Abstract
Background
FOXP3+ regulatory T cells (Tregs) are critical for controlling inflammation in the gastrointestinal (GI) tract. There is a paradoxical increase of mucosal FOXP3+ T cells in patients with inflammatory bowel disease (IBD). These FOXP3+ cells were recently shown to include IL-17A-producing cells in Crohn’s disease, resembling Th17 cells implicated in autoimmune diseases. FOXP3 inhibits IL-17A production, but a naturally-occurring splice variant of FOXP3 lacking exon 2 (Δexon2) cannot.
Aims
We hypothesized that IBD patients preferentially express the Δexon2 variant of FOXP3 so the paradoxically increased mucosal Tregs in IBD could represent cells expressing only Δexon2.
Methods
We used antibodies and primers that can distinguish between the full-length and Δexon2 splice variant of FOXP3 to evaluate expression of these isoforms in human intestinal tissue by immunohistochemistry (IHC) and quantitative PCR, respectively.
Results
No difference in the expression pattern of Δexon2 relative to full length FOXP3 was seen in ulcerative colitis (UC) or Crohn’s disease versus non-IBD controls. By immunofluorescence microscopy and flow cytometry, we also did not find individual cells which expressed FOXP3 protein exclusively in the Δexon2 isoform in either IBD or control tissue. FOXP3+ mucosal CD4+ T cells from both IBD and control specimens were able to make IL-17A in vitro after PMA and ionomycin stimulation, but these cells did not preferentially express Δexon2.
Conclusions
Our data do not support the hypothesis that selective expression of FOXP3 in the Δexon2 isoform accounts for the inability of copious FOXP3+ T cells to inhibit inflammation or IL-17 expression in IBD.
Keywords: FOXP3, Interleukin-17A, Th17, Treg
Introduction
FOXP3 is a nuclear transcription factor, which plays a central role in the differentiation of CD4+ T cells into CD25+ regulatory T cells (Tregs), to whom its expression is largely restricted[1]. Tregs play an essential role in regulating inflammation in the gastrointestinal tract, as humans born with mutations in FOXP3 and mice engineered to lack Tregs both develop severe intestinal inflammation [2–5]. However, humans with the inflammatory bowel diseases (IBD) Crohn’s disease (CD) and ulcerative colitis (UC) do not lack mucosal FOXP3+ cells, but rather have a large number of them in the lamina propria and mesenteric lymph nodes, particularly in areas of active inflammation[6–9].
In healthy humans (but not mice), roughly half of all FOXP3 is expressed as an alternatively spliced isoform lacking exon 2 (Δexon 2)[10;11]. It is not known whether the two isoforms are coexpressed or expressed in different cells. When transfected into T cells, both full-length and Δexon2 versions of FOXP3 cause the cells to acquire Treg markers and lose their cytokine-secreting capacity[10]. However, there are mutations within exon 2 of FOXP3 that are associated with immune-mediated, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome in humans.[12;13] This suggests that the exon 2 sequence found exclusively within full-length FOXP3 plays a unique and critical role in the maintenance of immune homeostasis in the gut and elsewhere.
Th17 cells are IL-17A-secreting CD4+ T cells that have been shown to play a pathogenic role in several models of autoimmunity[14]. Th17’s are enriched in the intestinal mucosa of IBD patients[15], and may play a role in promoting the neutrophilic inflammation that is typical in active IBD[16]. Furthermore, several lines of evidence have implicated a Th17 survival factor, IL-23,[17] in the pathogenesis of IBD. Genetic correlations have been identified between IBD and polymorphisms in or near the receptor for IL-23[18;19], as well as shared components of IL-23 and the IL-23 receptor’s signal transduction cascade [20]. Additionally, clinical trials of an antibody directed at the shared p40 subunit of IL-12 and IL-23 have shown efficacy in treating Crohn’s disease [21]. Thus, Th17 cells are likely central mediators of IBD. Although they seem to have diametrically opposed roles in inflammation, Th17 cells and Tregs can both be generated from naïve T cells activated in the presence of TGF-β[22], a cytokine common to the bowel microenvironment in IBD[23]. Thus, the balance between whether T cells become pro-inflammatory Th17 cells or anti-inflammatory Tregs may be delicate and critical to maintaining gut immune homeostasis.
