Abstract
Purpose
To compare the prevalence of endothelial rejection episodes and probability of graft survival after initial and repeat penetrating keratoplasty in keratoconic patients with and without atopy.
Methods
A retrospective review was conducted of all patients receiving penetrating keratoplasty (PK) for keratoconus at the University of Texas Southwestern Medical Center at Dallas from 1988–2009. Inclusion criteria involved those with both an ICD-9 code for keratoconus and a CPT code for penetrating keratoplasty based on a computer data base search. Patients less than 18 years of age were excluded. These records were then reviewed for a history of atopic disorders. The main outcome measures included the prevalence of endothelial rejection episodes and the probability of graft survival. The probability of corneal graft survival in patients with and without a history of atopy was compared using the Kaplan-Meier method.
Results
There were 168 grafts in 122 patients. There were 66 (39.2%) and 102 (60.8%) grafts with and without a history of atopy, respectively. Bilateral first grafts were required in 32 patients, 14 and 18 with and without a history of atopy, respectively. The atopic and nonatopic groups had no significant differences with respect to: age, preexisting ocular conditions, concomitant surgical procedures, and length of follow up. Males received first grafts significantly more frequently than females in the nonatopic group (p=0.029); however, there was no gender difference in repeat grafts. There were no significant differences in the prevalence of endothelial rejection episodes after the first (p= 0.716), second (p>0.999) and three of more grafts (p>0.999). Graft survival between the atopic and nonatopic groups did not differ significantly in the first (p=0.881), second (p=0.752) or three or more graft groups (p=.157). Among first grafts in the atopic group, no statistically significant difference in survival existed among patients analyzed with different manifestations of atopy (p=0.061). One episode of allograft endothelial rejection created a statistically significant difference in ultimate graft survival probability in both the atopic (p=0.003) and nonatopic (p=0.002) groups.
Conclusion
Among keratoconus patients receiving penetrating keratoplasty, there is no statistically significant difference in the prevalence of endothelial graft rejection episodes or probability of graft survival between patients with and without a clinical history of atopy.
Keywords: atopy, keratoconus, penetrating keratoplasty, corneal graft survival
Introduction
Corneal transplantation has been successfully performed for over 100 years and is among the most common forms of solid tissue transplantation with an estimated 42,606 corneal transplants performed in the United States in 2009.1–3 The probability of corneal graft survival in first time recipients approaches 90%, making penetrating keratoplasty (PK) one of the most successful forms of organ transplantation.2,4,5 Immunologic endothelial rejection is the leading cause of corneal graft failure.4, 6,7
Keratoconus is a progressive, noninflammatory corneal ectasia with central thinning. The incidence is approximately 1 per 2,000 in the general population.8 Penetrating keratoplasty (PK) may be indicated to treat keratoconus for various reasons including scarring in the visual axis, insufficient visual acuity with contact lens correction or contact lens intolerance.8–10 Patients undergoing PK for keratoconus can expect a clear corneal graft in greater than 90% of cases.6,11,12
There is a known link between keratoconus and atopic disease.13–17 Atopic patients have immune phenotypes skewed toward the type 2 T helper (Th2) cellular response.13,18 Clinical manifestations of atopy include asthma, allergic rhinitis, seasonal allergic conjunctivitis, atopic keratoconjunctivitis (AKC) and eczema.13, 18
Corneal allograft rejection classically has been considered a type 1 T helper (Th1) cellular response.2,13,14, 19 However, investigators have recently suggested the Th2 cell response could also play a role in mediating transplant rejection after observing eosinophils in rejected cardiac and skin allografts in mice.20,21 To explore the role of atopy and the Th2 immune response in corneal graft rejection, investigators have created murine models that negate the Th1 immune response and are skewed toward the Th2 immune system.2,13,14,19 Grafts in these models experienced rejection suggesting a role for the Th2 immune response in corneal graft rejection.2,13,14, 19 One investigation using a murine model of atopic conjunctivitis suggested that this disorder induced a systemic Th2 response to corneal graft alloantigens with an associated increased incidence and tempo of graft rejection.19 Others have shown in murine models of allergic airway hyperreactivity (AHR) that grafts transplanted during allergic exacerbations failed significantly more frequently than those placed during periods of allergic quiescence.22 This implies that transplantation occurring during periods of active allergy could have a decreased survival probability.
Given these data from animal models, investigators have explored the role of atopy in human corneal endothelial graft rejection and survival. Hargrave et al. noted the presence of eosinophils in failed corneal graft pathology specimens, which implies that the Th2 immune response could play a role in endothelial graft rejection in humans. 14Wagoner et al. investigated the role of atopy in corneal graft rejection and failure by using survival analysis to compare 80 grafts in keratoconus with a history of vernal keratoconjunctivitis (VKC) and 384 cases without VKC.23 VKC is an atopic disorder with a Th2 immune bias.23 No difference in postoperative complications, corneal graft survival or graft rejection was found in this study. 23Yildiz et al. also explored the role of atopy in corneal graft rejection in keratoconus and observed an increased incidence of corneal graft rejection in atopic patients, but this difference failed to reach statistical significance.13
The current study uses survival analysis to assess endothelial rejection episodes and the probability of graft survival in keratoconus patients with and without a history of atopy. Survival analysis will be utilized to determine if there are differences among the various manifestations of atopy including asthma, eczema, allergic rhinitis, seasonal allergic conjunctivitis and AKC.
