatment, even though she knows that her life will never be the same. She has not seen her regular doctor since the breast cancer was detected and is wondering how her follow-up care is going to be handled. Zoe also worries about how she is going to manage her persistent fatigue and other symptoms, particularly as she is having difficulty returning to work as corporate counsel for a movie studio in Los Angeles.
This scenario of a cancer patient making the posttreatment transition from curative therapy to survivorship raises numerous issues that can be analyzed from the ecological perspective. At the individual level, this woman has courageously faced a cancer diagnosis and received standard contemporary care that consumed a good deal of time and resources, including financial and personal. What we do not see is how her individual biology affected her treatment. The growing interest in personalizing care has increased the need to understand the biology of her tumor and tailor her therapy accordingly (42). However, her therapy as provided exacted a cost in terms of physical and psychological long-term effects. She faces fatigue, weight gain, menopausal symptoms, anxiety, and fear of recurrence, for which effective interventions are available. Furthermore, the management of these conditions is affected by her choice of where to seek care. Although the quality of her oncology treatment may have been excellent, she appears at a loss in terms of how to coordinate her posttreatment care.
The family and social levels represent contextual influence on her care. Zoe thinks she is not living up to the expectations of her family, who do not appear to understand that the effects of cancer treatment can take time to resolve and that she will face ongoing anxieties about the risk of recurrence. Family can help by recognizing the psychological stress of cancer and facilitating access to psychosocial services and/or other resources, such as exercise programs. In addition, Zoe may have family concerns about the possible hereditary nature of this cancer. Consultation with a genetic counselor and possible genetic testing for BRCA1/2 may be helpful, but consideration of how to anticipate family needs and incorporate them into care planning is rare.
At the provider/team level, Zoe's health-care providers are a key resource, but she is uncertain about to whom she should turn. Medical oncologists see themselves as competent internists and often will manage common medical problems that arise during treatment (eg, hypertension, hyperglycemia, thrombosis) (43). During the acute phase of treatment, intense emotional bonding often develops between oncologist and patient. Because of the significant risk of cancer recurrence, oncologists frequently serve as both specialist and PCP because they see the patient regularly (44). This may be good for cancer surveillance, but it may lead to neglect of other routine and important medical care, such as health promotion and disease prevention, and to unnecessary visits to numerous providers (45–47). Evidence suggests that primary care is an effective place to go for survivorship care, including other health prevention needs, but Zoe may not consider this and/or her primary care physician may not be prepared (44,48). As a breast cancer survivor, Zoe represents less than 2% of the women aged 40–49 years, and primary care practices may have few such women and be unfamiliar with posttreatment needs (49). Breast cancer is infrequent in younger women, and it may take explicit communication and planning between primary care and oncology resources to deliver optimal care (47).
At the organizational level, Zoe's case exemplifies the challenge of delivering postcancer treatment care in a fragmented health-care system. The intensity, complexity, and length of treatment magnify the need for coordination during the survivorship phase, including follow-up for potential late effects and recurrence. An Institute of Medicine (IOM) monograph stresses that people in the United States may get state-of-the-science biomedical care, but attention to psychological and social needs is woefully inadequate (50). Patients may become so focused on the cancer and the risk of recurrence that they may neglect other aspects of their health and have limited follow-up with their regular provider. Furthermore, unless the patient is being cared for within an integrated health-care system with a single medical record, the PCP may have no idea what transpired (50,51). In addition, unlike treatment in the hospital, where a discharge summary is required, no such documentation is routine after outpatient cancer therapy. There is increasing recognition that many communication issues can be moderated by an organization's commitment to implementing a cancer treatment summary and survivorship care plan (52).
At the community level, resources exist that can influence Zoe's care. The IOM report emphasizes that local community resources for cancer survivors are essential and underused. These resources include Internet sites with online support and information, support groups, assistance with prosthetic devices, and exercise programs adapted to meet survivors’ specialized needs (49,50).