A unique subset of IL17-expressing FoxP3+ T cells was recently described in the intestinal mucosa, and found to be more common in Crohn’s patients than in controls, particularly in inflamed tissues [24]. These cells resemble both Tregs and Th17 cells in their surface protein expression profile, and they coexpress both FOXP3 and the nuclear transcription factor RORγt[24], which plays a central role in the differentiation of CD4+ T cells into Th17 cells[25]. FOXP3 can prevent RORγt from promoting IL-17A expression in CD4+ T cells by a direct interaction[26] involving a region of FOXP3 encoded by its second exon[27]. Thus, while full-length FOXP3 inhibits RORγt from promoting Th17 differentiation, the Δexon 2 splice-variant lacking this exon does not[27].
We hypothesized that the paradoxically increased number of FOXP3+ T cells in the inflamed mucosa of IBD patients may be preferentially expressing the Δexon2 isoform of FOXP3. This could potentially allow these cells to become pathogenic Th17 cells, or otherwise compromise their immunoregulatory potential. However, through immunohistochemistry and real-time PCR, we found no reduction of full-length FOXP3 expression in IBD, nor any preferential expression of Δexon2 transcripts. Furthermore, through immunofluorescence microscopy, we were unable to identify cells that exclusively expressed Δexon2 FOXP3. In cells isolated from colectomy specimens, we did identify FOXP3+ cells capable of expressing IL-17A in vitro, but these cells were seen both in patients with and without IBD, and did not preferentially express Δexon 2, although they were unusually capable of producing TNF-α. Thus neither the paradoxically plentiful FOXP3+ cells nor the FOXP3+ IL-17-expressing cells in the inflamed intestines of IBD patients express exclusively the Δexon2 form of FOXP3.
Methods
Human Subjects
All experiments were performed according to protocols approved by the Institutional Review Board (IRB) at Virginia Mason Medical Center/Benaroya Research Institute. For real-time quantitative polymerase chain reaction (qPCR), immunofluorescence, and flow cytometry experiments, specimens were obtained from sequential consenting patients undergoing bowel resection for Crohn’s disease (n=10), UC (n=7) or a non-IBD indication (n=8, consisting of one case each of HNPCC, diverticulosis, colon cancer, colonic inertia, and rectal prolapse, and 3 cases of FAP) by a single colorectal surgeon (RT). Non-IBD subject tissue was obtained from healthy, non-neoplastic portions of bowel. IBD subject tissue was from inflamed segments of bowel.
For immunohistochemistry (IHC) analyses, billing records were reviewed to identify patients with Crohn’s disease (ICD9 555.#), ulcerative colitis (IC9 556.#) or neither of the above who underwent colonoscopy with biopsy (CPT code 45380). Electronic medical records of patients thus identified were reviewed to confirm diagnosis accuracy and abstract basic demographic information as well as clinical data, including disease characteristics, duration, and treatment. Results were crossed with pathology records to select and retrieve archived colonoscopic biopsies for analysis from 31 patients with Crohn’s, 41 with UC, and 14 without IBD (1 with C. difficile colitis, 2 with collagenous colitis, 2 with chronic diverticulitis, 1 with rectal prolapse, and 7 with no identifiable pathology). Sections from historical archived tissue blocks were deidentified for research to be compliant with privacy laws.
Real-Time PCR
Samples of human intestine were obtained fresh from the operating room, immediately snap-frozen in liquid nitrogen, and later thawed, homogenized with an RNEasy kit (Quiagen), and converted to cDNA. cDNA was probed with commercial primer sets specific to CD4, GAPDH, or FOXP3 using TaqMan (Applied Biosystems) and a 7900HT real time PCR machine (ABI). FOXP3 probes were selected which span C-terminal sequence (exons 11–12), found in all FOXP3 transcripts, or could discriminate between full-length and Δexon2 transcripts by spanning either exon 2 or a junction between exons 1 and 3, respectively.
Histology
Sections of archived, formalin-fixed, paraffin embedded intestinal tissue were stained with hematoxylin and eosin using an Autostainer XL (Leica Microsystems). These slides were then evaluated by a blinded single pathologist (HH) to grade inflammation based upon neutrophilic infiltrate. Biopsies without neutrophilic inflammation were scored as “0”. Biopsies showing neutrophilic inflammation (infiltration of surface or crypt epithelium with associated epithelial injury) but without crypt abscesses were scored as “1+”. Biopsies with neutrophilic inflammation and crypt abscess formation were scored as “2+”. Biopsies with neutrophilic inflammation with severe epithelial injury resulting in mucosal ulceration were scored as “3+”.
