Abstract
Background
We performed a pilot study to test the hypothesis that acute oral ingestion of tetrahydrobiopterin (BH4), a key cofactor modulating vascular nitric oxide (NO) synthase activity, improves large elastic artery stiffness with aging in men.
Methods
Healthy older (63 ± 2 years; n = 8) and young (age 25 ± 1 years; n = 6) men were studied 3 h after ingestion of BH4 (10 mg·kg−1 body weight) or placebo on separate days in a randomized, placebo-controlled, double-blind study.
Results
Baseline carotid artery compliance was 37% lower (0.17 ± 0.02 vs. 0.22 ± 0.02 mm/mm Hg·10−1) and β-stiffness was 42% higher (7.3 ± 1.1 vs. 4.2 ± 0.5 AU) in the older men (both P < 0.05). BH4 ingestion markedly increased circulating BH4 concentrations in both groups (17–19-fold, P < 0.05), but increased compliance (+39% to 0.23 ± 0.02 mm/mm Hg.10−1, P < 0.01) and decreased β-stiffness index (–27% to 5.3 ± 0.7 AU, P < 0.01) only in the older men. BH4 also reduced carotid systolic blood pressure (SBP) in the older men (P < 0.05).
Conclusions
These preliminary results support the possibility that limited BH4 bioavailability contributes to impaired carotid artery compliance in healthy older men. Further studies are needed to determine if increasing BH4 bioavailability though oral BH4 supplementation may have therapeutic efficacy for improving large elastic artery compliance and reducing central SBP with aging.
American Journal of Hypertension, advance online publication 7 June 2012; doi:10.1038/ajh.2012.70
Keywords: arterial stiffness, blood pressure, cardiovascular disease, central blood pressure, hypertension, nitric oxide
Aging is associated with progressive stiffening of the large elastic arteries of the cardiothoracic region (aorta, carotids) in humans, even in the absence of conventional risk factors for cardiovascular diseases (CVD).1 Decreased compliance of the carotid artery and increased stiffness of the aorta act to increase central and peripheral systolic blood pressure (SBP) and pulse pressure (PP), as well as risk of CVD-related events such as myocardial infarction, stroke, and heart failure.2,3 As such, insight into the mechanisms involved in reduced large elastic artery compliance with aging may be clinically valuable for identifying possible therapeutic targets.
The mechanisms in question are thought to include so-called “functional influences” that increase smooth muscle vascular tone as a result of decreased nitric oxide (NO)-mediated vasodilation4,5 and/or increased endothelin-1 vasoconstrictor tone.6 Reduced vascular NO bioavailability with aging is mediated in part by reductions in the concentration or bioactivity of tetrahydrobiopterin (BH4), the essential cofactor for NO synthase.7 Therefore, reduced vascular BH4 bioavailability may be one mechanism contributing to decreased large elastic artery compliance with aging.
We performed a pilot study to test the hypothesis that acute oral ingestion of BH4 improves large elastic artery stiffness with aging in men. In a randomized, placebo-controlled, double-blinded cross-over pilot study, we assessed carotid artery compliance and β-stiffness index, a less BP-dependent expression of compliance, in groups of healthy older and young men after administering a single therapeutic dose of BH4 shown previously to restore peripheral vascular endothelial function in this group.8 Central (carotid) and peripheral (brachial) SBP and PP also were assessed.
Methods
Subjects. Eight older (age 55–75 years) and six young (age 19–30 years) healthy men participated in the protocol. Subjects were nonsmokers, nonobese (body mass index <30 kg.m−2), had brachial artery BP <150/ <90 mm Hg and circulating total (<240 mg.dl−1) and low-density lipoprotein (<160 mg.dl−1) cholesterol in the clinically normal range. All study procedures complied with the Declaration of Helsinki and the informed consent and study documents were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation. See Supplemental Methods (32KB, doc) online for additional information.
General experimental procedures. All BP measurements were performed at the University of Colorado at Boulder Clinical and Translational Research Center (CTRC) after a 12-h overnight fast and abstention from caffeine, alcohol and exercise for at least 24 h. See Supplemental Methods (32KB, doc) online for additional information.
