Abstract
Advancing age is the major risk factor for the development of CVD (cardiovascular diseases). This is attributable, in part, to the development of vascular endothelial dysfunction, as indicated by reduced peripheral artery EDD (endothelium-dependent dilation) in response to chemical [typically ACh (acetylcholine)] or mechanical (intravascular shear) stimuli. Reduced bioavailability of the endothelium-synthesized dilating molecule NO (nitric oxide) as a result of oxidative stress is the key mechanism mediating reduced EDD with aging. Vascular oxidative stress increases with age as a consequence of greater production of reactive oxygen species (e.g. superoxide) without a compensatory increase in antioxidant defences. Sources of increased superoxide production include up-regulation of the oxidant enzyme NADPH oxidase, uncoupling of the normally NO-producing enzyme, eNOS (endothelial NO synthase) (due to reduced availability of the cofactor tetrahydrobiopterin) and increased mitochondrial synthesis during oxidative phosphorylation. Increased bioactivity of the potent endothelial-derived constricting factor ET-1 (endothelin-1), reduced endothelial production of/responsiveness to dilatory prostaglandins, the development of vascular inflammation, formation of AGEs (advanced glycation end-products), an increased rate of endothelial apoptosis and reduced expression of oestrogen receptor α (in postmenopausal females) also probably contribute to impaired EDD with aging. Several lifestyle and biological factors modulate vascular endothelial function with aging, including regular aerobic exercise, dietary factors (e.g. processed compared with non-processed foods), body weight/fatness, vitamin D status, menopause/oestrogen deficiency and a number of conventional and non-conventional risk factors for CVD. Given the number of older adults now and in the future, more information is needed on effective strategies for the prevention and treatment of vascular endothelial aging.
Keywords: aging, endothelium-dependent dilation, exercise, inflammation, nitric oxide, oxidative stress
INTRODUCTION
Despite reductions in death rates from CVD (cardiovascular diseases) over the last four decades, CVD remain the leading cause of morbidity and mortality in modern societies [1]. What is less appreciated, perhaps, is that the great majority of CVD are associated with dysfunction of arteries [1].
The effect of aging on CVD is illustrated simply, but powerfully, by the observation that the risk of CVD increases progressively with age [1]. As such, advancing age is the major risk factor for CVD, and in a broad sense, CVD are diseases of aging. Taken together, these facts lead to the conclusion that there is something about aging that causes dysfunction of arteries, which, in turn, increases the risk of developing CVD [2].
Given the current and projected increases in the number of older adults, we face the possibility of a ‘new wave’ of CVD in the near future with an associated increase in healthcare burden. As such, establishing a better understanding of the relationship between arterial aging and CVD represents one of our most important clinical challenges. Determining how arteries change with age to increase our risk of CVD, the mechanisms by which these changes are mediated and strategies for the prevention and treatment of arterial aging are, therefore, among our highest biomedical priorities.
VASCULAR ENDOTHELIAL DYSFUNCTION AND CVD RISK
Several changes to arteries probably contribute to the increase in CVD risk with aging. One of the most clinically important of these is the development of vascular endothelial dysfunction [2,3].
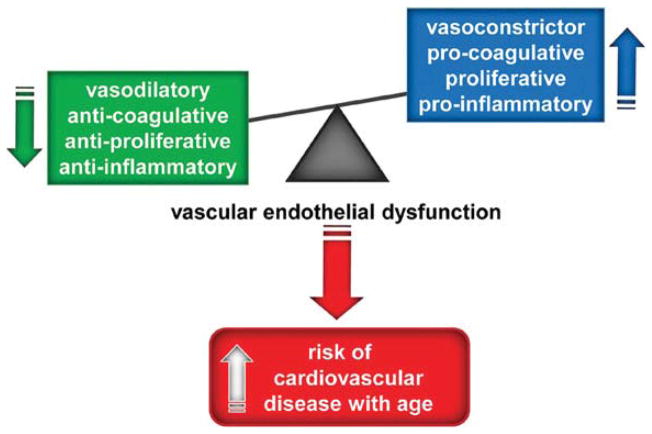
The vascular endothelium is a single layer of cells lining blood vessels that plays a key role in regulating the function and health of arteries [4,5]. Vascular endothelial cells synthesize and release a wide array of biologically active molecules that act in an autocrine or paracrine fashion to modulate arterial structure and vasodilatory, thrombolytic and vasoprotective functions. Arterial endothelial dysfunction refers to functional alterations in the normal endothelial phenotype of arteries that may contribute to the development and clinical expression of atherosclerosis and other vascular disorders [4–6]. These alterations include a shift to a vasoconstrictor, procoagulation, proliferative and pro-inflammatory state [7,8] (Figure 1).
Figure 1. Vascular endothelial dysfunction and risk of CVD with aging.
Vascular endothelial dysfunction is characterized by a shift from a vasodilatory, anti-coagulative, anti-proliferative and anti-inflammatory state to a vasoconstrictor, pro-coagulative, pro-proliferative and pro-inflammatory state, leading to an increased risk of cardiovascular disease with aging.
Vascular endothelial dysfunction is observed in several forms of clinical CVD [4,5,9]. Endothelial dysfunction is also associated with major CVD risk factors including smoking, hypercholesterolaemia, hypertension, hyperglycaemia, diabetes, obesity, chronic inflammation and a family history of premature vascular occlusive diseases [4,5]. Vascular endothelial dysfunction is viewed as a key antecedent of clinical arterial diseases and serves as a marker of the inherent risk of developing CVD in an individual or group [5,10]. Given its central role in the development of clinical coronary, cerebrovascular and peripheral artery diseases, vascular endothelial dysfunction is considered an important therapeutic target for reducing the risk of CVD morbidity and mortality [5,11].
ASSESSMENT OF VASCULAR ENDOTHELIAL FUNCTION
Because of the range of biological effects of the vascular endothelium, arterial endothelial function can be assessed using several different approaches that include measuring fibrinolytic function, leucocyte adhesion and inflammatory markers [12–14]. However, the most common approach is to determine vasodilation in response to an endothelium-dependent stimulus, i.e. EDD (endothelium-dependent dilation) [5,8]. Although coronary EDD has been assessed in patients with heart disease and in subjects undergoing diagnosis for coronary disease [15–17], in general, EDD of peripheral arteries has been used to assess vascular endothelial function in humans [4,8]. There are limited data suggesting that peripheral EDD correlates with EDD measured in the coronary arteries [18,19] and, thus, may reflect disease processes in the coronary circulation.
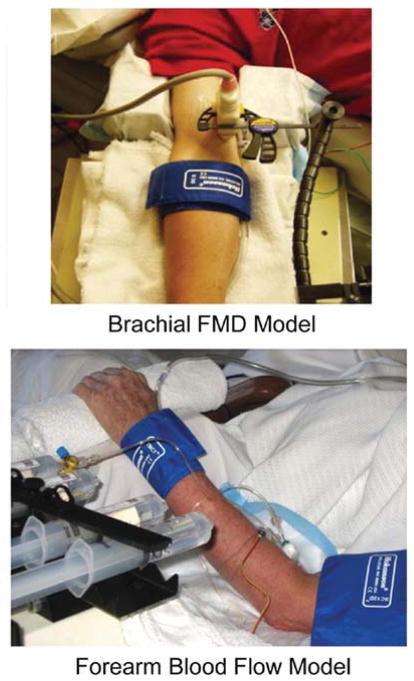
In human subjects, peripheral artery EDD is assessed by two primary methods (Figure 2). These methods, including their respective strengths and limitations, have been described in detail elsewhere [5,8,21,22,26].
Figure 2. Models to assess EDD in humans.
Brachial artery FMD and FBF models for assessing EDD.
One approach is to use a chemical stimulus to evoke EDD [20]. A pharmacological agonist for NO (nitric oxide) synthesis and release from the vascular endothelium [most often ACh (acetylcholine)] is infused into an artery of a limb (usually the brachial artery), and the consequent increase in blood flow to the distal portion of the limb (usually the forearm) is measured using venous occlusion plethysmography [21,22]. A dose–response relationship is established, and group or condition differences are identified either by the slopes of the dose–response curves or the peak blood flows attained. The increase in blood flow reflects the dilation occurring in the resistance vessels (arterioles) of the distal limb. Thus this technique measures EDD of peripheral resistance vessels in response to a chemical stimulus.
The other method uses a mechanical stimulus to evoke an EDD [23]. This approach involves inflating a cuff on a limb (typically the upper forearm) to a suprasystolic external pressure for several minutes and measuring the dilation in a segment of an artery (typically the brachial artery) proximal to the occlusion in response to the acute increase in blood flow produced by rapid deflation of the cuff [24]. The ischaemia-evoked dilation of resistance vessels distal to the occlusion produces a marked temporary increase in blood flow (‘hyperaemic stimulus’) in the proximal conduit arteries that, in turn, causes a FMD (‘flow-mediated dilation’) of those arteries. Thus this procedure assesses the ability of peripheral conduit arteries to dilate in response to the physiological stimulus of an acute increase in intravascular shear produced by an increase in blood flow [25]. Because FMD is a function of the hyperaemic stimulus [25], for proper interpretation, hyperaemia should be assessed and used as a covariate if differences exits. [26–28].