Finally, policy recommendations that encourage coordination and communication among providers could include requirements for an end-of-treatment summary and care plan for all patients completing cancer treatment to facilitate patient understanding and the sharing of care with multiple specialists and the PCP (49). This summary would document what transpired during treatment, describe potential late effects of treatments, communicate to the survivor and other providers what has been done and what needs to be done in the future, and promote a healthy lifestyle to prevent recurrence and reduce risk of comorbid conditions. Professional organizations are recognizing these issues, and emerging guidelines now are advocating reporting standards to improve quality of care. For example, efforts by the American Society of Clinical Oncology (ASCO) and its Quality Oncology Practice Initiative (QOPI) are leading the way toward monitoring clinical practice with a focus on quality improvement [see http://qopi.asco.org, (53)]. Although specific research to evaluate the impact of treatment summaries and care plans has not been reported (51), this recommendation flows directly from other IOM work directed at how the health-care system can ensure quality care.
Much still needs to be done to improve care in the transition between treatment and survivorship, but the case of Zoe Adler illustrates that success will require understanding the influences at multiple levels to provide optimum care to the growing population of cancer survivors.
Discussion and Implications
Levels, Complexity, and Variability
These two scenarios demonstrate the complex and interactive factors that affect the quality of cancer care and optimal patient outcomes. They highlight some but not all of the participants and activities within and between levels of care. Moreover, each type of care varies substantially, increasing complexity. Table 1 highlights not only the multilevel issues from the specific scenarios but also exemplifies other potential factors. These factors represent challenges to designing and testing interventions that improve care for each patient and are at the same time sufficiently sustainable, flexible, and generalizable to affect population groups.
The Mandate for Multilevel Intervention Research
Multilevel intervention strategies could achieve optimal care for Ms Smith and Ms Adler. So, where to begin? Strong conceptual frameworks for developing interventions targeted at behavior change in individuals and organizations have been developed (54–56). In designing multilevel interventions, both the targeted levels of care and the timing of each intervention component are critical (57). Interest in complex adaptive systems is growing as many challenges confront the development of multilevel intervention strategies (58). Complex models and frameworks integrate numerous theoretical perspectives that must be considered in view of efficacy evidence. As Weiner et al. (59) emphasize, strikingly little practical guidance is available for making choices and designing strategies. They emphasize that future research will require detailed familiarity with existing evidence to justify which combinations of which strategies, at which level, most likely will affect outcomes synergistically. An understanding of biological, individual, social, and organizational epidemiology and related theories is needed to guide understanding of potential moderating and mediating effects on intervention outcomes. Weiner et al. (59) outline five strategies for combining interventions at multiple levels: accumulation, facilitation, amplification, cascade, and convergence. Building on our case scenarios, we now describe hypothetical examples of multilevel interventions, understanding that final design decisions are predicated on a careful review of the contextual factors and existing evidence on intervention components as well as consideration of relevant theory.
Scenario 1.
In this scenario, the general issue is underuse of screening. We know that 55% of women aged 65–79 years have not been screened for colon cancer within the past 10 years, 22% have not been screened for cervical cancer, and 19% of women of above-average health have not been screened for breast cancer within 2 years (60). Parenthetically, it also is true, however, that screening is overused (61–63). To optimize care, interventions addressing Ms Smith's age group should consider both underuse and overuse of screening services.
Example of a multilevel intervention.
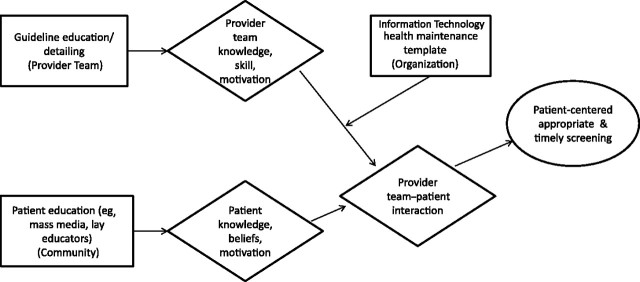
A consortium of Community Health Centers (CHCs) partnering with a state's department of public health (DPH) could plan a trial that addresses at least three levels: individuals seeking care, health-care organizations, and the community. The interventions could address patient knowledge, beliefs, and motivation; provider knowledge and motivation; organizational change; and community support of the interventions. Figure 1 is a schematic of potential converging and facilitating strategies (59).