Study design. Subjects were randomly assigned to ingest BH4 (Schircks Laboratories, Jona, Switzerland) pills (single oral dose of 10 mg·kg−1 body weight) or placebo (double-blinded, cross-over study design) on separate days (4–5 days apart) between 7–8 AM at the CTRC and then returned to the CTRC for experimental measurements 3–3½ h after ingestion. The placebo and BH4 pills were encapsulated to look identical to each other. Pharmacokinetic studies in humans demonstrate that plasma BH4 concentrations peak between 3 and 5 h after oral ingestion and that 4–5 days is an adequate washout period.9 All BH4 tablets were stored at −20 °C until immediately before ingestion by the subject. This dose increases plasma biopterin concentrations ~25-fold,9 improves endothelium-dependent dilation in healthy middle-aged/older adults,8 and is within the dosing range used therapeutically to treat adults with phenylketonuria.10
BP and carotid artery measurements. BP was measured by oscillometry over the brachial artery according to established procedures as previously described our laboratory. Carotid arterial compliance and β-stiffness index were assessed noninvasively as previously described in detail by our laboratory11,12 and others.13,14 See Supplemental Methods (32KB, doc) online for details.
Measurement of circulating factors. See Supplemental Methods (32KB, doc) online for details.
Statistical analysis. All analyses were performed using IBM SPSS Statistics 19.0 (IBM, Armonk, NY). All data are presented as mean ± s.e. An independent t-test was used to compare older men (i.e., during placebo) to young men at baseline with statistical significance was set at P < 0.05. A 2 × 2 repeated-measures analysis of variance was used for between-group (older men, younger men) and within-group (placebo condition, BH4 condition) comparisons. When a significant condition × group interaction was revealed (P < 0.05) within-group paired t-tests with Bonferonni correction for multiple comparisons were performed with statistical significance set at P < 0.025. Bivariate pearson correlation analyses were performed to examine relations between variables of interest.
Results