Studies performed in experimental animals have established that the responses evoked by both approaches are ‘endothelium-dependent’ because they are abolished after removal of the vascular endothelium [20,29]. Moreover, the responses are primarily (although not completely) mediated by vascular endothelial production and release of NO because they are markedly attenuated by administration of agents that inhibit NO synthesis by eNOS (endothelial NO synthase), such as L-NMMA (NG-monomethyl-L-arginine) [30,31]. Vasodilatory prostaglandins and endothelium-derived hyperpolarizing factors are considered to be the other endothelium-derived dilators that contribute to EDD [32,33].
In both experimental approaches, the possibility that group or condition differences observed are due to other (i.e. ‘endothelium-independent’) mechanisms is assessed by determining the vasodilatory responses to intra-arterial infusion of SNP (sodium nitroprusside) (i.a. infusion model) or sublingual administration of nitroglycerine (FMD model). These drugs serve as ‘NO donors’, thus providing a measure of the sensitivity of the vascular smooth muscle cells in the arterial wall to NO [29,34]. An absence of group or condition differences in response to endothelium-independent stimuli in the presence of clear differences in endothelium-dependent responses are interpreted as indicative of vascular endothelium-specific abnormalities in vasodilatory responsiveness.
Both ACh-induced increases in FBFACh [FBF (forearm blood flow) in response to ACh infusion] and brachial artery FMD provide important clinical insight into the overall health and functional integrity of the vascular endothelium. This conclusion is supported by the facts that both methods identify differences in vascular endothelial function in healthy adults in response to acute conditions that impair or augment EDD [35,36] or compared with adults with risk factors (e.g. chronic smoking, insulin resistance, etc.) and patients with CVD [4,30]. These techniques also predict future CV events, disease and/or prognosis in adults who are healthy at baseline [37–39] as well as in patients with CVD [40–42].
Despite these common features, however, the two methods appear to measure different properties of vascular endothelial vasodilatory capacity in that the responses are not consistently related within the same individuals [43–45]. This may be due, in part, to the fact that arteriolar and conduit (large) artery function respectively is being assessed [5,43].
FOCUS OF THE PRESENT REVIEW
The present review will focus on the development of vascular endothelial dysfunction with human aging as reflected by impaired EDD. Our emphasis will be on findings available in healthy adults to provide as much insight as possible into the effects of aging as opposed to co-morbidities associated with aging. Observations made from experiments on animal models of arterial aging will be used to highlight cellular and molecular mechanisms. The latter portion of the review will emphasize modulating factors and strategies for the prevention and treatment of vascular endothelial dysfunction with aging.
VASCULAR ENDOTHELIAL DYSFUNCTION WITH AGING
Several lines of experimental evidence indicate that vascular endothelial dysfunction develops with aging in humans in the absence of clinical CVD and major risk factors for CVD. Impaired EDD, reduced fibrinolytic function, increased leucocyte adhesion and/or other markers of endothelial dysfunction have been observed in older compared with young adult humans, as well as rodents and non-human primates [3,46,47].
Chemical stimulation of peripheral artery EDD with aging
In humans, peak FBFACh decreases progressively with age, and this is observed in both sexes [48–52]. Based on available data, the slope of the decline appears to be less steep in women during the premenopausal years compared with men of similar age, whereas the rate of decrease with age in postmenopausal women is similar to men [48,49,51]. In humans, the impairment in chemically stimulated EDD appears to be agonist specific, as it is not observed with other endothelium-dependent dilators including bradykinin, substance P and isoproterenol [53]. Moreover, unlike FBF, responses to ACh are not obviously reduced with aging in the femoral artery of humans [52], although a ‘systemic’ arterial impairment of EDD has been established with age in mice [54]. These observations suggest that aging may have less of an effect on arteries in the leg compared with the arm, possibly as a result of differences in hydrostatic forces, activity patterns or other reasons. Although physiologically interesting, the clinical importance of such differences are less certain because the forearm, not the femoral, blood flow response to ACh is a predictor of future CVD risk [42,55,56].
FMD of peripheral arteries with aging
Brachial artery FMD is impaired in older compared with young healthy adults [57–63], although such observations can be affected by the methods used [28]. Brachial artery FMD may be preserved in men until approx. 40 years of age and in women until their early 50s [57]. Thereafter the rate of decline may be greater in men than women, although brachial artery FMD consistently is reduced even in healthy men and women by approx. 65 years of age [57]. Although data exist to the contrary [64], decreases in brachial artery FMD with aging appear to be independent of any reductions in the hyperaemic stimulus [60,63,65,66].
Impaired FMD with age also has been observed in the leg in humans [64]. In experimental animals, heterogeneity has been reported in the reductions of FMD in large arteries with age, and this is associated with artery-specific differences in enzymes involved in producing endothelium-modulating factors, including eNOS and SOD (superoxide dismutase) [67]. In rodents, age-related impairments in EDD differ between arterioles in the same tissue or organ (e.g. skeletal muscle) as a result of differences in oxidative capacity and basal blood flow patterns [31].
Coronary and renal artery EDD with aging
EDD in response to ACh is reduced with age in adult humans in both the large epicardial coronary arteries and in coronary resistance vessels [68–70]. In some cases, a paradoxical vasoconstriction of the coronary arteries is observed in older adults [17]. Unlike the large arteries, coronary resistance arteries do not develop atherosclerosis, suggesting that impaired EDD may precede this pathophysiological process. As observed in peripheral arteries, reductions in EDD with age in the coronary circulation are observed in adults without major risk factors or clinical disease [71], consistent with a primary effect of aging.
There is evidence that EDD in the renal circulation also is reduced with aging [72]. Moreover, recent findings indicate that EDD in response to ACh in peripheral arteries is a predictor of future decline in glomerular filtration rate in patients with essential hypertension [73]. However, presently, there is little information concerning the relationship between vascular endothelial and renal dysfunction with aging in the absence of clinical disease.
MECHANISMS OF IMPAIRED EDD WITH AGING
Vascular smooth muscle sensitivity to NO
In general, evidence from studies performed on healthy adults indicates that the vasodilatory responses to NO donors are unchanged with age [50,51,74]. Data from earlier investigations suggested a decrease in endothelium-independent dilation with age, albeit not as great as observed for EDD [48,49,52,75]; however, the subjects studied tended to have a more adverse CVD risk factor profile compared with those assessed in more recent investigations. Overall, it does not appear that reduced vascular smooth muscle sensitivity to NO contributes to reductions in EDD with aging in healthy adults.
NO bioavailability
Data in both humans and experimental animals indicate that impaired EDD with aging is mediated by a decrease in NO bioavailability [31,54,76,77]. This is supported by the facts that the reduction in EDD produced by pharmacological inhibition of NO production by eNOS is smaller with advancing age, and there no longer are significant age group differences in EDD in the absence of NO synthesis [31,54,76].
The mechanisms underlying reduced NO-mediated EDD with age could involve decreased stimulus (pharmacological or flow)-evoked NO production, increased NO removal (see below) or both. The exact contributions of altered NO production compared with removal during EDD are unknown. NO production is reduced in older compared with young adults under baseline resting conditions, as indicated by reduced vasoconstriction in response to infusion of L-NMMA [74]. Moreover, NO production in response to an increase in shear stress is reduced in old animals and contributes to impaired NO-mediated EDD [78].
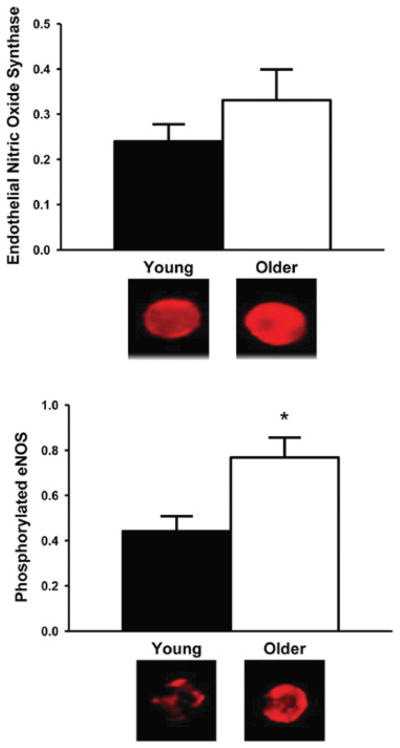
Despite consistent observations of reduced NO bioavailability, analysis of arterial tissue in experimental animals indicates decreased, increased or unchanged eNOS expression and/or activation (i.e. phosphorylation at Ser1177) with aging [76,79–81]. In healthy humans, eNOS protein expression tends to be greater in vascular endothelial cells obtained from the brachial artery of older compared with young adults [65], whereas eNOS phosphorylated at Ser1177 is significantly increased, suggesting a greater state of activation of the enzyme with aging [65] (Figure 3). If so, such activation with age in healthy adults may represent an attempt to compensate for low NO bioavailability.