Figure 1.
Convergence and facilitation intervention strategies—screening. Box represents intervention and level of influence (in parentheses); diamond represents mediator; oval represents outcome. Convergence intervention strategies are those interventions at multiple levels that mutually reinforce each other by altering patterns of interaction among two or more target audiences. A facilitation strategy is one that enables another strategy to reach an objective.
A community education strategy intervention could motivate women such as Ms Smith to seek a medical provider. Such a campaign could use mass media or lay educators and be facilitated by the national policy change assuring coverage for screening.
Once a woman is associated with a medical team, it is important that she is approached about screening. Recall Ms Smith's distrust of providers; to counteract this, an educational strategy could be directed at key members of the provider team through academic detailing (64) to individualize care so as to recognize unique patient concerns relevant to both over- and underuse (60,65). Additionally, patient-centeredness requires skill to detect and improve individual perceptions related to such issues as fear and mistrust (16) and to improve communication (66). The staff training also could include group sessions to promote consensus about integrating recommendations into the practice's processes (22). For example, women with no evidence of human papillomavirus who are in monogamous relationships do not need annual Pap smears (67), but older women actively dating may need this screening (68).
At the organizational level, an automated health maintenance template could be designed and integrated into the EMR. Such systems enable decision support screening at point of contact (69), and current efforts prompted by the American Recovery and Reinvestment Act (ARRA) (70) encourage use of information technology (IT) and such electronic templates. The investment in software, however, needs justification at the leadership level. This CHC-DPH intervention, therefore, could include a leadership strategy that builds a business case for the reminder system, identifies available EMR reminder software, or identifies an appropriate record to install. The intervention could include training in the use of the EMR and examination of patient flow to identify how staff could use it efficiently.
As shown in Figure 1, this example of a three-component intervention could directly affect mediators of physician knowledge, skills, and motivation as well as patient knowledge, beliefs, and access to resources. Accompanying organizational changes at the CHCs could facilitate improved scheduling of medical appointments, where patients and providers interact. A research trial would test whether these changes lead to improved patient-centered, timely, and appropriate screening.
Scenario 2.
In this scenario, the broad issue is management and communication about posttreatment survivorship care. With current demographic trends—an aging population and the decline in cardiovascular disease—we can expect significant growth in new cancer cases, especially those that peak in older age (eg, breast, colon, prostate). This will seriously challenge the capacity of the health-care system, with its limited workforce of medical and nursing professionals (71,72). Building on prior IOM reports (13), we emphasized the posttreatment phase of cancer care due to the heightened need for coordination and patient-centered care, to enable exchanging information and responsibility (5).
Example of a multilevel intervention.
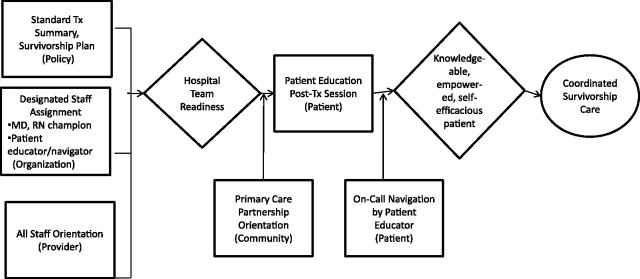
A Cancer Center could undertake significant changes in structure and processes of care and actively promote a partnership with area PCPs. Figure 2 illustrates this potential accumulation and cascade design (59).
Figure 2.
Accumulation and cascade intervention strategies—survivorship. Box represents intervention and level of influence (in parentheses); diamond represents mediator; oval represents outcome. Accumulation intervention strategies are those interventions at multiple levels that produce a cumulative impact on a common mediating pathway. A cascade strategy is one that affects the desired outcome in and through one or more interventions at other levels of influence.