For IHC, serial sections of formalin-fixed, paraffin-embedded intestinal mucosa were deparaffinized, antigen-retrieved, and stained on a BOND-MAX immunostainer (Leica Microsystems), using the manufacturer’s proprietary reagents. Primary antibodies used for IHC included a murine IgG to the c-terminus of FOXP3, which recognizes both full-length and Δexon2 versions of FOXP3 (clone 236A/E7, eBioscience), a murine IgG specific to sequence corresponding to exon 2 of FOXP3, which therefore cannot recognize the Δexon2 variant (clone 150D, eBioscience), or murine anti-CD4 (clone 4D11, Vector Labs). Sections were photographed in their entirety at low power, and digitally analyzed with ImageJ software (NIH), using a color deconvolution plugin to objectively count individual cells (in the case of FOXP3) or pixels (in the case of CD4, due to cytoplasmic staining and confluency) staining brown (DAB+) for a given marker. The average number of pixels per CD4+ cell was then determined in areas where CD4+ cells were not confluent, and then the total number of CD4+ pixels per slide was divided by the average number of pixels per CD4+ cell to estimate the number of CD4+ cells per slide. The number of FOXP3+ cells was then divided by the number of CD4+ cells to estimate the relative fraction of intramucosal CD4+ T cells expressing FOXP3.
Flow Cytometry
Fresh human intestine from the operating room was sharp dissected to isolate mucosa and remove as much muscle and connective tissue as possible. Then tissue was washed and incubated three times with DTT and EDTA in calcium and magnesium-free PBS at 37° to remove epithelium and mucous. The remaining lamina propria was then washed three times with RPMI, chopped, and incubated for 90 minutes at 37° with RPMI containing collagenase and DNAse. Liberated cells were then washed with PBS and incubated overnight in RPMI containing 10% fetal calf serum, L-glutamine, penicillin, streptomycin, amphotericin-B, non-essential amino acids, beta-mercaptoethanol, and sodium pyruvate, to recover from collagenase treatment. Cells were then stimulated with PMA and ionomycin in the presence of brefeldin A and Qdot 655-conjugated anti-CD4 (Invitrogen), and then stained extracellularly with anti-CD3 (Beckman-Coulter) and anti-CD8 (eBioscience), and intracellularly with antibodies to FOXP3 (clone 236A/E7 or clone 150D, eBioscience), TNF-α, IL-2 (BioLegend), IL-4, IL-17A, and IFN-γ (eBioscience), using a FOXP3 intracellular staining kit (eBioscience). Stained cells were run through an LSR II flow cytometer (Beckton Dickenson) and analyzed with FlowJo software (TreeStar).
Results
FOXP3+ cells are plentiful in IBD
Using primers recognizing the C-terminal transcript of FOXP3, we performed real-time qPCR for FOXP3 on mRNA isolated from frozen intestine surgically resected from patients with UC, CD, or no IBD. In addition to the housekeeping gene GAPDH, expression of CD4 was likewise quantified by qPCR, as CD4 was used to standardize FOXP3 expression to total number of CD4 T cells instead of total cellularity in each specimen. FOXP3 expression was no lower in IBD specimens than controls, and relative to CD4, there was a paradoxical increase in the frequency of FOXP3 transcripts in tissue from Crohn’s patients or the inflamed portion of UC specimens relative to uninflamed colon from UC patients or controls (figure 1A).
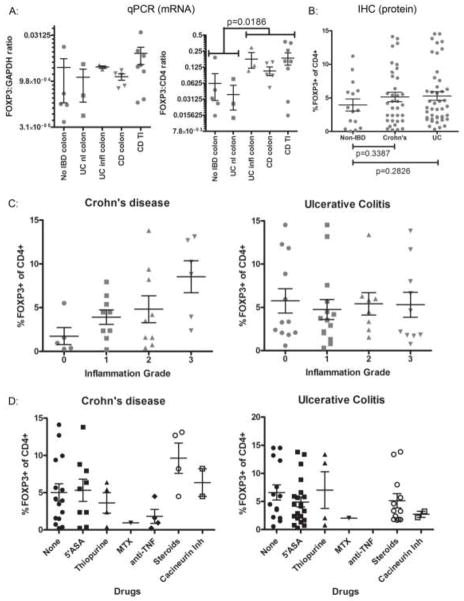
Figure 1. FOXP3+ cells are plentiful in IBD.