Methods and Materials
This study was approved by the University of Texas Southwestern (UTSW) Medical Center Institutional Review Board (IRB). The clinical records of patients from six cornea and external disease faculty surgeons at UTSW Medical Center who underwent PK for keratoconus were located by a computerized review based on ICD-9 and CPT codes. This yielded records of 122 patients who received 168 corneal allografts between 1988–2009. The records of each patient were reviewed to determine the presence of a clinical history of atopic disorders including asthma, eczema, allergic rhinitis, seasonal allergic conjunctivitis and AKC.
The diagnosis of keratoconus was based on previously described clinical features.8 Indications for surgery included visual axis scarring, contact lens intolerance or insufficient best corrected visual acuity with spectacles or contact lenses. The procedures were classified as primary grafts or regrafts. Each graft was considered separately for data analysis purposes. Information was gathered on associated preoperative diagnoses, surgical procedures and postoperative courses. Graft rejection episodes were studied separately and in detail. Graft clarity rather than visual acuity was used as the measurement of success.
Donor corneas, surgical technique, and postoperative care
All surgeries were performed by faculty surgeons at the UTSW Medical Center. Donor tissue was obtained through the UTSW transplant services and stored in a standard fashion. Donor buttons were manually trephined with a disposable blade from the endothelial side, 0.25–0.5mm larger than the recipient bed. Recipient tissue was removed by partial thickness trephination and excision with corneal scissors, diamond knives or microrazor blades. Simultaneous cataract extraction with or without intraocular lens placement or other indicated procedures were performed as indicated at the time of PK. The donor button was secured with 10–0 nylon sutures using a variable combination of interrupted and running suture techniques depending upon surgeon preference. Subconjunctival injections of antibiotics and corticosteroids were administered at the conclusion of the procedure and included a combination of gentamicin, vancomycin or cefazolin and methylprednisolone. Postoperative management included topical antibiotics and corticosteroids used at the discretion of the operating surgeon. Typical regimens included topical antibiotics for 1–2weeks and corticosteroids administered hourly for 1–2weeks and gradually tapered at the discretion of the operating surgeon.
Patients were monitored for surgical complications and other comorbidities throughout the postoperative course. Sutures were removed at the surgeon's discretion with most sutures typically being removed by 24 months postoperatively. Cataract was managed surgically when indicated. Glaucoma was managed initially with medical therapy, and surgically when indicated. Wound complications such as dehiscence were repaired as indicated. Phototherapeutic keratectomy (PTK) was performed as necessary to manage postoperative astigmatism. Grafts complicated by infectious processes were cultured and antimicrobial therapy was administered based on these results or the presumptive microbial organism. Cases of herpes simplex keratitis (HSV) were treated with variable combinations of systemic and topical antiviral therapies administered at the surgeon's discretion.
Diagnosis and treatment of allograft endothelial rejection and failure
Endothelial rejection was diagnosed using established criteria.6 Possible endothelial rejection was defined as an acute onset of ciliary flush, anterior chamber reaction and/or keratic precipitates with or without corneal edema in a previously clear graft. Definite rejection was characterized by an endothelial rejection line. Rejections episodes were treated with intensive topical corticosteroids, typically with 1% prednisolone acetate. In some cases, periocular or systemic corticosteroids were administered. The duration and intensity of corticosteroid therapy was adjusted based on the clinical response.
Graft failure was defined as an irreversible loss of clarity in a graft which had previously been clear after surgery. These criteria were used for all causes of failure whether from endothelial rejection or from others causes such as infection. Irreversible rejection was diagnosed if the graft remained edematous after four weeks of intensive topical and/or systemic corticosteroid therapy. The date of failure was estimated from available clinical information. If not documented precisely, it was assumed to have occurred midway between the time of the last examination when the graft was clear and the first examination when the graft was noted to be cloudy.
Statistical and survival analysis
SigmaPlot 11.0 was used for statistical analysis. Comparisons were made between the atopic and nonatopic groups with respect to endothelial rejection episodes in each graft and the Kaplan-Meier probability of graft survival after the initial graft, second graft and third or more grafts. Statistical significance with the Kaplan-Meier method was determined with the Breslow method (p<0.05). Pairwise multiple comparison procedures (Holm-Sidak method) were used to compare multiple curves to determine significance. Continuous variables were compared with the two-tailed Student's t-test. Categorical data were compared with the Fisher exact test. Values were considered statistically significant if p<0.05.