Figure 3. Endothelial eNOS protein expression and activation with aging in men.
In vascular endothelial cells collected from the brachial artery of healthy human subjects, eNOS protein expression tends to be greater in older compared with young subjects, whereas eNOS phosphorylated at Ser1177 is significantly increased, suggesting a greater state of activation of the enzyme with aging. *P < 0.05 compared with young subjects; values are ratios to HUVEC control; representative images are shown below the histograms. Reproduced from [65], with permission. © (2009) The American Physiological Society.
BH4 (tetrahydrobiopterin) bioactivity
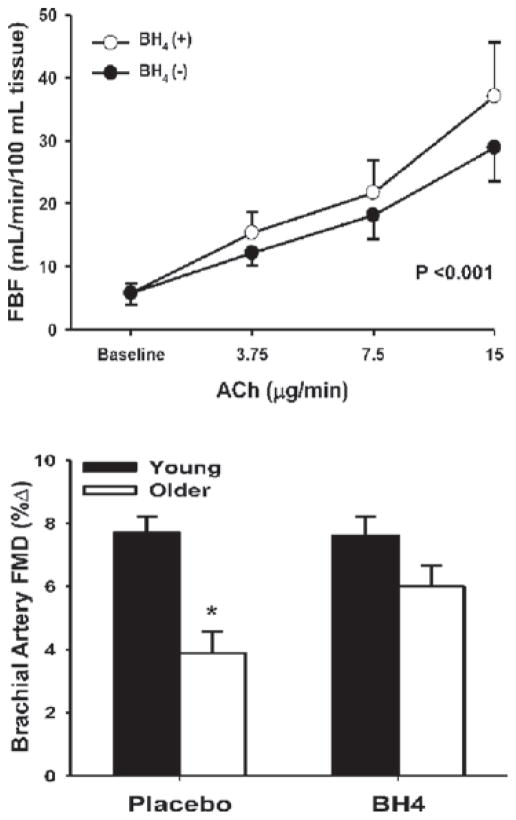
BH4 is an essential cofactor for NO synthesis by eNOS [82]. Inadequate availability of BH4 results in ‘uncoupling’ of eNOS and synthesis of superoxide anion instead of NO [83–85]. Administration of BH4 to young and older humans causes a selective improvement in EDD in older adults [60,86] (Figure 4). The EDD-enhancing effect of BH4 administration in older adults is abolished by L-NMMA [86], suggesting that it is mediated by augmenting NO bioavailability. The mechanism responsible for the reduction in BH4 bioactivity with aging is unclear. In rodents, BH4 concentrations in arteries have been reported to be either reduced [87,88] or unchanged [89] with aging. However, consistent with observations in humans, augmenting vascular BH4 bioavailability in skeletal muscle arterioles of old rats restores NO-mediated flow-induced dilation [87].
Figure 4. BH4 and impaired EDD with aging in men.
Administration of BH4 improves endothelium-dependent dilation in middle-aged/older adults, as measured by FBF in response to ACh (upper panel) and brachial artery FMD (lower panel). *P < 0.05 compared with young adults. The upper panel was reprinted from Atherosclerosis, volume 186, Higashi, Y., Sasaki, S., Nakagawa, K., Kimura, M., Noma, K., Hara, K., Jitsuiki, D., Goto, C., Oshima, T., Chayama, K. and Yoshizumi, M., Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production, pp. 390–395, copyright (2006), with permission from Elsevier (http://www.sciencedirect.com/science/journal/00219150). The lower panel was re-drawn from data in [60].
ADMA (asymmetric dimethylarginine) and arginase
ADMA, which reduces NO synthesis by competing with the substrate L-arginine for binding sites on eNOS, is increased in some CVD states. However, ADMA is not obviously increased with aging in the absence of disease [63]. Acute L-arginine administration, which should restore any deficit in L-arginine competitive binding to eNOS, does not increase brachial artery FMD in healthy older adults [63], although small increases in FMD have been reported in adults >70 years of age after 14 days of oral supplementation [90]. The lack of a clear role for ADMA in mediating impaired EDD with aging in humans may be explained, in part, by the fact that ADMA and the enzyme that controls its degradation, dimethylarginine dimethylaminohydrolase II, do not differ in vascular endothelial cells from older compared with young adults [63].
There is evidence in rats that the activity of arginase, an enzyme that competes with eNOS for L-arginine, is increased in arteries with aging and contributes to impaired EDD in aortic rings [91–94]. However, neither inhibition of arginase nor administration of L-arginine improves flow-mediated EDD in skeletal muscle arterioles of old rats, whereas increasing BH4 restores function [87]. As emphasized elsewhere [92], it is possible that differences in the arteries studied contribute to such differences in mechanisms of vascular endothelial dysfunction with aging.
Oxidative stress
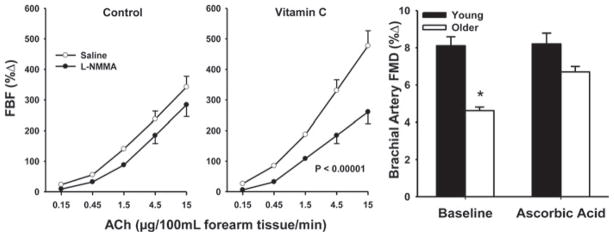
Based on markers of oxidant damage such as nitration of tyrosine residues on proteins (nitrotyrosine), oxidative stress is observed with aging in vascular endothelial cells of humans [59] and arteries of experimental animals [54,76,80,95,96]. Several lines of evidence suggest that development of oxidative stress contributes to vascular endothelial dysfunction with aging. In healthy adults varying in age, brachial artery FMD is inversely related to circulating markers of oxidative stress [61,97], as well as to nitrotyrosine staining in vascular endothelial cells [59] (Figure 5). Acute administration of antioxidants such as vitamin C selectively improves or restores EDD in older adults [58,74] (Figure 6).
Figure 5. Endothelial nitrotyrosine and EDD with aging in men.
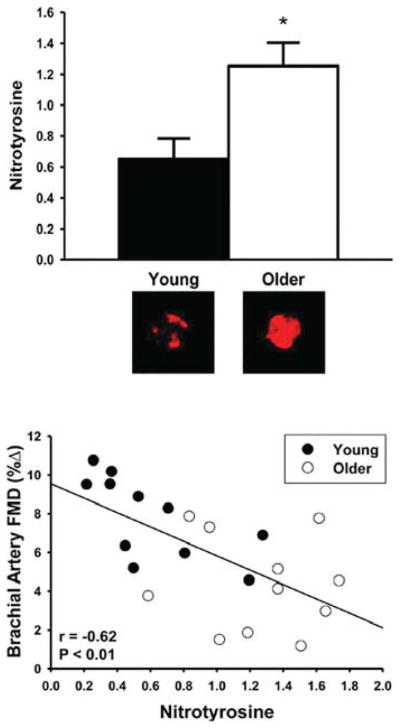
Brachial artery endothelial cell staining for nitrotyrosine is greater in older compared with young healthy subjects (upper panel) and is inversely related to brachial artery FMD in the overall group (lower panel). *P = 0.01 compared with young adults. Values are ratios to HUVEC control and representative images are shown below the histogram. Reproduced with permission from Donato, A.J., Eskurza, I., Silver, A.E., Levy, A.S., Pierce, G.L., Gates, P.E. and Seals, D.R., Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB, Circ. Res. 100 (11), pp. 1659–1666. © (2007) Wolters Kluwer Health.
Figure 6. Oxidative-stress-related impairment of EDD with aging in humans.
Acute administration of ascorbic acid (vitamin C) improves EDD in older adults, as measured by FBF to ACh (left-hand and middle panels) and brachial artery FMD (right-hand panel). *P < 0.05 compared with young adults. The left-hand and middle panels were reproduced with permission from Taddei, S., Galetta, F., Virdis, A., Ghiadoni, L., Salvetti, G., Franzoni, F., Giusti, C. and Salvetti, A., Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes, Circulation 101 (25), pp. 2896–2901. © (2000) Wolters Kluwer Health. The right-hand panel was re-drawn from data in [58].
The mechanisms contributing to arterial oxidative stress-associated vascular endothelial dysfunction with aging appear to involve increased production of reactive oxygen species in the face of unchanged or reduced antioxidant defences. The bioactivity of superoxide and other free radicals are increased with aging in skeletal muscle of humans [98] and in arteries of rodents [80,99,100]. Antioxidant enzyme expression in vascular endothelial cells is not different in young and older healthy adults [59], whereas expression and activity of these enzymes generally are unchanged or reduced with aging in arteries of experimental animals [76,80,101,102]. That SOD mimetics restore EDD in arteries of old rodents [54,80,103] supports a key role for increased superoxide in age-associated vascular endothelial dysfunction.