At the organizational level, a three-component intervention could be carried out. This intervention could involve designing a standardized treatment summary and survivorship plan (policy); designating staff responsibility for discrete survivorship services, including physician and nurse champions; and instituting a Patient Educator/Navigator position (73,74). An all-staff orientation to the survivorship initiative also could be designed and implemented. These ambitious components could enable and reinforce hospital team readiness by improving knowledge, skill, and dedicated supportive resources. Although the treatment summary and survivorship care plan model still needs field testing, the IOM report concluded that such a plan would facilitate the sharing and coordination of care as patients transition out of the treatment phase. The designated staff assignments could improve care by establishing expectations around the performance of essential processes of care. The Patient Educator/Navigator would understand the medical, palliative, and psychosocial needs of the patient. This specialist would not necessarily undertake the management of all of these needs but would assess and acknowledge the multiple issues and make appropriate recommendations and referrals.
At the community level, a Primary Care Partnership initiative could integrate local primary care resources. Research has documented the added value of comanagement between oncology specialists and PCPs (45,75,76). Work done by Grunfeld et al. (48,77) suggests that for patients with early-stage breast cancer, follow-up care can be performed successfully by PCPs. Whether this is true of other cancer sites has not been studied. However, a recent qualitative study with primary care physicians who were receiving treatment summaries and survivorship plans at an academic institution (78) suggests that PCPs feel both equipped and empowered to take charge of follow-up care with a variety of survivors after receipt of a comprehensive consultation with recommendations. The Primary Care Partnership initiative could include mailings and academic detailing about the treatment summary plan and encourage PCPs to initiate calls with specialists if questions arise.
At the individual level, patient education could be a key intervention component. Education and guidance to patients at the end of active treatment—something that is very effective at diagnosis and during treatment—will help patients avoid feeling that they are “lost in transition” (79). An explicit posttreatment educational session with the treatment summary and survivorship plan could be tested. Oncology specialists are not always able to predict the time course of recovery, and many patients need increased psychological support during this time—something that is not always forthcoming (50). To address this reality of variable and evolving needs, the on-call navigation availability with the Patient Educator system represents a potential accumulating strategy.
As shown in Figure 2, this multilevel approach could first prepare hospital teams for survivorship care by adopting structural and policy changes and improving staff education. In addition, education at the end of treatment could prepare patients for the journey ahead by influencing important mediators of knowledge, skills, and self-efficacy, whereas a community effort could facilitate PCP–specialist transitions. These combined efforts could lead to improved patient-centered coordinated survivorship care.
Conclusions From the Multilevel Intervention Examples
As described in the scenarios, a consideration of the quality of care domains (6) can guide decisions about priorities and help investigators prioritize theory-guided interventions. The screening scenario illustrated how interventions could promote equity of access to reduce disparities and encourage strategies to improve adherence and timeliness. The posttreatment scenario stressed the importance of patient–provider communication to improve patient-centered care and symptom management as well as assure timely and effective surveillance care.
Beyond the quality-of-care domains, it is important to consider important moderators of the impact of the intervention, including race/ethnicity, physician and other manpower levels, geographic location, and organizational networks, to name a few. Thus, with intensive investigation of potential determinants, a combination of interventions at the organization, provider, and patient levels could evolve.
Other Methodological Challenges
Research design and measurement issues are compounded in multilevel research. Priority may be given to estimating the relative contribution of strategies aimed at different levels, a perspective common within the research community. In pragmatic trials, case–control studies, and outcomes research, however, it is not always possible to determine which of the interventions contributed the most. Indeed, implementation research suggests that it may be difficult to determine which intervention has greater effect than any other. This lack of certainty about effects at specific levels challenges the current paradigm for research designs (80). Design, of course, affects decisions about unit of randomization and statistical power. Because interventions are driven by theories related to a certain level (eg, intrapersonal, interpersonal, and organization theory), measurement challenges include the availability of valid and reliable outcome measures and data sources, respondent burden, multiple data sources requiring resource-intensive management, and other concerns (81). Multilevel studies therefore require statistical methods that consider interdependence of measures at multiple levels (80).
Despite these challenges, it is an exciting time to push forward with building an evidence base about the potential of multilevel interventions along the cancer continuum to promote improved care and outcomes which address all segments of the population (82).