Results
Demographic Data
Recipient demographic data are summarized in Table 1. The records of 122 patients were reviewed and yielded data from 168 total grafts. A clinical history of atopy was reported in 66 (39.2%) of all grafts. Among those with a history of atopy, there were 59 first grafts, 5 second grafts and 2 three or more grafts. There were 102 patients without a history of atopy among which there were 92 first grafts, 6 second grafts and 4 three or more grafts. Bilateral first grafts were performed in 32 patients of which 14 and 18 were atopic and nonatopic, respectively. Bilateral second grafts were required in 1 nonatopic. Among patients receiving first grafts, a statistically significant gender difference existed between the atopic and nonatopic groups with more males receiving grafts in the nonatopic group (p=0.029). Otherwise there was no significant difference in gender, age or duration of follow up between the atopic and the nonatopic groups for first, second or three or more grafts.
Table 1.
Recipient Profile
| First Grafts | Second Grafts | ≥3 grafts | ||||
|---|---|---|---|---|---|---|
| Atopy | No Atopy | Atopy | No Atopy | Atopy | No Atopy | |
| Total Grafts | 59 | 92 | 5 | 6 | 2 | 4 |
| Gender | ||||||
| Male | 26 | 58† | 3 | 4 | 2 | 0 |
| Female | 33 | 34 | 2 | 2 | 0 | 4 |
| Bilateral Grafts | 14 | 18 | 0 | 1 | 0 | 0 |
| Average Age(yrs) | 37.6±12.5 | 39.1±11.8 | 46.8±9.7 | 43.83±17.7 | 38.5±19.1 | 45.75±10.7 |
| Range(yrs) | 14–71 | 18–74 | 36–61 | 27–74 | 25–52 | 30–54 |
| Average follow-up (mos) | 64.6±52.6 | 61.6 ± 50.9 | 56±66.6 | 29±21.2 | 81.5±58.7 | 13.3±6.85 |
Student's t test p>0.05 for all mean values
p<0.05 for Fisher's exact test (gender analysis)
Among the 59 first grafts from patients with manifestations of atopy, 15 (25.4%) had a history of asthma, 15 (25.4%) allergic rhinitis, 20 (33.8%) seasonal allergic conjunctivitis and 2 (3.39%) AKC (Table 2). There were 7 (11.9%) patients with multiple atopic manifestations including asthma with allergic rhinitis (2patients), asthma with seasonal allergic conjunctivitis (3patients), asthma with eczema (1 patient) and allergic rhinitis and seasonal allergic conjunctivitis (1patient). Manifestations of atopy in second grafts included 1 asthma, 2 allergic rhinitis, 1 AKC and 1 with multiple features. Manifestations of atopy in those with three or more grafts included 1 AKC and 1 allergic rhinitis.
Table 2.
Clinical Manifestations of Atopy
| First Grafts | Second Grafts | Three or More Grafts | |
|---|---|---|---|
| Manifestation | |||
| Asthma (%) | 15 (25.4) | 1 (20) | 0 |
| Allergic Rhinitis (%) | 15 (25.4) | 2 (40) | 1 (50) |
| Seasonal Allergic Conjunctivitis (%) | 20 (33.9) | 0 | 0 |
| Atopic Keratoconjunctivitis (%) | 2 (3.4) | 1 (20) | 1 (50) |
| Multiple Atopic Manifestations (%) | 7 (11.9) | 1 (20) | 0 |
| Total | 59 | 5 | 2 |
Associated Diagnoses and Procedures
The atopic and nonatopic groups receiving first grafts (Table 3) did not have a statistically significant difference in preoperative diagnoses of cataract, glaucoma or pseudophakia. The atopic and nonatopic groups receiving regrafts also did not differ significantly with regard to preoperative diagnoses.
Table 3.
| Associated Diagnoses | First graft | Regrafts | ||
|---|---|---|---|---|
| Preoperative Diagnoses | Atopy (n=59) | No Atopy (n=92) | Atopy (n=7) | No Atopy (n=10) |
| Cataract (%) | 3 (5.0) | 5 (5.4) | 0 | 1 (10) |
| Glaucoma (%) | 0 | 0 | 2 (28.6) | 3 (30) |
| Pseudophakia (%) | 0 | 0 | 1 (14.3%) | 2 (20) |
-The difference between the atopic and nonatopic groups for each diagnosis was not significant (P>0.05).
Various surgical procedures were performed concomitantly at the time of initial PK as indicated based on the discretion of the attending surgeon (Table 4). Among first grafts, the atopic and nonatopic groups did not differ significantly with regard to the incidence of PK with concomitant cataract extraction with and without intraocular lens (IOL) placement, peripheral iridectomy, tarsorrhaphy or glaucoma tube shunt (p>0.05). Among those receiving regrafts, there was not a significant difference between the atopic and nonatopic groups with regard to concomitant procedures at the time of PK. The postoperative courses were reviewed in all patients (Table 5). The postoperative courses for first grafts in both the atopic and nonatopic groups did not differ significantly in terms of postoperative cataract, glaucoma, anterior synechia, dehiscence, HSV keratitis, corneal infiltrate, fungal keratitis or retinal detachment (p>0.05). Regrafts in the atopic and nonatopic groups also failed to show a significant difference in the incidence of these postoperative diagnoses (p>0.05). Surgical procedures were performed when indicated in the postoperative period (Table 6). The postoperative courses for first grafts did not differ significantly between the atopic and nonatopic groups with regard to the incidence of cataract surgery, glaucoma tube shut procedure, enucleation, wound dehiscence repair, astigmatism modifying procedures or retinal detachment repair. Enucleation was performed in one case within the nonatopic group due to fungal keratitis that resulted in the patient having no light perception and intractable pain.