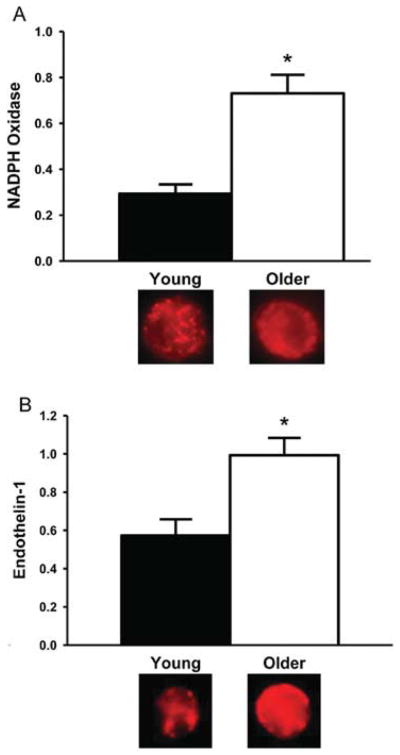
The sources of superoxide mediating impaired EDD with aging include up-regulation of the oxidant enzyme NADPH oxidase (Figure 7A), uncoupling of eNOS and increased mitochondrial production [59,76,104,105]. In contrast, the available evidence does not support a role for the oxidant enzymes xanthine oxidase or cytochrome P450 epoxygenase 2C9, at least in humans [61,106]. Increased superoxide could reduce NO bioavailability and impair EDD with aging by reacting with NO to form peroxynitrite. In turn, peroxynitrite oxidizes BH4 to its inactive form, which both reduces NO production and increases superoxide production by eNOS [85].
Figure 7. Endothelial NADPH oxidase and ET-1 with aging in humans.
Brachial artery endothelial cell protein expression of (A) NADPH oxidase and (B) ET-1 are greater in older and young healthy adults. *P < 0.05 compared with young adults. Values are ratios to HUVEC control, and representative images are shown below the histograms. The results in (A) are from A.J. Donato, G.L. Pierce and D.R. Seals, unpublished work. Panel (B) was reproduced from [65], with permission. © (2009) The American Physiological Society.
ET-1 (endothelin-1)
ET-1 is the most potent vasoconstrictor molecule produced by the vascular endothelium and is implicated in various CVD states [107]. Plasma ET-1 concentrations increase with age in some adults [65,108], ET-1-mediated vasoconstriction is augmented in older adults [109,110] and synthesis of ET-1 is greater in cultured aortic endothelial cells obtained from older compared with young donors [111]. Recent evidence implicates ET-1 in vascular endothelial dysfunction and oxidative stress with aging. Expression of ET-1 is increased in vascular endothelial cells obtained from brachial arteries and antecubital veins of older compared with young adults (Figure 7B), is inversely related to EDD and is positively related to endothelial cell staining for nitrotyrosine, a marker of oxidant stress [65]. Inhibition of ET-1 signalling with an ETA receptor antagonist improves EDD in arteries from old mice, while not affecting dilation in young controls [65], and new work indicates that this is mediated, in part, by increased endothelial production and exocytotic release of ET-1 [112].
Inflammation
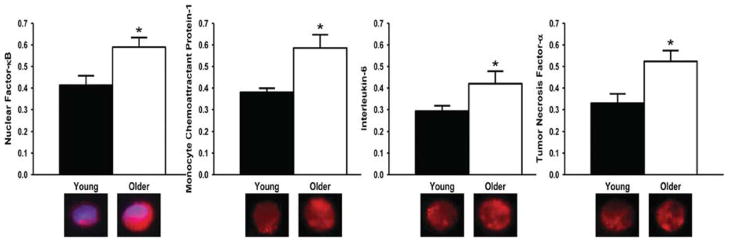
Inflammation is believed to play an essential role in the aetiology of many CVD [113,114], and evidence is accumulating for similar involvement in vascular aging. Plasma concentrations of inflammatory proteins can increase with age even in healthy adults [59,62,115]. In vascular endothelial cells obtained from the brachial artery and/or antecubital veins of humans, expression of the pro-inflammatory nuclear transcription factor NF-κB (nuclear factor κB; total and nuclear) and pro-inflammatory cytokines IL-6 (interleukin-6), TNF-α (tumour necrosis factor-α) and MCP-1 (monocyte chemoattractant protein-1) are increased in older adults [59,62] (Figure 8). Expression of MCP-1 and matrix metalloproteinases are greater in the thickened arterial intima of older compared with young adult donors obtained during autopsy [116]. Similar observations have been made in experimental animals [117–120].
Figure 8. Endothelial pro-inflammatory proteins and aging in humans.
In vascular endothelial cells obtained from an antecubital vein, expression of NF-κB, MCP-1, IL-6 and TNF-α are greater in older compared with young adults. *P < 0.05 compared with young adults. Values are ratios to HUVEC control, and representative images are shown below the histograms. Reproduced from Donato, A.J., Black, A.D., Jablonski, K.L., Gano, L.B. and Seals, D.R., Aging Cell, with permission. © (2008) Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland (http://onlinelibrary.wiley.com/journal/10.1111/%28ISSN%291474-9726).
Among middle-aged and older adults in the Framingham Heart Study [121], brachial FMD is inversely related to plasma markers of inflammation, including CRP (C-reactive protein), IL-6 and ICAM-1 (intercellular adhesion molecule-1). These relationships were no longer significant after correcting for conventional risk factors, suggesting that the latter may be an important stimulus for inflammation with aging. In otherwise healthy overweight and obese middle-aged and older adults, inhibition of NF-κB signalling improves brachial artery FMD to near-normal young control levels by reducing oxidative stress [122], whereas inhibition of TNF-α restores EDD in old rodents [118]. Taken together, these observations suggest that inflammation contributes to the tonic suppression of EDD with aging, perhaps by inducing oxidative stress (and vice versa).
Prostaglandins
EDD in response to prostacyclin is impaired in older compared with young adults, and this is NO-dependent, as the difference is abolished by L-NMMA [123]. Basal (tonic) prostanoid vasodilation also is reduced in older adults [124]. These observations in humans are consistent with data obtained from animal models, which suggest that the mechanisms involved may include increased expression of prostanoid vasoconstrictor proteins, and altered COX (cyclo-oxygenase) and prostaglandin H synthase activities [125–128].
Other mechanisms of vascular endothelial dysfunction with aging
Renin–angiotensin system activity
Increased renin–angiotensin system activity is implicated in several vascular disorders, and there is evidence for increased vascular expression of AngII (angiotensin II) and ACE (angiotensin-converting enzyme) with aging [129,130]. However, losartan, an AT1R (AngII type 1 receptor) antagonist, has no effect on brachial FMD in older adults, despite reducing blood pressure and circulating inflammatory markers [131].
AGEs (advanced glycation end-products)
AGEs accumulate in arteries with aging and are believed to contribute to vascular dysfunction, perhaps via fibrosis and remodelling [132,133]. Consistent with this, the AGE cross-link breaker, alagebrium, improves brachial artery FMD, independent of changes in intravascular shear, in older adults with isolated systolic hypertension [134]. Expression of RAGE (receptor for AGEs) is not obviously increased with age in vascular endothelial cells from healthy adults [62]. However, it is possible that increased bioavailability of agonists with aging stimulates signalling with an unchanged expression of the receptor. In any case, increased concentrations of AGEs may be an important mechanism in age-associated endothelial dysfunction.
Increased apoptosis
Apoptosis, i.e. programmed cell death, is thought to be accelerated in the endothelium of several CVD states associated with vascular dysfunction [135]. Non-human primates demonstrate increased apoptosis and reduced density of endothelial cells with aging, and this is associated with impaired systemic EDD [136]. Thus an increased rate of endothelial apoptosis may decrease the number of healthy, normally functioning, vascular endothelial cells with aging and contribute to vascular endothelial dysfunction.
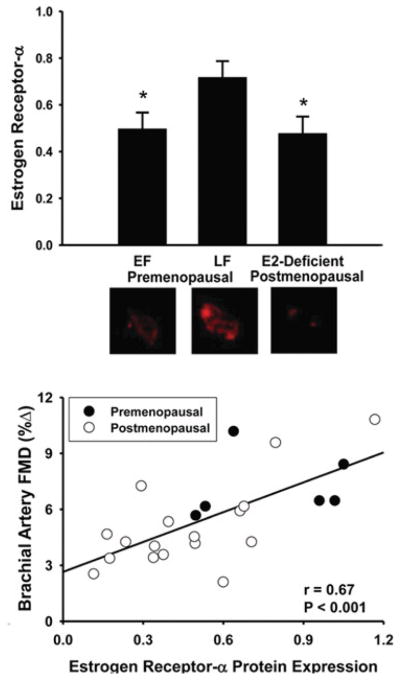
Reduced ERα (oestrogen receptor α) signalling
Recent findings indicate that expression of ERα, the primary receptor involved in oestrogen modulation of vascular function [137], is lower in vascular endothelial cells obtained from oestrogen-deficient postmenopausal women compared with premenopausal women [138]. In the overall group, ERα expression was positively related to brachial artery FMD, as well as to vascular endothelial cell expression of eNOS and eNOS phosphorylated at Ser1177 [138] (Figure 9). These observations are consistent with the idea that circulating oestrogen deficiency may lead to down-regulation of ERα and impaired EDD in postmenopausal women, in part as a result of reduced eNOS expression and activation.