Funding
This work was funded by the National Cancer Institute through direct salary (ST) and IPA Contract (JZ). The content of this paper does not necessarily reflect the views or policies of the US Department of Health and Human Services. EG is supported by a clinician scientist award from the Ontario Institute for Cancer Research (OICR) with funds from the Ontario Ministry of Research and Innovation (OMRI). The research is independent, and no endorsement by OICR or OMRI is intended or should be inferred.
Footnotes
We thank Marvella Ford, for her consultation on Case Scenario 1 and acknowledge the assistance of Marcia Feinleib and Joanne Brodsky, for assistance with manuscript preparation.
References
- 1.American Cancer Society. Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 2.Hewitt M, Simone JV, editors. Institute of Medicine. Ensuring Quality Cancer Care. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 3.Aday LA, Begley CE, Lairson DR, Balkrishnan R. Evaluating the Healthcare System: Effectiveness, Efficiency, and Equity. Chicago, IL: Health Administration Press; 2004. [Google Scholar]
- 4.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4–13. [PubMed] [Google Scholar]
- 5.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;(40):3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;(44):2–10. doi: 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips KA. The intersection of biotechnology and pharmacogenomics: health policy implications. Health Aff (Millwood). 2006;25(5):1271–1280. doi: 10.1377/hlthaff.25.5.1271. [DOI] [PubMed] [Google Scholar]
- 8.Hesse BW, Hanna C, Massett HA, Hesse NK. Outside the box: will information technology be a viable intervention to improve the quality of cancer care? J Natl Cancer Inst Monogr. 2010;(40):81–89. doi: 10.1093/jncimonographs/lgq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilo CM, Wasson JH. Practice redesign and the patient-centered medical home: history, promises, and challenges. Health Aff (Millwood). 2010;29(5):773–778. doi: 10.1377/hlthaff.2010.0012. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman A, Pearson SD. ‘Marginal medicine’: targeting comparative effectiveness research to reduce waste. Health Aff (Millwood). 2009;28(4):w710–w718. doi: 10.1377/hlthaff.28.4.w710. [DOI] [PubMed] [Google Scholar]
- 11.Taplin SH, Clauser S, Rodgers AB, Breslau E, Rayson D. Interfaces across the cancer continuum offer opportunities to improve the process of care. J Natl Cancer Inst Monogr. 2010;(40):104–110. doi: 10.1093/jncimonographs/lgq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clauser SB, Taplin SH, Foster MK, Fagan P, Kaluzny AD. Multilevel intervention research: lessons learned and pathways forward. J Natl Cancer Inst Monogr. 2012;(44):127–133. doi: 10.1093/jncimonographs/lgs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 14.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 15.Purnell JQ, Katz ML, Andersen BL, et al. Social and cultural factors are related to perceived colorectal cancer screening benefits and intentions in African Americans. J Behav Med. 2010;33(1):24–34. doi: 10.1007/s10865-009-9231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klassen AC, Smith KC, Shariff-Marco S, Juon H. A healthy mistrust: how worldview relates to attitudes about breast cancer screening in a cross-sectional survey of low-income women. Int J Equity Health. 2008;7:5. doi: 10.1186/1475-9276-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116(11):2531–2542. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sieverding M, Matterne U, Ciccarello L. What role do social norms play in the context of men's cancer screening intention and behavior? Application of an extended theory of planned behavior. Health Psychol. 2010;29(1):72–81. doi: 10.1037/a0016941. [DOI] [PubMed] [Google Scholar]