Table 4.
| Initial Procedures | First graft | Regrafts | ||
|---|---|---|---|---|
| Atopy (n=59) | No Atopy (n=92) | Atopy (n=7) | No Atopy (n=10) | |
| PK with CE/PCIOL (%) | 3 (5.08) | 3 (3.26) | 0 | 1 (10) |
| PK with CE/aphakia (%) | 0 | 2 (2.17) | 0 | 0 |
| Peripheral Iridectomy (%) | 2 (3.44) | 1 (10.8) | 0 | 0 |
| Tarsorrhaphy (%) | 1 (1.69) | 0 | 0 | 1 (10) |
| Glaucoma Tube Shunt (%) | 0 | 0 | 1 (14.3%) | 0 |
The difference between the atopy and no atopy groups for each diagnosis was not significant (P>0.05).
Table 5.
| Postoperative Diagnoses | First graft | Regrafts | ||
|---|---|---|---|---|
| Atopy (n=59) | No Atopy (n=92) | Atopy (n=7) | No Atopy (n=10) | |
| Cataract (%) | 10 (16.9) | 12 (13.0) | 0 | 3 (30) |
| Glaucoma (%) | 12 (20.3) | 14 (15.2) | 2 (28.6) | 3 (30) |
| Anterior synechia (%) | 0 | 3 (3.2) | 1 (14.3) | 0 |
| Dehiscence (%) | 1 (1.69) | 3 (3.26) | 0 | 0 |
| HSV (keratitis) (%) | 1 (1.69) | 1 (1.09) | 2 (28.6) | 0 |
| Infiltrate (bacterial) (%) | 0 | 2 (2.17) | 0 | 0 |
| Fungal keratitis (%) | 0 | 2 (2.17) | 0 | 0 |
| Retinal Detachment (%) | 0 | 1 (1.09) | 0 | 0 |
-The difference between the atopy and no atopy groups for each diagnosis was not significant (P>0.05).
Table 6.
| Procedures in Postoperative Course | First graft | Regrafts | ||
|---|---|---|---|---|
| Atopy (n=59) | No Atopy (n=92) | Atopy (n=7) | No Atopy (n=10) | |
| Cataract extraction with PCIOL (%) | 6 (10.2) | 4 (4.34) | 0 | 2 (20) |
| Glaucoma Tube Shunt Surgery (%) | 2 (3.44) | 1 (1.09) | 0 | 0 |
| Enucleation (%) | 0 | 1 (1.09) | 0 | 0 |
| Wound Dehiscence Repair (%) | 1 (1.69) | 3 (3.26) | 0 | 0 |
| Astigmatism Modifying Procedure (%) | 2 (3.44) | 2 (2.17) | 0 | 0 |
| Retinal Detachment Repair (%) | 0 | 1 (1.09) | 0 | 0 |
The difference between the atopy and no atopy groups for each diagnosis was not significant (P>0.05).
Survival Analysis
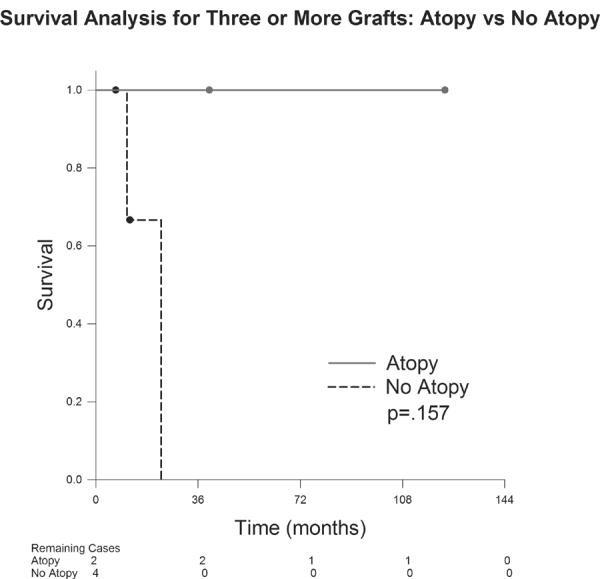
The graft survival probability for first grafts in the atopic group was 98.3% at 12months, 98.3% at 24 months, 95.6% at 60months and 82.1% at 90months (Figure 1). The probability of survival for first grafts in the nonatopic group was 97.8% at 12months, 96.5% at 24months, 93.4% at 60months and 85.7% at 126months. There was not a statistically significant difference in survival probability in first grafts between the atopic and nonatopic groups (p=0.881). Regarding second grafts (Figure 2), the probability of survival in the atopic group was 100% at 12months, 80% at 24months, 80% at 60months and 80% at 174months. The nonatopic group survival probability was 100% at 12months, 80% at 24months, 80% at 60months and 80% at 70months. There was not a statistically significant difference in survival probability between the atopic and nonatopic groups (p=0.752). Patients receiving three or more corneal grafts (Figure 3) in the atopic group demonstrated a 100% survival probability at 12months, 24 months, 60months and at 123months. Those in the nonatopic group demonstrated a 1year survival of 66.7%. No grafts survived beyond 23 months in this group. There was no statistically significant difference in survival probability between the atopic and nonatopic groups (p=0.157).