A. mRNA was harvested from intestinal mucosa snap-frozen from surgically resected specimens, and evaluated by qPCR for the quantity of FOXP3 transcripts relative to CD4 (right) or GAPDH (left) transcripts. In the right panel, a p-value is shown for a Student’s t-test comparing pooled data points under the two brackets shown. B. Serial sections of FFPE colonoscopic biopsies were stained by IHC with antibodies to FOXP3 or CD4 and digitally photographed, and the total number of FOXP3+ or CD4+ cells were quantified by ImageJ, and used to estimate the percent of total CD4+ cells that were FOXP3+ Tregs. P-values are shown for Student’s t-tests comparing non-IBD specimens with either Crohn’s or UC specimens. C. H&E-stained serial sections of the samples in B from IBD patients were graded for neutrophilic inflammation by a single, blinded GI pathologist, and the Treg frequency in B was re-plotted relative to Crohn’s (left) or UC (right) inflammatory grade. D. Data in B was re-plotted relative to whether a patient was using a particular medication at the time of biopsy (note: some patients were using more than one agent, and so may be represented in multiple columns). For all IHC plots, each dot represents a unique colonoscopy.
We also performed immunohistochemistry (IHC) for FOXP3 and CD4 on serial sections of intestinal biopsies obtained colonoscopically from patients with versus without UC or CD (figure 2B, upper panels, shows representative FOXP3 IHC). FOXP3+ cells were electronically counted, relative to the density of CD4+ T cells in the sections, and were at least as large a fraction of total CD4+ T cells in IBD patients as in controls (figure 1B), as has been described previously[6–9]. When the degree of neutrophilic inflammation present in these sections was graded by a blinded pathologist, and treated as a continuous variable for linear regression analysis, it was found to correlate with FOXP3+ T cell frequency in specimens from patients with Crohn’s disease (figure 1C left panel, Pearson’s r = 0.5135, p = 0.0044), but not UC (figure 1C right panel, r = −0.01984, p = 0.8983). Thus, not only were FOXP3+ T cells plentiful in the mucosa of Crohn’s patients, but they were paradoxically more common in more severely inflamed tissue. No correlations were found between FOXP3+ T cell frequency and patient age, gender, disease duration, biopsy location, Crohn’s complications (strictures, abscesses or fistulas) (data not shown), or treatment (figure 1D) by linear regression analyses, ANOVA, or Student’s t-test, as appropriate.
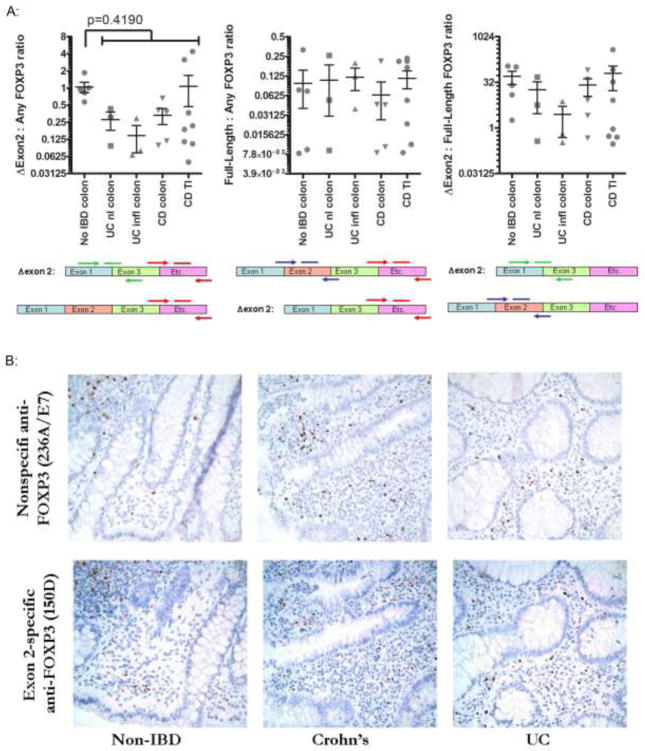
Figure 2. Cells expressing full-length FOXP3 are plentiful in IBD.