- 19.Burke NJ, Bird JA, Clark MA, et al. Social and cultural meanings of self-efficacy. Health Educ Behav. 2009;36(5 suppl):111S–128S. doi: 10.1177/1090198109338916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanio H, Bianchini F, editors. IARC Handbooks of Cancer Prevention, Volume 7, Breast Cancer Screening. Lyon, France: IARC Press; 2002. Chapter 3. Use of breast cancer screening; p. 51. [Google Scholar]
- 21.Miles A, Cockburn J, Smith RA, Wardle J. A perspective from countries using organized screening programs. Cancer. 2004;101(5 suppl):1201–1213. doi: 10.1002/cncr.20505. [DOI] [PubMed] [Google Scholar]
- 22.Ornstein S, Nemeth LS, Jenkins RG, Nietert PJ. Colorectal cancer screening in primary care: translating research into practice. Med Care. 2010;48(10):900–906. doi: 10.1097/MLR.0b013e3181ec5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasick RJ, Hiatt RA, Paskett ED. Lessons learned from community-based cancer screening intervention research. Cancer. 2004;101(5 suppl):1146–1164. doi: 10.1002/cncr.20508. [DOI] [PubMed] [Google Scholar]
- 24.Cram P, Fendrick AM, Inadomi J, Cowen ME, Carpenter D, Vijan S. The impact of a celebrity promotional campaign on the use of colon cancer screening: the Katie Couric effect. Arch Intern Med. 2003;163(13):1601–1605. doi: 10.1001/archinte.163.13.1601. [DOI] [PubMed] [Google Scholar]
- 25.Sauaia A, Min SJ, Lack D, et al. Church-based breast cancer screening education: impact of two approaches on Latinas enrolled in public and private health insurance plans. Prev Chronic Dis. 2007;4(4):A99. [PMC free article] [PubMed] [Google Scholar]
- 26.Holt CL, Klem PR. As you go, spread the word: spiritually based breast cancer education for African American women. Gynecol Oncol. 2005;99(3 supp1):S141–S142. doi: 10.1016/j.ygyno.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 27.Tseng TS, Holt CL, Shipp M, et al. Predictors of colorectal cancer knowledge and screening among church-attending African Americans and Whites in the Deep South. J Community Health. 2009;34(2):90–97. doi: 10.1007/s10900-008-9128-2. [DOI] [PubMed] [Google Scholar]
- 28.Andrews JO, Felton G, Wewers ME, Heath J. Use of community health workers in research with ethnic minority women. J Nurs Scholarsh. 2004;36(4):358–365. doi: 10.1111/j.1547-5069.2004.04064.x. [DOI] [PubMed] [Google Scholar]
- 29.Wells KJ, Luque JS, Miladinovic B, et al. Do community health worker interventions improve rates of screening mammography in the United States? A systematic review [published online ahead of print June 5, 2011] Cancer Epidemiol Biomarkers Prev. 2011;20(8):1580–1598. doi: 10.1158/1055-9965.EPI-11-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paskett ED, Tatum CM, D’Agostino R, Jr, et al. Community-based interventions to improve breast and cervical cancer screening: results of the Forsyth County Cancer Screening (FoCaS) Project. Cancer Epidemiol Biomarkers Prev. 1999;8(5):453–459. [PubMed] [Google Scholar]
- 31.Martinez-Donate AP. Using lay health advisors to promote breast and cervical cancer screening among Latinas: a review. WMJ. 2009;108(5):259–262. [PubMed] [Google Scholar]
- 32.McWilliams JM. Health consequences of uninsurance among adults in the United States: recent evidence and implications. Milbank Q. 2009;87(2):443–494. doi: 10.1111/j.1468-0009.2009.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabatino SA, Coates RJ, Uhler RJ, Breen N, Tangka F, Shaw KM. Disparities in mammography use among US women aged 40-64 years, by race, ethnicity, income, and health insurance status, 1993 and 2005. Med Care. 2008;46(7):692–700. doi: 10.1097/MLR.0b013e31817893b1. [DOI] [PubMed] [Google Scholar]
- 34.Thorpe KE, Ogden LL. Analysis & commentary. The foundation that health reform lays for improved payment, care coordination, and prevention. Health Aff (Millwood). 2010;29(6):1183–1187. doi: 10.1377/hlthaff.2010.0415. [DOI] [PubMed] [Google Scholar]
- 35. US preventive services task force. Agency for Healthcare Research and Quality Web site. http://www.ahrq.gov/clinic/uspstfix.htm. Accessed March 28, 2011.