Figure 1.
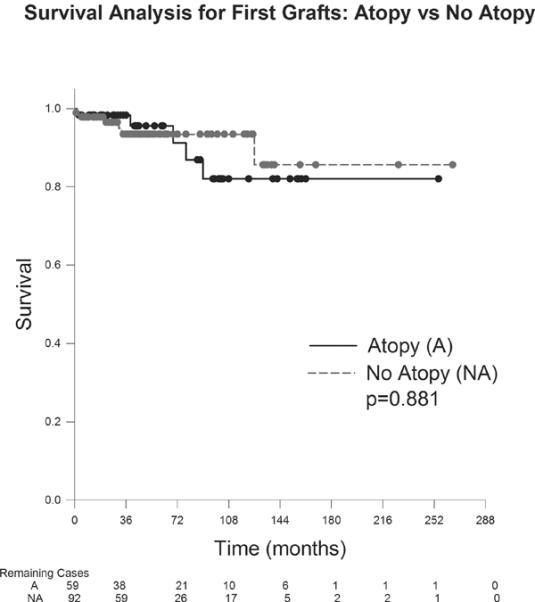
Figure 2.
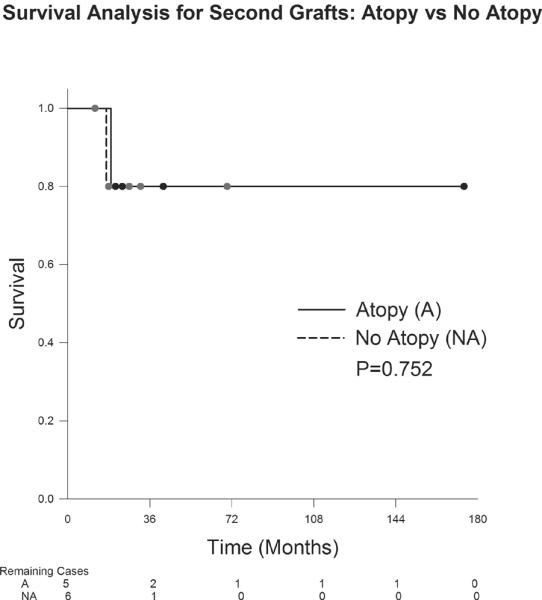
Figure 3.
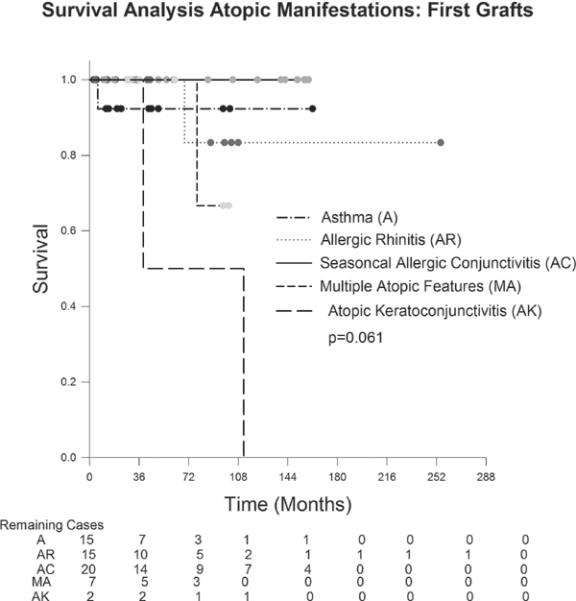
Survival analysis was also performed on first grafts in the atopic group to determine if the various manifestations of atopy had different survival probabilities. (Figure 4). Grafts survival probability for each manifestation of atopy did not differ significantly (p= 0.061). However, only 2 patients were included in the AKC analysis, both of which failed.
Figure 4.