Baseline values. Clinical characteristics and baseline function are shown in Table 1. Clinical characteristics were within normal ranges for both groups. Older men had greater waist circumference, waist:hip ratio, and plasma glucose (all P < 0.05), but did not differ in body mass index compared with young men (P = 0.46). Brachial artery diastolic BP (DBP) (P = 0.02), carotid end-diastolic diameter (P = 0.05), carotid end-systolic diameter (P = 0.03), carotid distension (P = 0.06) and carotid intimal-medial thickness (P < 0.01) were greater (or trended greater) in the older men, although mean values for BP were well within the normotensive range (Table 1). Older and young men did not differ in leisure time physical activity levels (P = 0.59). Carotid artery compliance and β-stiffness were 37% lower and 42% higher in the older men, respectively (P < 0.05, Figure 1).
Table 1.
Subject characteristics, hemodynamic, and circulating factors after oral ingestion of placebo and 10 mg.kg−1 tetrahydrobiopterin (BH4) in older and young men
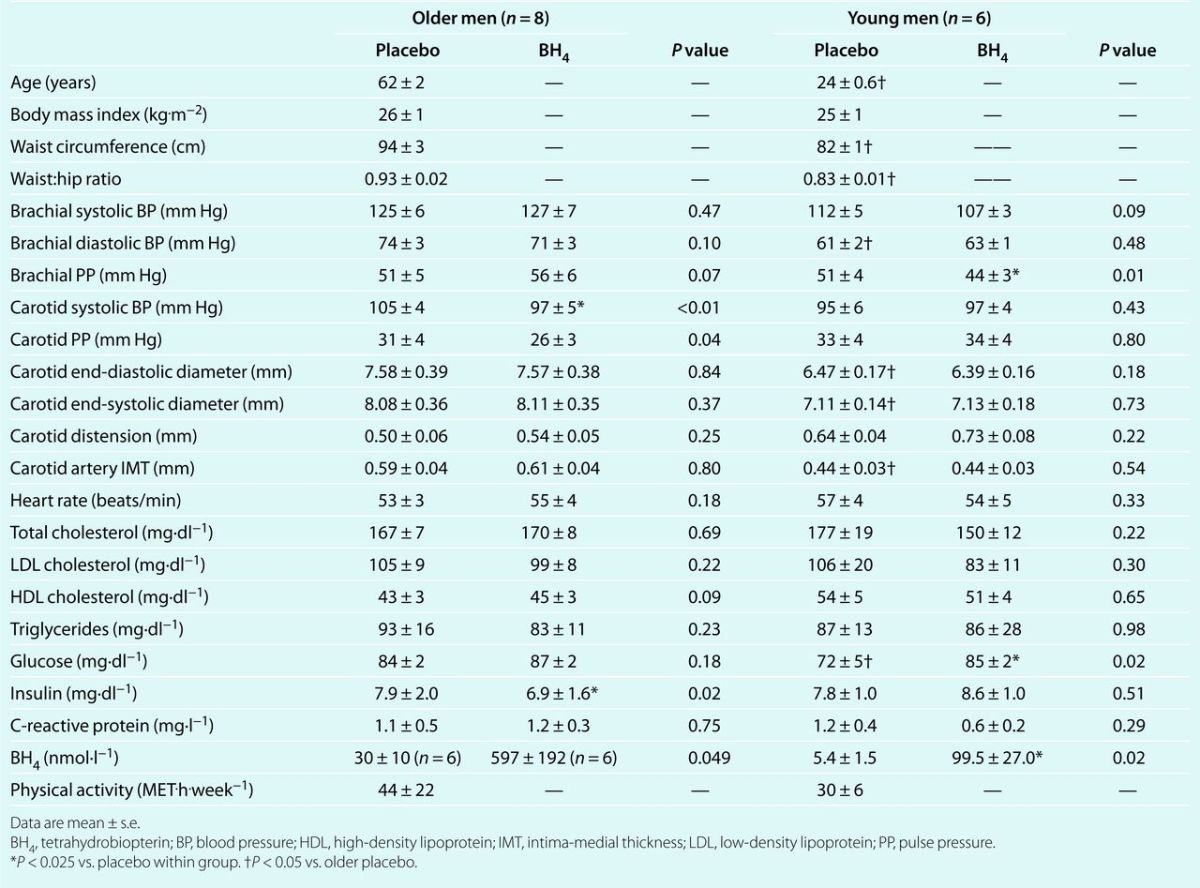
Figure 1. Carotid artery compliance and β-stiffness index after acute placebo and tetrahydrobiopterin (BH4) supplementation. (a) Carotid artery compliance and (b) β-stiffness index in older and young men 3 h after oral ingestion of placebo or 10 mg.kg−1 tetrahydrobiopterin (BH4). Individual data of older men on (c) carotid artery compliance and (d) β-stiffness index 3 h after oral ingestion of placebo or 10 mg·kg−1 tetrahydrobiopterin (BH4). *P < 0.05 vs. Placebo within group. †P < 0.05 vs. older placebo.
Circulating BH4 concentrations. BH4 ingestion increased circulating BH4 concentrations by 19-fold (30.0 ± 10.4–597 ± 192 nmol.l−1, P = 0.02) in the older men and ~17½-fold (5.4 ± 1.5–99.5 ± 27 nmol.l−1, P = 0.049) in the younger men (the group × condition interaction P = 0.06).
Effects of BH4 administration in older men. After BH4 there was no change in brachial artery SBP, DBP, PP, heart rate, or circulating factors compared with after placebo (Table 1). In contrast, there was a significant group × condition interaction for carotid compliance (P < 0.01) and β-stiffness index (P = 0.01) where BH4 increased carotid artery compliance by 39% (P = 0.02) and reduced β-stiffness index by 27% (P < 0.01, Figure 1). There was also a group × condition interaction for carotid artery SBP (P < 0.01) and a trend for carotid PP (P = 0.09), where BH4 decrease carotid SBP by ~6 mm Hg (P < 0.01, Table 1) compared with placebo. The effects of BH4 were consistent, with seven of the eight older men showing improvements in both carotid compliance and β-stiffness (Figure 1; bottom panels).