- 36.Anhang Price R, Zapka J, Edwards H, Taplin SH. Organizational factors and the cancer screening process. J Natl Cancer Inst Monogr. 2010;(40):38–57. doi: 10.1093/jncimonographs/lgq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emmons KM, Lobb R, Puleo E, Bennett G, Stoffel E, Syngal S. Colorectal cancer screening: prevalence among low-income groups with health insurance. Health Aff (Millwood). 2009;28(1):169–177. doi: 10.1377/hlthaff.28.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park AN, Buist DS, Tiro JA, Taplin SH. Mediating factors in the relationship between income and mammography use in low-income insured women. J Womens Health (Larchmt). 2008;17(8):1371–1378. doi: 10.1089/jwh.2007.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yabroff KR, Washington KS, Leader A, Neilson E, Mandelblatt J. Is the promise of cancer-screening programs being compromised? Quality of follow-up care after abnormal screening results. Med Care Res Rev. 2003;60(3):294–331. doi: 10.1177/1077558703254698. [DOI] [PubMed] [Google Scholar]
- 40.Kelly CM, Hortobagyi GN. Adjuvant chemotherapy in early-stage breast cancer: what, when, and for whom? Surg Oncol Clin N Am. 2010;19(3):649–668. doi: 10.1016/j.soc.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Taplin SH, Ichikawa L, Buist DS, Seger D, White E. Evaluating organized breast cancer screening implementation: the prevention of late-stage disease? Cancer Epidemiol Biomarkers Prev. 2004;13(2):225–234. doi: 10.1158/1055-9965.epi-03-0206. [DOI] [PubMed] [Google Scholar]
- 42.Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97(9):675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 43.Ganz PA. Monitoring the physical health of cancer survivors: a survivorship-focused medical history. J Clin Oncol. 2006;24(32):5105–5111. doi: 10.1200/JCO.2006.06.0541. [DOI] [PubMed] [Google Scholar]
- 44.Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;(40):25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–1719. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 46.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21(8):1447–1451. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 47.Grunfeld E, Hodgson DC, Del Giudice ME, Moineddin R. Population-based longitudinal study of follow-up care for breast cancer survivors. J Oncol Pract. 2010;6(4):174–181. doi: 10.1200/JOP.200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol. 2006;24(6):848–855. doi: 10.1200/JCO.2005.03.2235. [DOI] [PubMed] [Google Scholar]
- 49.Hewitt M, Greenfield S, Stovall E, editors. Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 50.Adler NE, Page AEK, editors. Institute of Medicine. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 51.Grunfeld E, Levine MN, Julian J, et al. FUP 2 Trial Investigators. Breast cancer survivors perception of family physician (FP) or specialist as principal provider of routine follow-up care. J Clin Oncol. 2010;28(15_suppl):9090. [Google Scholar]
- 52.Ganz PA, Hahn EE. Implementing a survivorship care plan for patients with breast cancer. J Clin Oncol. 2008;26(5):759–767. doi: 10.1200/JCO.2007.14.2851. [DOI] [PubMed] [Google Scholar]
- 53. American Society of Clinical Oncology. QOPI: The Quality Oncology Practice Initiative Web site. http://qopi.asco.org. Accessed March 28, 2011.