Allograft Rejection
Grafts in the atopic and nonatopic groups experienced endothelial rejection in 20 (30.4%) and 32 (31.3%) cases, respectively. This provided a cumulative 52(30.9%) prevalence of endothelial graft rejection in this study. Among first grafts in the atopic group, (Table 7), 16 (27.1%) experienced one episode of rejection and 1 (1.69%) experienced two or more episodes. Among first grafts in the nonatopic group, one rejection episode occurred in 28 (30.4%) of cases and two or more rejection episodes occurred in 1 (1.09%) case. The prevalence of endothelial rejection episodes in first grafts did not differ significantly between the atopic and nonatopic groups (p=0.7161). Regrafts in the atopic group experienced one rejection episode in 4 (57.1%) cases and no cases of two or more episodes occurred. Among regrafts in the nonatopic group, 4 (40%) experienced one episode of rejection and none experienced two or more rejections. There was no statistically significant difference in the prevalence of endothelial rejection episodes in the regrafts between the atopic and nonatopic groups (p>0.999). The incidence of two or more rejection episodes also did not differ significantly for first grafts (p>0.999) or regrafts (p>0.999) between the atopic and nonatopic groups.
Table 7.
| Allograft Rejection Episodes | First Grafts | Regrafts | ||
|---|---|---|---|---|
| Atopy | No Atopy | Atopy | No Atopy | |
| Total No. of grafts | 59 | 92 | 7 | 10 |
| Grafts with 1 rejection episode | 16 (27.1) | 28 (30.4) | 4 (57.1) | 4 (40) |
| ≥2rejection episode | 1 (1.69) | 1 (1.09) | 0 | 0 |
The difference between first grafts and regrafts for both groups was not significant.
The difference between the atopy and no atopy groups, for first grafts and regrafts, was not significant (p>.05, Fisher Exact test)
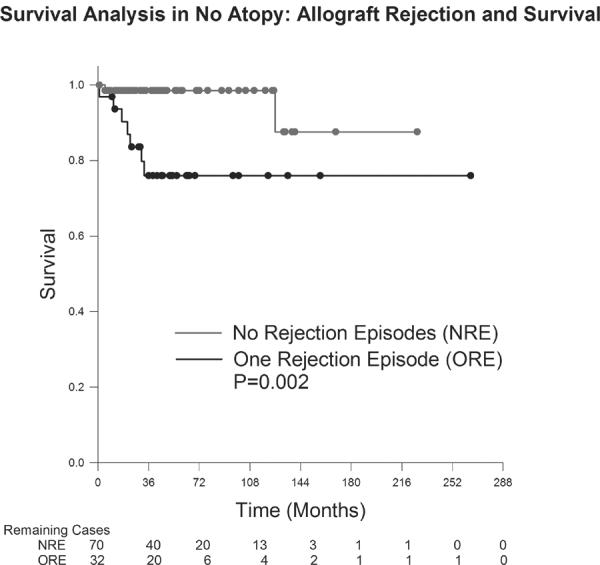
Figures 5 and 6 demonstrate the influence of endothelial rejection on survival. A single episode of endothelial rejection significantly affected the survival probability of grafts in the atopic group (p=0.003); this findings was also seen in the nonatopic group with a single episode of rejection significantly influencing survival (p=0.002). Both the atopic and nonatopic group involved only one case each of 2 episodes of allograft rejection and neither of these grafts failed.
Figure 5.
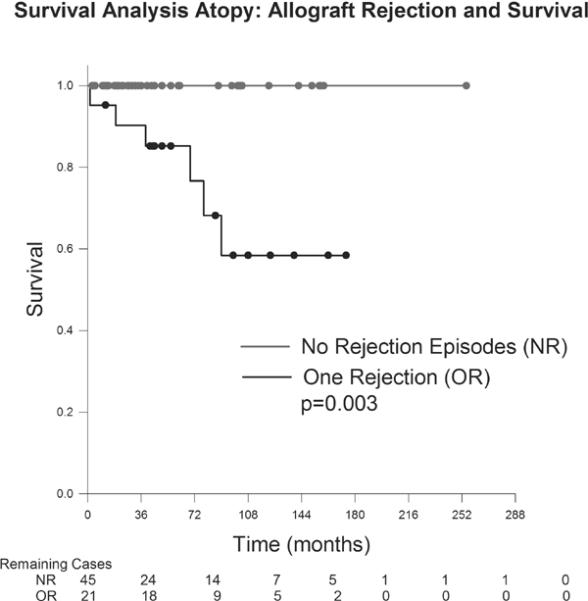
Figure 6.
Causes of graft failure
All 6 cases of graft failure in the atopic group resulted from endothelial graft rejection. The nonatopic group involved 9 graft failures, 7 (77.8%) from rejection and 2 (22.2%) from fungal keratitis. One of these cases of fungal keratitis resulted in an eye with no light perception and intractable pain leading to enucleation. Grafts experiencing endothelial rejection episodes were significantly more likely to fail in the atopic (p= 0.0017) and nonatopic groups (p= 0.0278). Fungal keratitis was a significant risk factor for graft failure in the nonatopic group (p= 0.0278). Corticosteroid therapy was too variable to enable the determination of its impact on the incidence or timing of rejection episodes or to comment on differences between the various groups.
Discussion
Our study compared the preoperative and postoperative courses of the atopic and nonatopic groups. No statistically significant difference in terms of preoperative diagnoses, initial procedures, postoperative complications or procedures was noted between the atopic and nonatopic group. Wagoner et al. explored the postoperative course of patients receiving PK for keratoconus with and without VKC and found no difference in the incidence of postoperative glaucoma, cataract, secondary cataract and infectious keratitis between the two groups.23 These results are consistent with our study.