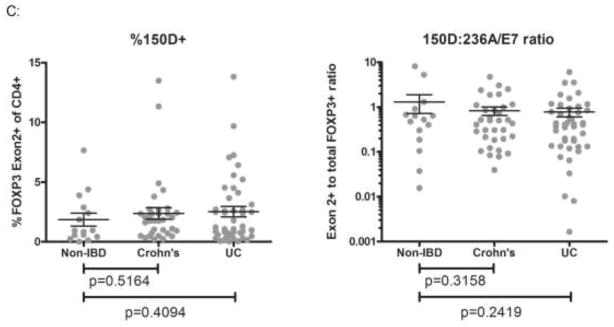
A. mRNA was harvested from intestinal mucosa snap-frozen from surgically resected intestine specimens, and evaluated by qPCR using primers to FOXP3 transcripts that either overly an exon 1-exon 3 splice junction (and therefore will only amplify Δexon 2 transcripts) (green arrows), bind within exon 2 (and thus will only amplify full-length FOXP3 transcripts) (blue arrows), or recognize sequence corresponding to C-terminal sequence (and so will amplify both Δexon 2 and full-length FOXP3 transcripts) (red arrows). The ratio of each of these qPCR results to one another are shown for each patient (represented by a unique dot). In the left panel, a p-value for a Student’s t-test comparing no IBD colon to pooled data from all IBD specimens is shown. B. Representative fields are shown from serial sections of colon biopsies from a patient with UC (right), Crohn’s (middle) or no IBD (left) stained by IHC for FOXP3 with antibodies that recognize either the C-terminus of FOXP3 (and thus will not discriminate between full-length and Δexon 2 proteins, clone 236A/E7, upper panels) or sequence encoded by its exon 2 (and thus will only bind full-length protein, not Δexon 2, clone 150D, lower panels). C. Biopsy serial sections from figure 1B were stained by IHC with the FOXP3 exon 2-specific antibody clone 150D, and positive cells were quantified as in figure 1B. The ratio of exon 2-positive cells to either CD4+ (left) or total FOXP3+ (right) cells is shown for each patient (represented as a unique dot). P-values are shown for Student’s t-tests comparing non-IBD specimens with either Crohn’s or UC specimens.
Cells expressing full-length FOXP3 are plentiful in IBD
To determine the fraction of FOXP3 transcripts encoding full-length versus Δexon 2 FOXP3, we performed real-time PCR on the mRNA from figure 1A, using primers that spanned either exon 2 (present only in full-length FOXP3 transcripts), or the junction between exons 1 and 3 (present only in Δexon2 transcripts). When divided by the frequency of total FOXP3 transcripts (from which the data in figure 1A was generated), transcripts containing exon 2 (and thus full-length FOXP3) were no less common, and those containing the Δexon 2 splice junction were no more common, in IBD than in controls (figure 2A). In fact, there was a nonsignificant trend towards fewer Δexon2 transcripts in IBD.
To confirm this finding at the protein level, and demonstrate the histological distribution of cells expressing full-length FOXP3, we also performed IHC with an antibody (clone 150D) recognizing only the exon 2 domain (and thus only full-length FOXP3, not Δexon 2). Using serial sections from the same biopsies analyzed in figure 1B–D, we found copious full-length-FOXP3+ cells present in the lamina propria and lymphoid follicles of both IBD patients and controls, with a tissue distribution similar to total FOXP3+ cells in figure 1 (figure 2B). Again, when divided by the CD4+ T cell density in sections, there were no fewer full-length-FOXP3+ cells in IBD patients than in controls (figure 2C, left panel). When the ratio of full-length to total FOXP3+ cells was calculated for each specimen, there was no qualitative difference between IBD patients and controls (figure 2C, right panel).