Figure 9. ERα and EDD with aging in women.
In vascular endothelial cells obtained from an antecubital vein, ERα expression is lower in oestrogen-deficient postmenopausal women compared with premenopausal women in the late follicular (LF; high oestrogen), but not early follicular (EF; low oestrogen) phase (upper panel) and is related to brachial artery FMD in the overall group (lower panel). *P < 0.001 compared with LF. Values are ratios to HUVEC controls, and representative images are shown below the histograms. Reproduced with permission from Gavin, K.M., Seals, D.R., Silver, A.E. and Moreau, K.L., J. Clin. Endocrinol. Metab., Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women, volume 94 (9), September 2009, pp. 3513–3520. Copyright 2009, The Endocrine Society.
MODULATING FACTORS AND STRATEGIES FOR PREVENTION AND TREATMENT
Several factors appear to modulate EDD with aging, and strategies aimed at modifying these factors may have efficacy in the prevention and treatment of age-related vascular endothelial dysfunction.
Regular exercise
This topic has been reviewed in detail recently [139,140]. The results of both cross-section comparisons of exercise-trained and sedentary adults and intervention studies clearly demonstrate that regular aerobic exercise is associated with enhanced EDD (both brachial FMD and FBFACh) compared with the sedentary state in middle-aged and older men [50,58,60,75] (Figure 10). A similarly consistent effect of aerobic exercise on EDD has not been established in postmenopausal women [66,141,142]. Indeed, recent evidence indicates that brachial artery FMD is not influenced by moderate or vigorous aerobic exercise in many/most healthy postmenopausal women [141]. The mechanisms underlying possible sex-specific effects are not presently understood. The minimal available evidence suggests that regular resistance exercise has no influence on EDD, at least in postmenopausal women [142].
Figure 10. Regular aerobic exercise and EDD with aging in men.
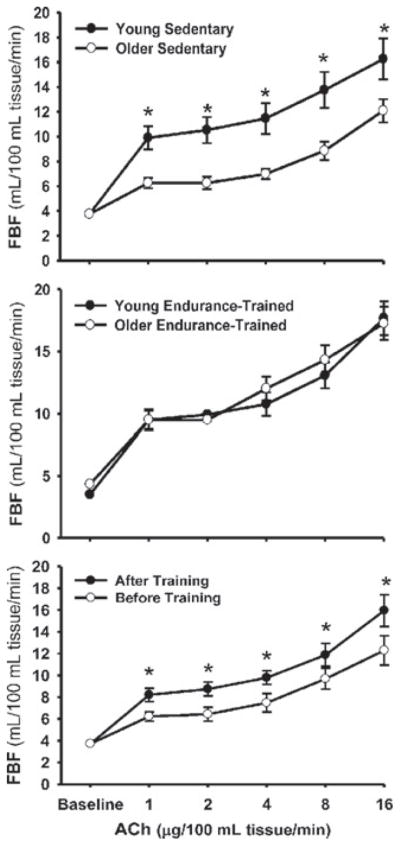
FBF responses to ACh in sedentary (top panel) and endurance-exercise-trained (middle panel) healthy young and middle-aged/older men and before and after an aerobic exercise intervention in older men (bottom panel). *P < 0.05. Reproduced from DeSouza, C.A., Shapiro, L.F., Clevenger, C.M., Dinenno, F.A., Monahan, K.D., Tanaka, H. and Seals, D.R., Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men, Circulation, volume 102 (12), pp. 1351–1357. © (2000) Wolters Kluwer Health.
In middle-aged and older men, regular aerobic exercise enhances EDD by increasing NO bioavailability [75], as a consequence of reduced oxidative stress [58,75], perhaps mediated, in part, via preservation of BH4 bioactivity [60]. Basal NO production also is maintained with aging in men who exercise [139]. Results of studies on rodents indicate that the cellular and molecular mechanisms involved include increases in eNOS expression and activity, reduced expression and activity of NADPH oxidase and increased SOD activity [76,143], as well as enhanced prostacyclin activity [144]. The stimulus for these changes may be intravascular shear, as experimental increases in flow induce eNOS and improve NO bioavailability and EDD in arteries from old rats [145].
Regular exercise also may preserve EDD with aging by protecting arteries against the deleterious effects of potentially ‘adverse’ factors, including conventional CVD risk factors such as LDL (low-density lipoprotein)-cholesterol [140,146].
Dietary factors
Several dietary factors may influence vascular endothelial function with aging. Low-sodium intake is associated with enhanced brachial artery FMD in middle-aged and older adults with elevated systolic blood pressure [147] (Figure 11), and dietary sodium restriction improves brachial FMD in overweight and obese adults [148]. Increasing servings of fruits and vegetables improves FBFACh in middle-aged and older adults with systolic hypertension [149], and a DASH (Dietary Approaches to Stop Hypertension) diet emphasizing reduced total and saturated fat and cholesterol and increased dietary fibre, potassium, magnesium and calcium improves brachial FMD in non-medicated middle-aged and older adults with modestly elevated systolic blood pressure [150].
Figure 11. Dietary sodium intake and EDD in middle-aged and older adults.
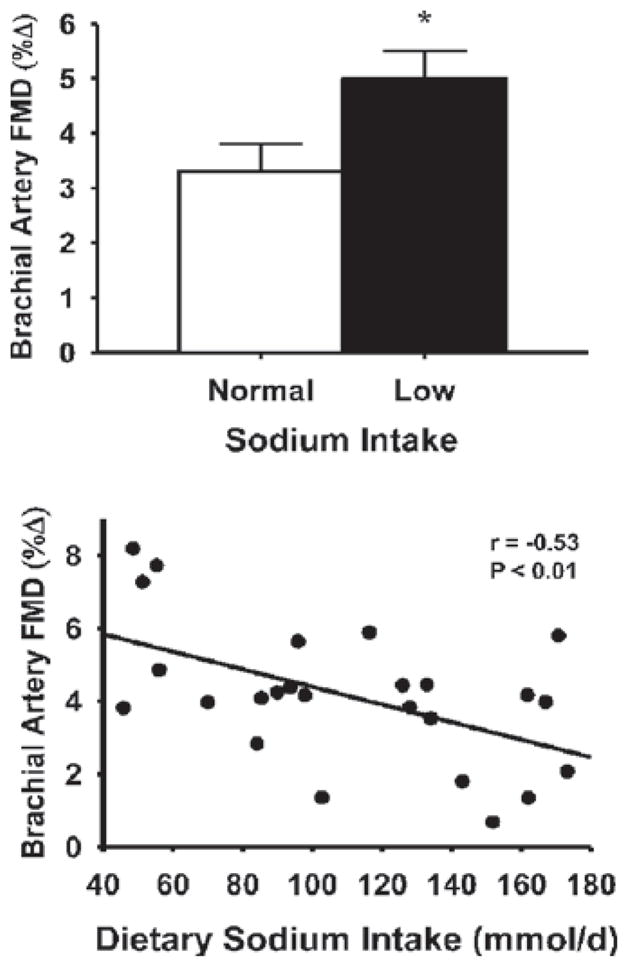
Brachial artery FMD is greater in middle-aged/older adults consuming low (73 ± 6 mmol/day) compared with normal (144 ± 6 mmol/day) sodium in their diet (upper panel) and is inversely related to dietary sodium intake among individuals (lower panel). *P < 0.05. Reproduced with permission from Jablonski, K.L., Gates, P.E., Pierce, G.L. and Seals, D.R., Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure, Ther. Adv. Cardiovasc. Dis., volume 3, pp. 347–356. Copyright © (2009) by SAGE Publications (http://online.sagepub.com).