- 54.Green LW, Kreuter MW. Health Program Planning: An Educational and Ecological Approach. 4th ed. Boston, MA: McGraw Hill; 2005. [Google Scholar]
- 55.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 56.Bartholomew LK, Parcel GS, Kok G, Gottleib N. Planning Health Promotion Programs: Intervention Mapping. 2nd ed. San Francisco, CA: Jossey-Bass; 2006. [Google Scholar]
- 57.Alexander J, Prabhu Das I, Johnson TP. Time issues in multilevel interventions for cancer treatment and prevention. J Natl Cancer Inst Monogr. 2012;(44):42–48. doi: 10.1093/jncimonographs/lgs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott WR. Organizations: Rational, Natural, and Open Systems. 5th ed. Old Tappan, NJ: Prentice Hall; 2003. [Google Scholar]
- 59.Weiner BJ, Lewis MA, Clauser SB, Stitzenberg KB. In search of synergy: strategies for combining interventions at multiple levels. J Natl Cancer Inst Monogr. 2012;(44):34–41. doi: 10.1093/jncimonographs/lgs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schonberg MA, Leveille SG, Marcantonio ER. Preventive health care among older women: missed opportunities and poor targeting. Am J Med. 2008;121(11):974–981. doi: 10.1016/j.amjmed.2008.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yabroff KR, Saraiya M, Meissner HI, et al. Specialty differences in primary care physician reports of papanicolaou test screening practices: a national survey, 2006 to 2007. Ann Intern Med. 2009;151(9):602–611. doi: 10.7326/0003-4819-151-9-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 62.Yabroff KR, Klabunde CN, Yuan G, et al. Are physicians’ recommendations for colorectal cancer screening guideline-consistent? J Gen Intern Med. 2011;26(2):177–184. doi: 10.1007/s11606-010-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98(11):774–782. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- 64.Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263(4):549–556. [PubMed] [Google Scholar]
- 65.Mehta KM, Fung KZ, Kistler CE, Chang A, Walter LC. Impact of cognitive impairment on screening mammography use in older US women. Am J Public Health. 2010;100(10):1917–1923. doi: 10.2105/AJPH.2008.158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zapka JM, Klabunde CN, Aurora NK, Yuan G, Smith JL, Kobrin SC. Physicians’ colorectal cancer screening discussion and recommendation patterns. Cancer Epidemiol Biomarkers Prev. 2011;20(3):509–521. doi: 10.1158/1055-9965.EPI-10-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med. 2009;360(14):1453–1455. doi: 10.1056/NEJMe0901167. [DOI] [PubMed] [Google Scholar]
- 68.Kearney F, Moore AR, Donegan CF, Lambert J. The ageing of HIV: implications for geriatric medicine. Age Ageing. 2010;39(5):536–541. doi: 10.1093/ageing/afq083. [DOI] [PubMed] [Google Scholar]
- 69.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 70. US Department of Health and Human Services. http://www.healthcare.gov. Accessed March 29, 2011.
- 71.Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Oncol Pract. 2007;3(2):79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patlak M, Levit L. Ensuring Quality Cancer Care Through the Oncology Workforce: Sustaining Care in the 21st Century: Workshop Summary. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 73.Freund KM, Battaglia TA, Calhoun E, et al. Patient Navigation Research Program Group. National Cancer Institute Patient Navigation Research Program: methods, protocol, and measures. Cancer. 2008;113(12):3391–3399. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin. 2011;61(4):237–249. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sny der CF, Frick KD, Kantsiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27(7):1054–1061. doi: 10.1200/JCO.2008.18.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snyder CF, Frick KD, Peairs KS, et al. Comparing care for breast cancer survivors to non-cancer controls: a five-year longitudinal study. J Gen Intern Med. 2009;24(4):469–474. doi: 10.1007/s11606-009-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grunfeld E, Mant D, Yudkin P, et al. Routine follow up of breast cancer in primary care: randomised trial. BMJ. 1996;313(7058):665–669. doi: 10.1136/bmj.313.7058.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mor-Shalom M, Hahn EE, Casillas JN, Ganz PA. Do survivorship care plans make a difference? The primary care physician perspective. Abstract presented at National Cancer Institute Biennial Cancer Survivor Research Conference; June 17–19, 2010; Washington, DC. [Google Scholar]
- 79.Earle CC. Failing to plan is planning to fail: improving the quality of care with survivorship care plans. J Clin Oncol. 2006;24(32):5112–5116. doi: 10.1200/JCO.2006.06.5284. [DOI] [PubMed] [Google Scholar]
- 80.Cleary PD, Gross CP, Zaslavsky AM, Taplin SH. Multilevel interventions: study design and analysis issues. J Natl Cancer Inst Monogr. 2012;(44):49–55. doi: 10.1093/jncimonographs/lgs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Charns MP, Foster MK, Alligood EC, et al. Mulitlevel interventions: measurement and measures. J Natl Cancer Inst Monogr. 2012;(44):67–77. doi: 10.1093/jncimonographs/lgs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]