FOXP3+ cells in IBD are exon 2+
The data in figures 1 and 2 could not exclude the possibility that a small number of FOXP3+ cells in IBD patients expressed exclusively the Δexon 2 form of FOXP3. Therefore, we stained frozen sections from the surgically resected tissue of IBD patients with both nonspecific and exon 2 (eg: full-length) –specific antibodies to FOXP3, plus anti-CD3, by three-color immunofluorescence. Cells recognized by both anti-FOXP3 antibodies were readily identified (figure 3A), but not a single cell was found that would be recognized by C-terminus-specific anti-FOXP3 (clone PCH101) without also being labeled by exon 2-specific anti-FOXP3 (clone 150D). Thus no cells expressing exclusively Δexon2 were identified.
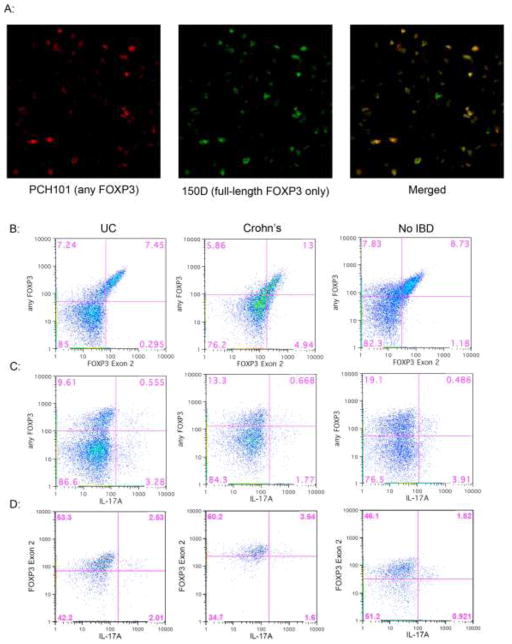
Figure 3. FOXP3+ cells in IBD are Exon 2+.
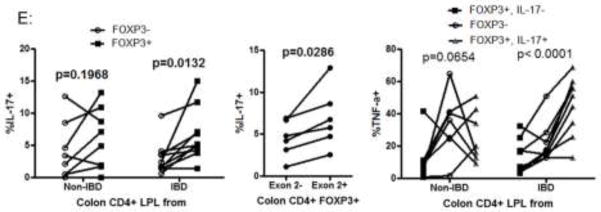
A. A frozen section from the surgically resected MLN of a patient with UC was simultaneously stained with exon-2 specific mouse anti-FOXP3 (clone 150D, green) and nonspecific rat anti-FOXP3 (clone PCH101, red), revealing no FOXP3+ cells recognizable by the latter which were not also expressing exon 2 (merged). B–D: Lamina propria lymphocytes (LPL) from the surgically resected intestines of patients with UC (left) Crohn’s (middle) or no IBD (right) were stimulated overnight with PMA and ionomycin in the presence of brefeldin A and then stained extracellularly for CD3, 4, and 8, and intracellularly with antibodies to the C-terminus (clone 236A/E7, Y-axis in B and C) and Exon 2 region (clone 150D, X-axis in B, Y-axis in D) of FOXP3, as well as IL-17A (X-axis in C and D). Data shown in B and C is gated to include CD3+4+8- cells within the live lymphocyte gate, while data in D is additionally gated to include only FOXP3+ (any isoform) cells. E. Colon LPL from patients without (n=8) or with IBD (n=6 UC, 4 Crohn’s) were stimulated as above and stained for intracellular cytokines and FOXP3. The percent of FOXP3+ and FOXP3- CD3+ CD4+ CD8- cells expressing IL-17 is shown in the left panel, with p-values representing paired t-test comparisons between FOXP3+ and FOXP3- populations. In a subset of the FOXP3+ populations (n=2 Crohn’s, 2 UC and 2 non-IBD patients) which were also stained for Exon2, the percent of Exon2+ and Exon 2- FOXP3+ cells expressing IL-17 is shown in the middle panel, with p-values representing paired t-test comparisons between Exon 2+ and Exon 2- populations. TNF-α expression by FOXP3- and either IL-17+ or IL-17- FOXP3+ CD4+ T cells is shown in the right panel, with p-values representing ANOVA calculations for each cohort.
For flow cytometry, we similarly stained collagenase-liberated cells from the surgically-resected colons of patients with or without IBD, using both nonspecific and exon 2-specific anti-FOXP3 antibodies. We again found few cells that were positive for nonspecific anti-FOXP3 but negative for exon 2-specific anti-FOXP3 (figure 3B), as there was a direct correlation in fluorescence between the two antibodies.