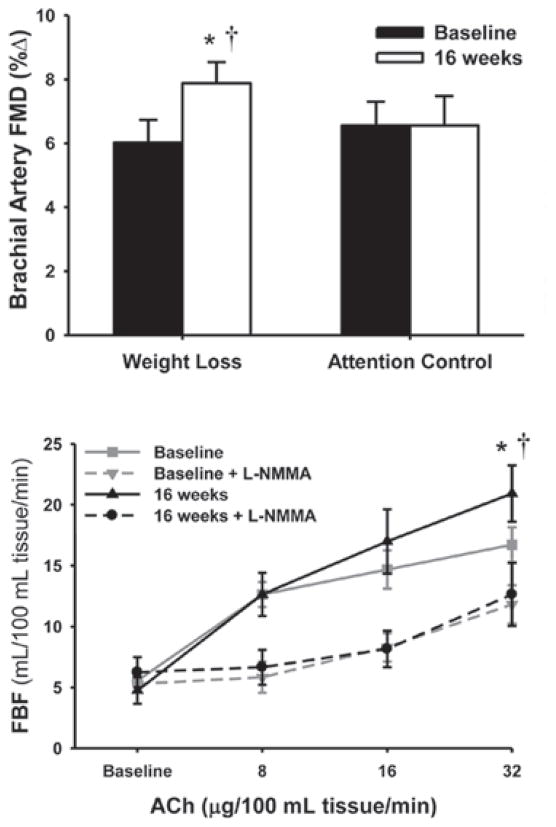
Weight loss/caloric restriction
Energy-intake-restriction-induced body weight loss alone improves both NO-mediated FBFACh and brachial FMD in young, middle-aged and older overweight and obese adults [44] (Figure 12), with improvements related, in part, to reductions in body fatness [44]. Consistent with this, short-term caloric restriction in old mice improves NO-mediated dilation to ACh by increasing eNOS protein, reducing oxidative stress (via reductions in NADPH oxidase-mediated superoxide production and stimulation of SOD antioxidant activity) and restoring expression of the anti-aging enzyme sirtuin-1 [80]. Lifelong caloric restriction also preserves EDD in rodents by what appears to be similar mechanisms of action [151,152].
Figure 12. Weight loss and NO-mediated EDD in young, middle-aged and older adults.
Energy-intake-restriction-induced body weight loss alone improves both brachial artery FMD (upper panel) and NO-mediated FBFACh (lower panel) in young and middle-aged/older overweight and obese adults. *P < 0.05 compared with baseline; †P < 0.05 for the dose–time interaction. Reproduced with permission from Pierce, G.L., Beske, S.D., Lawson, B.R., Southall, K.L., Benay, F.J., Donato, A.J. and Seals, D.R., Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults, Hypertension, volume 52 (1), pp. 1–8. © (2008) Wolters Kluwer Health.
Vitamin D status
Brachial artery FMD is lower in healthy middle-aged and older adults with insufficient or deficient circulating vitamin D (serum 25-hydroxyvitamin D) compared with their vitamin D-sufficient peers, and overall, FMD is positively related to vitamin D status in this group [153] (Figure 13). The lower FMD in vitamin D-insufficient and -deficient middle-aged/older adults is mediated, in part, by increased NF-κB-related pro-inflammatory signalling and is associated with reduced endothelial cell vitamin D receptor expression [153] (Figure 13).
Figure 13. Vitamin D status, receptor expression and EDD in middle-aged and older adults.
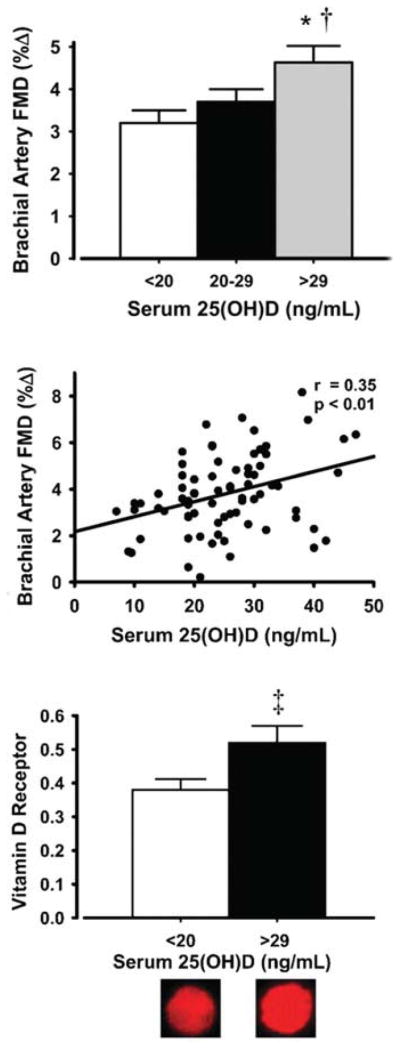
Brachial artery FMD is lower in vitamin D-deficient/-insufficient adults compared with sufficient middle-aged/older adults (top panel) and is positively related to serum 25-hydroxyvitamin D [25[OH)D] among individuals (middle panel). Vitamin D-deficient/-insufficient adults have lower endothelial cell vitamin D receptor expression (bottom panel). Values are means ± S.E.M. *P < 0.01 compared with deficient adults; †P < 0.05 compared with insufficient adults; ‡P < 0.05 compared with deficient adults. Values are ratios to HUVEC control, and representative images are shown below the histogram. Reproduced with permission from Jablonski, K.L., Chonchol, M., Pierce, G.L. Walker, A.E. and Seals, D.R., 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults, Hypertension, volume 57 (1), pp. 63–69. © (2011) Wolters Kluwer Health.
Menopause and oestrogen deficiency
EDD is reduced in oestrogen-deficient postmenopausal compared with premenopausal women [49,57,138], even early in menopause [49,57], and is related to severity of hot flushes [154,155]. This impairment in EDD appears to be dependent on circulating sex hormone status, particularly oestrogen. The primary evidence is that ovariectomy results in reduced EDD [156,157] and administration of oestrogen increases EDD (both brachial FMD and FBFACh) in some postmenopausal women [156–159] as a result of enhanced NO bioavailability [157,160,161] and reduced COX activity [157]. However, oestrogen replacement does not always improve vascular endothelial function in this group [158,162–164]. The age of the postmenopausal women [165,166], the number of years postmenopausal (i.e. oestrogen-deficient) [166,167], the presence/absence of CVD [165,168] and the inclusion/exclusion of progestin [169–171] probably contribute to the surprisingly inconsistent findings on this topic.
Risk factors for CVD
Several conventional risk factors for CVD that are independently associated with EDD change with advancing age in a manner that could contribute to age-related vascular endothelial dysfunction.
Hypertension
Hypertension is associated with vascular endothelial dysfunction as indicated by impaired EDD [48,172]. Arterial blood pressure, particularly systolic pressure, increases with age [2], and middle-aged and older patients with essential hypertension have lower EDD than age-and sex-matched adults with normal blood pressure [173,174] (Figure 14). Therefore, maintaining arterial blood pressure within the normal range may act to preserve endothelial function during aging.
Figure 14. Blood pressure and EDD with aging in humans.
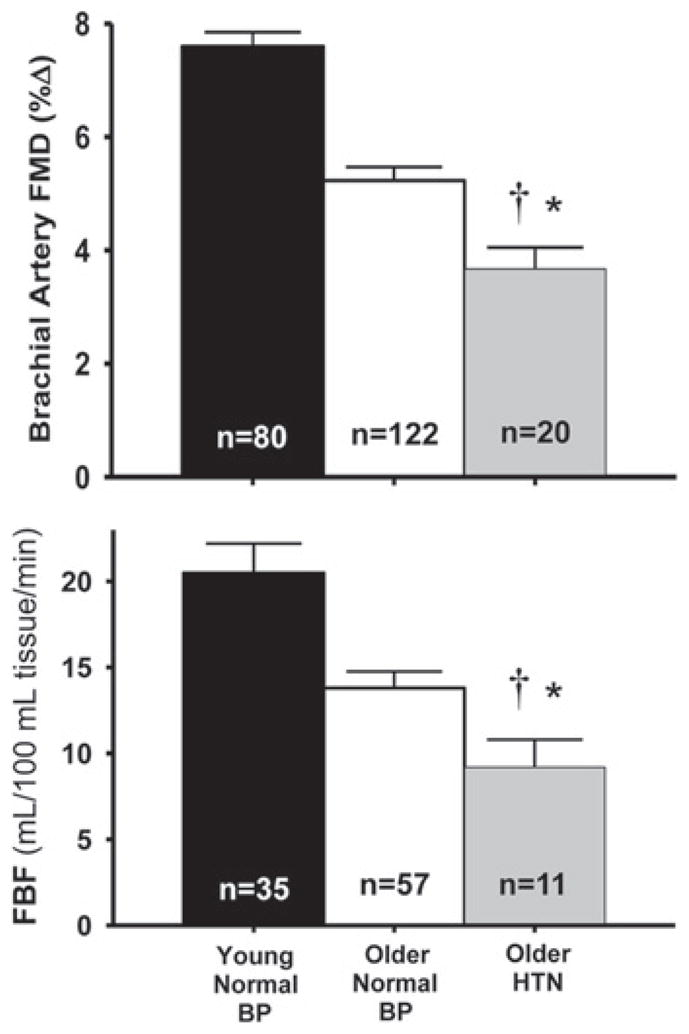
Older patients with essential hypertension (HTN) have lower brachial artery FMD (upper panel) and peak FBF to ACh (lower panel) compared with older adults with normal blood pressure. *P < 0.05 compared with older normal adults; †P < 0.01 compared with young adults. BP, arterial blood pressure. These results are from K.L. Jablonski, G.L. Pierce, A.J. Donato, A.E. Walker and D.R. Seals, unpublished work.