Effects of BH4 administration in young men. There was no effect of BH4 on brachial or carotid SBP or DBP, heart rate or circulating factors compared with placebo in the young men. In contrast, there was a significant group × condition interaction for brachial PP (P < 0.01) where brachial PP was decreased in the younger men after BH4 compared with placebo (P < 0.025, Table 1). BH4 had no effect on carotid artery compliance or β-stiffness index compared with placebo in the young men (Figure 1; top panels; Supplementary Figure S1 (63.5KB, doc) online).
Discussion
The results of this pilot study provide preliminary evidence that reduced vascular BH4 bioavailability could be an important mechanism contributing to age-associated reductions in large elastic artery compliance (increases in stiffness) in men. Specifically, we found that a single therapeutic dose of BH4 improved carotid artery compliance by ~40% and reduced β-stiffness index by almost 30% in a small group of healthy older men, such that values attained (compliance) or approached (β-stiffness index) those of young controls. In the older men, BH4 also reduced carotid (i.e., central) artery SBP in the absence of any changes in brachial (i.e., peripheral) artery SBP or PP. The latter observations suggest that reduced BH4 bioavailability may selectively contribute to increases in central SBP in older men, perhaps via effects on large elastic artery compliance. However, we cannot discount the possibility that BH4 induced a central BP-lowering effect, which, in turn, influenced carotid artery properties.
The acute BH4 administration used in the present study presumably exerted its carotid artery and central BP effects by affecting “functional” influences on compliance, perhaps related to NO modulation of vascular smooth muscle tone, rather than affecting structural components of the artery. Indeed, there is direct evidence that at least some portion of increased arterial stiffness is regulated by reduced endothelial NO bioavailability leading to enhanced vascular smooth muscle tone.4,5,15 Therefore, given the obligatory role of BH4 in maintaining vascular NO production we speculate that the improvement in carotid compliance in this case is a result of improvement in vascular tone in part from an increase in NO rather than alterations in vascular wall structural proteins elastin or collagen.
It also is possible that BH4 acted as an antioxidant and scavenged reactive oxygen species such as superoxide anion.16 However, paramagnetic resonance studies suggest that superoxide scavenging by BH4 is not a major reaction in vivo.17 Additionally, intra-brachial artery infusion of BH4 improves endothelium-dependent, NO-mediated dilation in smokers, whereas tetrahydroneopterin (NH4), a biopterin with similar antioxidant properties as BH4 but without the endothelial NO synthase coupling ability, has no effect.18 Moreover, although the formulation of BH4 used includes 50 mg of vitamin C, we have shown previously that a much higher oral dose of vitamin C (500 mg·day−1) administered for one month12 or acute intravenous infusion of supraphysiological dose of vitamin C12 does not alter carotid artery compliance or β-stiffness in older men.
The reasons for the difference in basal circulating BH4 concentrations between the older and young men is unclear. One possibility to explain this is the small sample size. With a larger sample size you might expect to see a regression to the mean resulting in circulating values that are not different. Another possibility is that basal circulating concentrations are inversely related with vascular BH4 levels,19 suggesting that vascular BH4 levels are lower in our cohort of older adults. However, both groups demonstrated an approximately 17–19-fold increase in circulating concentrations 3 h after oral BH4 ingestion that resulted in improvements in carotid compliance and β-stiffness only in the older group. Furthermore, baseline or the change in BH4 concentrations did not correlate with the change in carotid compliance or β-stiffness index, confirming that circulating BH4 concentrations may not be a good index of vascular BH4 concentrations.
There are several limitations to our pilot study that we wish to emphasize. First, we did not assess NO bioavailability or its effects on vascular smooth muscle relaxation. Second, because of this study's pilot nature, our sample size was, by definition, small and consisted only of healthy older and young men without major CVD risk factors or with known CVD which may limit generalizability. Despite this, the responses to BH4 were consistent among our healthy older men suggesting that similar results could be observed in a larger sample, including adults with more CVD risk factors. Finally, the reductions in carotid SBP (and trend for PP) in the absence of alterations in brachial SBP and PP makes it tempting to speculate that this was a direct result of a reduced amplitude or delayed return of the reflected wave to central arteries. However, we did not measure carotid-femoral pulse wave velocity or central augmentation index in the present study so it cannot be determined whether aortic stiffness or reflected wave properties were altered. Interestingly, a recent study in older adults with severe coronary artery disease demonstrated that 2–6 weeks of low- or high-dose BH4 treatment had no effect on aortic pulse velocity or aortic and carotid distensibility measured by magnetic resonance imaging.20 Future studies will be necessary to directly test whether chronic BH4 supplementation favorably modulates aortic pulse wave velocity and augmentation index in healthy older men and women.