Exon 2+ FOXP3+ T cells make IL-17
IL-17+ cells were present among FOXP3+ cells (figure 3C) with a greater percentage of FOXP3+ than FOXP3- T cells able to make IL-17 in specimens from IBD patients, although this difference was not statistically significant in patients without IBD (figure 3E, left panel). When gated on FOXP3+ cells, a fraction of those specifically expressing exon 2+ full-length FOXP3 (which were the majority of FOXP3+ cells) were likewise able to produce IL-17 (figure 3D), with more exon 2+ than exon 2- T cells able to make IL-17 (figure 3E, middle panel). No difference was noted between IL-17+ and IL-17-FOXP3+ cell in terms of IL-2, IL-4, or IFN-γ expression (data not shown). However, in specimens from IBD patients, IL-17-expressing FOXP3+ T cells expressed more TNF-α than FOXP3- T cells (p=0.0006 by paired t-test), which in turn expressed more TNF-α than FOXP3+ cells that did not express IL-17 (p=0.0368 by paired t-test) (figure 3E, right panel). Again, these differences were not significant in specimens from patients without IBD.
Discussion
FOXP3+ Tregs are critical mediators of intestinal immune homeostasis [2–5], and yet are paradoxically present in increased frequency in the bowels of IBD patients [6–9]. We show here that this paradox is not explained by a preferential expression of FOXP3 in its shortened Δexon 2 isoform in IBD. Furthermore, we find no evidence that T cells exclusively expressing Δexon 2 predominate among a subset of FOXP3+ T cells capable of producing IL-17 in vitro.
The latter cells have been described previously in the blood of normal donors [28;29] and in the intestinal mucosa of patients with Crohn’s disease, where expression of the Th17 differentiation transcription factor RORγt correlated with IL-17 production [24]. We confirmed that a higher fraction of FOXP3+ than FOXP3- T cells in the intestinal mucosa of IBD patients could make IL-17, although the lack of a similar correlation in patients without IBD was due more to a higher fraction of IL-17+ FOXP3- T cells and greater overall heterogeneity in these patients than a lower frequency of IL-17+ FOXP3+ T cells, as has been described [24]. We find that in normal PBMC, as in the intestine, these IL-17-producing FOXP3+ cells contain exon 2 (data not shown). The inability of endogenously expressed full-length FOXP3 to prevent IL-17 production by CD4+ T cells appears to contradict a model in which cotransfection of full-length FOXP3, but not Δexon 2 FOXP3, with RORγt prevented the latter from causing the T cells to secrete IL-17[27]. It is possible that in FOXP3+ PBMC and intestinal T cells, IL-17 secretion is mediated by an RORγt-independent mechanism, resistant to inhibition by FOXP3. Alternatively, endogenous FOXP3, in contrast to transfected FOXP3, may not be expressed at high enough levels to prevent RORγt from causing IL-17 secretion. Finally, it is possible that the in vitro stimulation with PMA and ionomycin we used to detect cytokine production by flow cytometry was too potent for FOXP3 to prevent IL-17 secretion.
We found that more IL-17+ than IL-17- FOXP3+ T cells were able to produce TNF-α, and in IBD patients we noted the former cells to even be more able to make TNF-α than FOXP3- T cells. TNF-α is presumed to be central to the pathogenesis of IBD, as it is a primary target of effective pharmacotherapy for both UC and Crohn’s disease. Thus it is paradoxical for a presumed regulatory population of FOXP3+ cells to be a potent source of such a pro-inflammatory cytokine. Future research may elucidate whether such a paradox is a cause or effect of disease pathogenesis.
We confirmed that the number of intramucosal FOXP3+ T cells correlates with the degree of tissue inflammation present. Furthermore, ex vivo analyses have not identified any specific defect in the ability of these cells to inhibit the proliferation of other T cells [6–8;30]. It is possible that these in vitro assays fail to capture some of the more subtle inhibitory effects through which Tregs function in vivo, and which could be defective in IBD. Alternatively, there may be cells or factors in the intestinal microenvironment which negate the inhibitory effect of Tregs in the mucosa. Clearly there are more pro-inflammatory cells and cytokines in the inflamed mucosa of IBD patients, against which even a robust Treg response may simply be insufficient. However, if there are specific molecular signals that prevent Tregs from controlling inflammation, they would represent attractive targets for therapeutic intervention, as any agent capable of blocking them could unfetter the central role Tregs normally play in maintaining gut immune homeostasis.