Hypercholesterolaemia
Hypercholesterolaemia also is associated with impaired EDD [175]. Plasma LDL-cholesterol increases with age [176], and middle-aged and older adults with even borderline elevations in LDL-cholesterol demonstrate impaired brachial artery FMD compared with their peers with optimal/near-optimal concentrations [146]. This modulatory influence is age-dependent in that it is not observed in young adults [51] (Figure 15). Lipid-lowering agents that activate selective energy-sensing pathways, such as fenofibrate and niacin, improve vascular function in middle-aged and older patients with Type 2 diabetes [177,178]. These and other agents may prove effective for improving age-associated vascular endothelial dysfunction, as recently shown for large elastic artery stiffness [179].
Figure 15. LDL-cholesterol and EDD with aging in men.
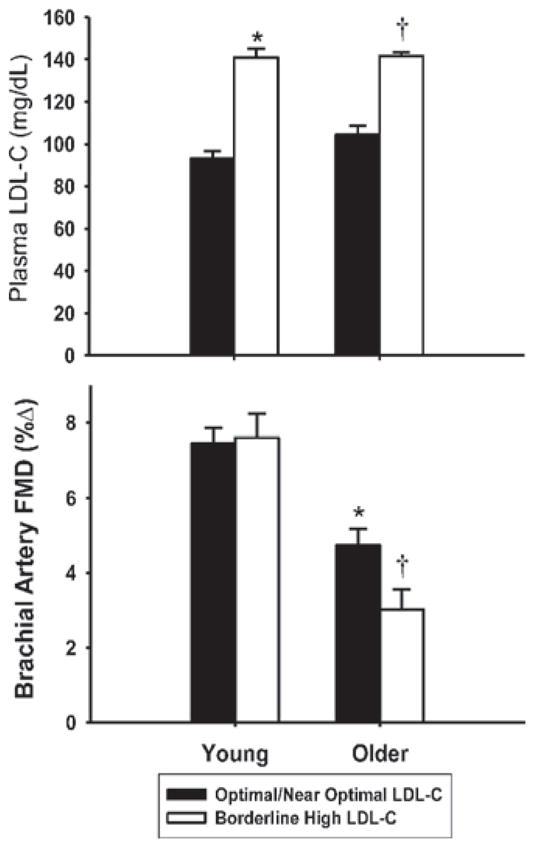
In contrast with young controls, middle-aged/older adults with borderline high LDL-cholesterol have impaired brachial artery FMD compared with peers with optimal/near-optimal LDL-cholesterol. *P < 0.05 compared with young adults with optimal/near-optimal LDL-cholesterol; †P < 0.05 compared with older with optimal/near-optimal LDL-cholesterol. Reprinted by permission from Macmillan Publishers Ltd: American Journal of Hypertension (Walker, A.E., Eskurza, I., Pierce, G.L., Gates, P.E. and Seals, D.R. (2009) Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise, volume 22, pp. 250–256), copyright (2009) (http://www.nature.com/ajh/index.html).
Overweight/obesity
Increased body fatness, particularly abdominal accumulation of fat, and obesity generally are associated with reduced EDD [180,181]. Because total and abdominal fatness and the prevalence of overweight/obesity increase with age, it is possible that changes in body fat or its distribution contribute to reductions in EDD with aging. Cross-sectional findings of inverse relationships between measures of EDD and body fat in small samples of adults varying with age are consistent with this possibility [50,182], as are the aforementioned results from weight loss intervention in humans and short-term calorie restriction in old mice [44]. However, recent evidence suggests that peripheral body fatness may actually be protective against the development of CVD [183–185]. Given that vascular endothelial dysfunction is a risk factor for CVD, more definitive insight as to the exact relationships between body fat, its distribution and EDD is needed in a larger cohort of subjects.
Non-conventional risk factors
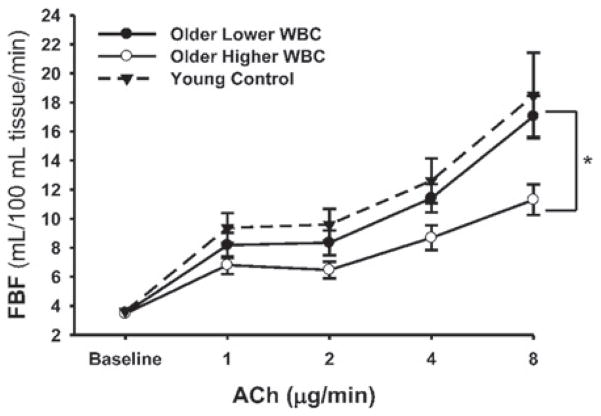
Finally, non-conventional risk factors also may modulate vascular endothelial function with aging. For example, among middle-aged and older adults, FBFACh is inversely related to white blood cell count (neutrophil, eosinophil and monocyte counts), even within the clinically normal range [186] (Figure 16). Impaired EDD in individuals with higher white blood cell count is associated with lower NO bioavailability linked to reduced BH4 bioactivity and with increased circulating concentrations of the oxidant enzyme myeloperoxidase. Data from experimental animals suggest that circadian dysregulation also may contribute to vascular endothelial dysfunction with aging by reducing eNOS-related NO production [187].
Figure 16. White blood cell count and EDD with aging in humans.
Older adults with a higher white blood cell (WBC) count have impaired FBFACh compared with older adults with lower WBC (*P < 0.05) and young adults. Reproduced with permission from Walker, A.E., Seibert, S.M., Donato, A.J., Pierce, G.L. and Seals, D.R., Vascular endothelial function is related to white blood cell count and myeloperoxidase among healthy middle-aged and older adults, Hypertension, volume 55 (2), pp. 363–369. © (2010) Wolters Kluwer Health.
Other interventions
Given the need to identify strategies that can be used to prevent and treat age-associated vascular endothelial dysfunction, more clinical research studies are needed to establish the efficacy of novel interventions. Among these, new approaches that are effective in reversing oxidative stress and inflammation in the vascular endothelium with aging would seem particularly compelling, as standard antioxidant treatments have proven ineffective [58,188]. Lifestyle-based interventions should continue to be investigated as first-line therapeutic options and the potential beneficial effects on vascular endothelial function in middle-aged and older adults established. However, given the limitations with adoption of and long-term adherence to lifestyle interventions, in general, and for older adults specifically (e.g. because of limited access to resources or disability), it is likely that innovative, low-cost pharmaceutical and ‘nutraceutical’ treatment options also will be needed. The efficacy of some oral agents will need to be established first using preclinical models, but several promising ‘anti-aging’ pharmacological agents, nutraceutical compounds and dietary interventions are ready for translational vascular health studies in middle-aged and older adults.
SUMMARY AND CONCLUSIONS
Aging is the primary risk factor for CVD, and the development of vascular endothelial dysfunction is a key mechanism linking older age to increased risk of clinical CVD. Age-associated endothelial dysfunction, as indicated most commonly by impaired EDD, is mediated by reduced NO bioavailability and also possibly by decreased responsiveness to endothelial-released vasodilatory prostaglandins. Oxidative stress and inflammation are major ‘macromechanisms’ by which aging leads to reduced NO bioavailability and EDD. Vascular oxidative stress develops with aging as a result of increased production of reactive oxygen species, such as superoxide anion, in the face of unchanged or reduced antioxidant defences. Oxidative stress may reduce NO bioavailability and EDD with aging, in part, via oxidation of BH4, which leads to uncoupling of eNOS. Increases in the endothelial vasoconstrictor molecule, endothelin-1, also appear to contribute to impaired EDD with aging. Several factors influence or may influence vascular endothelial function with aging, including regular aerobic exercise, dietary factors, body fatness, vitamin D status, menopause/oestrogen deficiency and conventional and non-conventional risk factors for CVD. Given the increasing numbers of older adults and associated health care burden, effective strategies are needed for the prevention and treatment of age-related vascular endothelial dysfunction.
Acknowledgments
We thank Matthew Racine for his technical assistance.
FUNDING
The authors’ work was supported by the National Institutes of Health [grant numbers AG006537, AG013038, AG022241, AG031141, AG029337, AG000279, AG033994 and RR00051].