In conclusion, the results of the present pilot study support the hypothesis that reduced BH4 bioavailability may be an important mechanism contributing to reductions in carotid artery compliance and increases in central SBP with aging in healthy men. These preliminary results provide the basis for larger investigations aimed at determining if increasing BH4 bioavailability through oral supplementation can improve large elastic artery compliance, lower central SBP and perhaps reduce the risk of age-associated CVD.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ajh
Acknowledgments
The authors thank Adam Bergquist, Livia Tsien, and the staff of the University of Colorado at Boulder Clinical and Translational Research Center for technical assistance. This work was supported by NIH awards AG013038, AG000279, UL1 RR025780, AG033994 and AHA 0715735Z.
Diclosure
The authors declared no conflict of interest.
References
- 1.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004;43:1239–1245 [DOI] [PubMed] [Google Scholar]
- 2.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension 1998;32:570–574 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010;121:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol 2006;101:1751–1759 [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation 2002;105:213–217 [DOI] [PubMed] [Google Scholar]
- 6.McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB. Endothelin-1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol 2003;42:1975–1981 [DOI] [PubMed] [Google Scholar]
- 7.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol (Lond) 2008;586:1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol (Lond) 2005;568:1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiege B, Ballhausen D, Kierat L, Leimbacher W, Goriounov D, Schircks B, Thöny B, Blau N. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genet Metab 2004;81:45–51 [DOI] [PubMed] [Google Scholar]
- 10.Levy HL, Milanowski A, Chakrapani A, Cleary M, Lee P, Trefz FK, Whitley CB, Feillet F, Feigenbaum AS, Bebchuk JD, Christ-Schmidt H, Dorenbaum A; Sapropterin Research Group Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet 2007;370:504–510 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000;102:1270–1275 [DOI] [PubMed] [Google Scholar]
- 12.Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol 2004;286:H1528–H1534 [DOI] [PubMed] [Google Scholar]
- 13.Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 1989;80:78–86 [DOI] [PubMed] [Google Scholar]
- 14.Armentano R, Megnien JL, Simon A, Bellenfant F, Barra J, Levenson J. Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension 1995;26:48–54 [DOI] [PubMed] [Google Scholar]
- 15.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Yokoi T, Otsuki T, Shimojo N, Miyauchi T, Maeda S, Tanaka H. Effect of systemic nitric oxide synthase inhibition on arterial stiffness in humans. Hypertens Res 2007;30:411–415 [DOI] [PubMed] [Google Scholar]
- 16.Wever RM, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun 1997;237:340–344 [DOI] [PubMed] [Google Scholar]
- 17.Vásquez-Vivar J, Martásek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 2002;362:733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Münzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 2000;86:E36–E41 [DOI] [PubMed] [Google Scholar]
- 19.Antoniades C, Shirodaria C, Crabtree M, Rinze R, Alp N, Cunnington C, Diesch J, Tousoulis D, Stefanadis C, Leeson P, Ratnatunga C, Pillai R, Channon KM. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation 2007;116:2851–2859 [DOI] [PubMed] [Google Scholar]
- 20.Cunnington C, Van Assche T, Shirodaria C, Kylintireas I, Lindsay AC, Lee JM, Antoniades C, Margaritis M, Lee R, Cerrato R, Crabtree MJ, Francis JM, Sayeed R, Ratnatunga C, Pillai R, Choudhury RP, Neubauer S, Channon KM. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation 2012;125:1356–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is linked to the online version of the paper at http://www.nature.com/ajh