Acknowledgments
Support: NIDDK/NIH 1K08DK081659
We wish to thank Sandhya Mishra for assistance with patient recruitment, and thank Patti Stewart, Joyce Matsuoka-Hayashi, Joelle Averbuch, Rachael Williams and all of the Anatomic Pathology attendings at Virginia Mason Medical Center for facilitating surgical specimen acquisition. We wish to thank Mary Beauchamps for assistance with histology preparation and K. Aru Arumuganathan for assistance with flow cytometry. Special thanks to Jane Buckner and Elisa Boden for manuscript review. This work was funded through a grant from the NIDDK/NIH, and internal resources at the Benaroya Research Institute.
Contributor Information
James D. Lord, Email: james.lord@vmmc.org, Translational Research Program, Benaroya Research Institute, Mailstop IN-RC, 1201 Ninth Ave., Seattle, WA 98101-2795, or Gastroenterology Division, Virginia Mason Medical Center, Mailstop C3-GAS, 1100 9th Ave., Seattle, WA 98111
Karine Valliant-Saunders, Email: kversus@gmail.com, Immunology Program, Benaroya Research Institute, Mailstop IN-RC, 1201 Ninth Ave., Seattle, WA 98101-2795.
Hejin Hahn, Email: hejin.hahn@vmmc.org, Pathology Department, Virginia Mason Medical Center, Mailstop C6-LAB, 1100 9th Ave., Seattle, WA 98111
Richard C. Thirlby, Email: richard.thirlby@vmmc.org, Surgery Department, Virginia Mason Medical Center, Mailstop C6-GS, 1100 9th Ave., Seattle, WA 98111
Steven F. Ziegler, Email: sziegler@benaroyaresearch.org, Immunology Program, Benaroya Research Institute, Mailstop IN-RC, 1201 Ninth Ave., Seattle, WA 98101-2795
Reference List
- 1.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 3.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 4.Patel DD. Escape from tolerance in the human X-linked autoimmunity-allergic disregulation syndrome and the Scurfy mouse. J Clin Invest. 2001;107:155–157. doi: 10.1172/JCI11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 6.Makita S, Kanai T, Oshima S, Uraushihara K, et al. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–3130. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 7.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Saruta M, Yu QT, Fleshner PR, et al. Characterization of FOXP3+CD4+ regulatory T cells in Crohn’s disease. Clin Immunol. 2007;125:281–290. doi: 10.1016/j.clim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allan SE, Passerini L, Bacchetta R, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mailer RK, Falk K, Rotzschke O. Absence of leucine zipper in the natural FOXP3Delta2Delta7 isoform does not affect dimerization but abrogates suppressive capacity. PLoS ONE. 2009;4:e6104. doi: 10.1371/journal.pone.0006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi I, Shiari R, Yamada M, et al. Novel mutations of FOXP3 in two Japanese patients with immune dysregulation, polyendocrinopathy, enteropathy, X linked syndrome (IPEX) J Med Genet. 2001;38:874–876. doi: 10.1136/jmg.38.12.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol. 2007;120:744–750. doi: 10.1016/j.jaci.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 14.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–iii29. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 15.Annunziata F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witowski J, Ksiazek K, Jorres A. Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci. 2004;61:567–579. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Dubinsky MC, Wang D, Picornell Y, et al. IL-23 receptor (IL-23R) gene protects against pediatric Crohn’s disease. Inflamm Bowel Dis. 2007;13:511–515. doi: 10.1002/ibd.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 23.McCabe RP, Secrist H, Botney M, Egan M, Peters MG. Cytokine mRNA expression in intestine from normal and inflammatory bowel disease patients. Clin Immunol Immunopathol. 1993;66:52–58. doi: 10.1006/clin.1993.1007. [DOI] [PubMed] [Google Scholar]
- 24.Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 26.Ichiyama K, Yoshida H, Wakabayashi Y, et al. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayyoub M, Deknuydt F, Raimbaud I, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voo KS, Wang YH, Santori FR, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–199. doi: 10.1002/ibd.20053. [DOI] [PubMed] [Google Scholar]