Survival analysis was performed to compare graft survival probability in the atopic and nonatopic groups. There was not a statistically significant difference in survival probability for the first, second or three or more grafts groups when the atopic and nonatopic groups were compared. This is consistent with the survival analysis performed by Wagoner et al. in which no difference was found between patients with and without a history of atopy.23Survival analysis for first grafts within the atopic group comparing the various manifestations of atopy failed to demonstrate a statistically significant difference in survival probability. The sample size of the AKC cohort receiving first grafts included only 2. The literature has reported a poor prognosis for cornea transplants in AKC.24 The limited sample size in the AKC cohort could explain why there was not a statistically significant difference between this group and the other atopic manifestations.
Despite the lack of survival difference between the atopic and nonatopic groups and the various manifestations of atopy,, one should still consider the data from Niederkorn et al. when considering PK in atopic patients.22 In this animal model of airway hyperreactivity, performing PK during periods of active allergy resulted in a significant increased incidence of graft rejection and failure. However, no difference in survival was noted if transplantation was delayed 30days from the time of allergic stimulation during a period of immunologic quiescence.22It may be prudent to extrapolate this concept to humans, and delay transplantation in atopic patients until periods of active allergy have resolved and immunologic quiescence is maximized.
The prevalence of endothelial graft rejection episodes in this study was 30.9% with graft rejection episodes occurring in 30.4% and 31.3% of the atopic and nonatopic groups, respectively. This difference was not statistically significant (p>0.999). The literature reports a variable prevalence of at least one graft rejection episode ranging from 4.3–31%.9, 13, 25, 26 Additionally, Yildiz et al. reported that atopic patients had an increased incidence of graft rejection; however, this difference failed to reach statistical significance.13In this study, endothelial rejection was also the most common cause of graft failure in both the atopic and nonatopic groups. Fungal keratitis was a significant risk factor for graft failure in the nonatopic group. One case of fungal keratitis required enucleation as the eye reached no light perception and developed intractable pain.
Survival analysis was performed comparing grafts with and without a history of endothelial graft rejection. In both the atopic and the nonatopic groups, those with one episode of endothelial rejection developed a statistically significant decrease in graft survival. This finding differs from the data from Epstein et al. in which a single rejection episode was not associated with a significant decrease in graft survival.6Limitations of this study include its retrospective design. Sample size also limited the statistical analysis in some subgroups. For example, the AKC cohort among first grafts did not demonstrate a statistically significant difference in survival compared to the other atopic manifestation despite the previously reported poor prognosis of grafts in this disorder.24 This is likely explained by the fact that there were only two primary grafts transplanted into patients with AKC in this study.
In conclusion, in keratoconus patients receiving PK, these data suggest that a clinical history of atopy does not provide a statistically significant difference in endothelial graft rejection prevalence or graft survival probability in comparison to those without a history of atopy. Furthermore, among those with a history of atopy, no statistically significant difference in survival was noted between the various manifestations of atopy.
Acknowledgments
Support in part by: National Institutes of Health Infrastructure Grant EY020799, and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Footnotes
Disclosures: The authors have no proprietary or commercial interest in any materials discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Niederkorn JY, Larkin DF. Immune privilege of corneal allografts. Ocul Immunol Inflamm. 2010 Jun;18(3):162–71. doi: 10.3109/09273948.2010.486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hargrave SL, Hay C, Mellon J, Mayhew E, Niederkorn JY. Fate of MHC-matched corneal allografts in Th1-deficient hosts. Invest Ophthalmol Vis Sci. 2004 Apr;45(4):1188–93. doi: 10.1167/iovs.03-0515. [DOI] [PubMed] [Google Scholar]
- 3.Eye Bank Association of America 2009 Statistics [Google Scholar]
- 4.Batchelor JR, Casey TA, Werb A, Gibbs DC, Prasad SS, Lloyd DF, James A. HLA matching and corneal grafting. Lancet. 1976 Mar 13;1(7959):551–4. doi: 10.1016/s0140-6736(76)90355-x. [DOI] [PubMed] [Google Scholar]
- 5.Völker-Dieben HJ, Schreuder GM, Claas FH, Doxiadis, Schipper RF, Pels E, Persijn GG, Smits J. D'Amaro Histocompatibility and corneal transplantation. J. Dev Ophthalmol. 2003;36:22–41. doi: 10.1159/000067653. [DOI] [PubMed] [Google Scholar]
- 6.Epstein RJ, Seedor JA, Dreizen NG, Stulting RD, Waring GO, III, Wilson LA, Cavanagh HD. Penetrating keratoplasty for herpes simplex keratitis and keratoconus. Allograft rejection and survival. Ophthalm. 1987 Aug;94(8):935–44. doi: 10.1016/s0161-6420(87)33356-1. [DOI] [PubMed] [Google Scholar]
- 7.Niederkorn JY. Immunology and immunomodulation of corneal transplantation. Int Rev Immunol. 2002 Mar-Jun;21(2–3):173–96. doi: 10.1080/08830180212064. [DOI] [PubMed] [Google Scholar]
- 8.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998 Jan-Feb;42(4):297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 9.Brierly SC, Izquierdo L, Jr, Mannis MJ. Penetrating keratoplasty for keratoconus. Cornea. 2000 May;19(3):329–32. doi: 10.1097/00003226-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Zadok D, Schwarts S, Marcovich A, Barkana Y, Morad Y, Eting E, Avni I. Penetrating keratoplasty for keratoconus: long-term results. Cornea. 2005 Nov;24(8):959–61. doi: 10.1097/01.ico.0000159729.51015.b4. [DOI] [PubMed] [Google Scholar]
- 11.Paglen PG, Fine M, Abbott RL, Webster RG., Jr The prognosis for keratoplasty in keratoconus. Ophthalmol. 1982;89:651–654. doi: 10.1016/s0161-6420(82)34753-3. [DOI] [PubMed] [Google Scholar]
- 12.Fukuoka S, Honda N, Ono K, Mimura T, Usui T, Amano S. Extended long-term results of penetrating keratoplasty for keratoconus. Cornea. 2010 May;29(5):528–30. doi: 10.1097/ICO.0b013e3181c29705. [DOI] [PubMed] [Google Scholar]
- 13.Yildiz EH, Erdurmus M, Hammersmith KM, Rapuano CJ, Laibson PR, Cohen EJ. Comparative study of graft rejection in keratoconus patients with and without self-reported atopy. Cornea. 2009 Sep;28(8):846–50. doi: 10.1097/ICO.0b013e318197eedc. [DOI] [PubMed] [Google Scholar]
- 14.Hargrave S, Chu Y, Mendelblatt D, Mayhew E, Niederkorn J. Preliminary findings in corneal allograft rejection in patients with keratoconus. Am J Ophthalmol. 2003;135:452–460. doi: 10.1016/s0002-9394(02)02055-x. [DOI] [PubMed] [Google Scholar]
- 15.Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991–994. [PubMed] [Google Scholar]
- 16.Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834–836. doi: 10.1136/bjo.84.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison RJ, Klouda PT, Easty DL, Manku M, Charles J, Stewart CM. Association between keratoconus and atopy. Br J Ophthalmol. 1989;73:816–82. doi: 10.1136/bjo.73.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hargrave SL, Mellon J, Niederkorn J. MHC matching improves corneal allograft survival in mice with Th2-immune bias. Transplant Proc. 2002 Dec;34(8):3413–5. doi: 10.1016/s0041-1345(02)03612-6. [DOI] [PubMed] [Google Scholar]
- 19.Beauregard C, Stevens C, Mayhew E, Niederkorn JY. Cutting edge: atopy promotes Th2 responses to alloantigens and increases the incidence and tempo of corneal allograft rejection. J Immunol. 2005 Jun 1;174(11):6577–81. doi: 10.4049/jimmunol.174.11.6577. [DOI] [PubMed] [Google Scholar]
- 20.Le Moine A, Flamand V, Demoor FX, Noël JC, Surquin M, Kiss R, Nahori MA, Pretolani M, Goldman M, Abramowicz D. Critical roles for IL-4, IL-5, and eosinophils in chronic skin allograft rejection. J Clin Invest. 1999 Jun;103(12):1659–67. doi: 10.1172/JCI5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanBuskirk AM, Wakely ME, Orosz CG. Transfusion of polarized TH2-like cell populations into SCID mouse cardiac allograft recipients results in acute allograft rejection. Transplantation. 1996 Jul 27;62(2):229–38. doi: 10.1097/00007890-199607270-00014. [DOI] [PubMed] [Google Scholar]
- 22.Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic airway hyperreactivity increases the risk for corneal allograft rejection. Am J Transplant. 2009 May;9(5):1017–26. doi: 10.1111/j.1600-6143.2009.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagoner MD, Ba-Abbad R, King Khaled Eye Specialist Hospital Cornea Transplant Study Group Penetrating keratoplasty for keratoconus with or without vernal keratoconjunctivitis. Cornea. 2009 Jan 28;1:14–8. doi: 10.1097/ICO.0b013e31818225dd. [DOI] [PubMed] [Google Scholar]
- 24.Ghoraishi M, Akova YA, Tugal-Tutkun I, Foster CS. Penetrating keratoplasty in atopic keratoconjunctivitis. Cornea. 1995 Nov;14(6):610–3. [PubMed] [Google Scholar]
- 25.Lim L, Pesudovs K, Coster DJ. Penetrating keratoplasty for keratoconus: visual outcome and success. Ophthalmology. 2000 Jun;107(6):1125–31. doi: 10.1016/s0161-6420(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 26.Sharif KW, Casey TA. Penetrating keratoplasty for keratoconus: complications and long term success. Br J Ophthalmol. 1991 Mar;75(3):142–6. doi: 10.1136/bjo.75.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]