Abbreviations
- ACh
acetylcholine
- ADMA
asymmetric dimethylarginine
- AGE
advanced glycation end-product
- AngII
angiotensin II
- BH4
tetrahydrobiopterin
- COX
cyclo-oxygenase
- CVD
cardiovascular disease(s)
- EDD
endothelium-dependent dilation
- eNOS
endothelial NO synthase
- ERα
oestrogen receptor α
- ET-1
endothelin-1
- FBF
forearm blood flow
- FBFAch
FBF in response to ACh
- FMD
flow-mediated dilation
- IL-6
interleukin-6
- LDL
low-density lipoprotein
- L-NMMA
NG-monomethyl-L-arginine
- MCP-1
monocyte chemoattractant protein-1
- NF-κB
nuclear factor-κB
- SOD
superoxide dismutase
- TNF-α
tumour necrosis factor-α
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Heart disease and stroke statistics –2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 3.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 5.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 6.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 7.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder. Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 8.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 9.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 10.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 11.Cooke JP. The endothelium: a new target for therapy. Vasc Med. 2000;5:49–53. doi: 10.1177/1358836X0000500108. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 13.Raitakari OT, Celermajer DS. Testing for endothelial dysfunction. Ann Med. 2000;32:293–304. doi: 10.3109/07853890008995931. [DOI] [PubMed] [Google Scholar]
- 14.Barac A, Campia U, Panza JA. Methods for evaluating endothelial function in humans. Hypertension. 2007;49:748–760. doi: 10.1161/01.HYP.0000259601.38807.a6. [DOI] [PubMed] [Google Scholar]
- 15.Zeiher AM, Drexler H, Wollschlager H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 16.Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 17.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 18.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 19.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82:1535–1539. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 20.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 21.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol. 2001;91:2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52:631–646. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerova M, Gero J, Barta E, Dolezel S, Smiesko V, Levicky V. Neurogenic and myogenic control of conduit coronary a. : a possible interference. Basic Res Cardiol. 1981;76:503–507. doi: 10.1007/BF01908351. [DOI] [PubMed] [Google Scholar]
- 24.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 25.Kelm M. Flow-mediated dilatation in human circulation: diagnostic and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2002;282:H1–H5. doi: 10.1152/ajpheart.2002.282.1.H1. [DOI] [PubMed] [Google Scholar]
- 26.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker BA, Trehearn TL, Meendering JR. Pick your Poiseuille: normalizing the shear stimulus in studies of flow-mediated dilation. J Appl Physiol. 2009;107:1357–1359. doi: 10.1152/japplphysiol.91302.2009. [DOI] [PubMed] [Google Scholar]
- 28.Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. Does arterial shear explain the magnitude of flow-mediated dilation? : a comparison between young and older humans. Am J Physiol Heart Circ Physiol. 2009;296:H57–H64. doi: 10.1152/ajpheart.00980.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- 32.Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res. 1993;72:1276–1284. doi: 10.1161/01.res.72.6.1276. [DOI] [PubMed] [Google Scholar]
- 34.Moncada S. The L-arginine: nitric oxide pathway, cellular transduction and immunological roles. Adv Second Messenger Phosphoprotein Res. 1993;28:97–99. [PubMed] [Google Scholar]
- 35.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 36.Duffy SJ, O’Brien RC, New G, Harper RW, Meredith IT. Effect of anti-oxidant treatment and cholesterol lowering on resting arterial tone, metabolic vasodilation and endothelial function in the human forearm: a randomized, placebo-controlled study. Clin Exp Pharmacol Physiol. 2001;28:409–418. doi: 10.1046/j.1440-1681.2001.03458.x. [DOI] [PubMed] [Google Scholar]
- 37.Halcox JPJ, Donald AE, Ellins E, Witte DR, Shipley MJ, Brunner EJ, Marmot MG, Deanfield JE. Endothelial function predicts progression of carotid intima–media thickness. Circulation. 2009;119:1005–1012. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- 38.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 39.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol. 2009;134:52–58. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 41.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 42.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 43.Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol. 2001;88:1067–1069. doi: 10.1016/s0002-9149(01)01997-x. [DOI] [PubMed] [Google Scholar]
- 44.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:1–8. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol. 2005;25:2368–2375. doi: 10.1161/01.ATV.0000184769.22061.da. [DOI] [PubMed] [Google Scholar]
- 46.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto K, Takeshita K, Kojima T, Takamatsu J, Saito H. Aging and plasminogen activator inhibitor-1 (PAI-1) regulation: implication in the pathogenesis of thrombotic disorders in the elderly. Cardiovasc Res. 2005;66:276–285. doi: 10.1016/j.cardiores.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 49.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28:576–582. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- 50.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 51.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 52.Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol. 2005;289:H308–H315. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- 53.DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–262. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 56.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the ‘vulnerable’ patient. Circulation. 2004;110:1926–1932. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]
- 57.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 58.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ. Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 60.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFκB, reduced IκBα, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol. 2007;102:63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- 64.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol. 2008;105:1661–1670. doi: 10.1152/japplphysiol.90612.2008. [DOI] [PubMed] [Google Scholar]
- 65.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297:H1109–H1116. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- 68.Yasue H, Matsuyama K, Matsuyama K, Okumura K, Morikami Y, Ogawa H. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment. Possible role of early coronary atherosclerosis. Circulation. 1990;81:482–490. doi: 10.1161/01.cir.81.2.482. [DOI] [PubMed] [Google Scholar]
- 69.Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 70.Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest. 1993;92:652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- 72.Higashi Y, Oshima T, Ozono R, Matsuura H, Kajiyama G. Aging and severity of hypertension attenuate endothelium-dependent renal vascular relaxation in humans. Hypertension. 1997;30:252–258. doi: 10.1161/01.hyp.30.2.252. [DOI] [PubMed] [Google Scholar]
- 73.Perticone F, Maio R, Perticone M, Sciacqua A, Shehaj E, Naccarato P, Sesti G. Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation. 2010;122:379–384. doi: 10.1161/CIRCULATIONAHA.110.940932. [DOI] [PubMed] [Google Scholar]
- 74.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 75.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 76.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tschudi MR, Barton M, Bersinger NA, Moreau P, Cosentino F, Noll G, Malinski T, Luscher TF. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, et al. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt K, Werner ER, Mayer B, Wachter H, Kukovetz WR. Tetrahydrobiopterin-dependent formation of endothelium-derived relaxing factor (nitric oxide) in aortic endothelial cells. Biochem J. 1992;281:297–300. doi: 10.1042/bj2810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Luscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- 85.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 87.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586:1161–1168. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–H2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- 90.Bode-Boger SM, Muke J, Surdacki A, Brabant G, Boger RH, Frolich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8:77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 91.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 92.Santhanam L, Christianson DW, Nyhan D, Berkowitz DE. Arginase and vascular aging. J Appl Physiol. 2008;105:1632–1642. doi: 10.1152/japplphysiol.90627.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, et al. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 94.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- 95.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 96.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 97.Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens. 2005;18:510–516. doi: 10.1016/j.amjhyper.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM, Pietri S, Young IS, Richardson RS. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol. 2010;109:449–456. doi: 10.1152/japplphysiol.00354.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2006;290:H2600–H2605. doi: 10.1152/ajpheart.00676.2005. [DOI] [PubMed] [Google Scholar]
- 100.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 101.Demaree SR, Lawler JM, Linehan J, Delp MD. Ageing alters aortic antioxidant enzyme activities in Fischer-344 rats. Acta Physiol Scand. 1999;166:203–208. doi: 10.1046/j.1365-201x.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- 102.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- 103.Tatchum-Talom R, Martin DS. Tempol improves vascular function in the mesenteric vascular bed of senescent ratsCan. J Physiol Pharmacol. 2004;82:200–207. doi: 10.1139/y04-010. [DOI] [PubMed] [Google Scholar]
- 104.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 105.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297:H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol. 2008;105:1359–1363. doi: 10.1152/japplphysiol.90629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 108.Battistelli S, Gori S, Borgogni T, Manasse G. Variation in the plasma endothelin levels in relation to age. Minerva Cardioangiol. 1996;44:111–114. [PubMed] [Google Scholar]
- 109.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50:403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- 110.Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol. 2007;103:852–857. doi: 10.1152/japplphysiol.00357.2007. [DOI] [PubMed] [Google Scholar]
- 111.Tokunaga O, Fan J, Watanabe T, Kobayashi M, Kumazaki T, Mitsui Y. Endothelin. Immunohistologic localization in aorta and biosynthesis by cultured human aortic endothelial cells. Lab Invest. 1992;67:210–217. [PubMed] [Google Scholar]
- 112.Goel A, Su B, Flavahan S, Lowenstein CJ, Berkowitz DE, Flavahan NA. Increased endothelial exocytosis and generation of endothelin-1 contributes to constriction of aged arteries. Circ Res. 2010;107:242–251. doi: 10.1161/CIRCRESAHA.109.210229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart. 2006;92:441–444. doi: 10.1136/hrt.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 115.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 116.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 117.Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Tumor necrosis factor-α and vascular angiotensin II in estrogen-deficient rats. Hypertension. 2006;48:497–503. doi: 10.1161/01.HYP.0000235865.03528.f1. [DOI] [PubMed] [Google Scholar]
- 118.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am J Pathol. 2007;170:388–398. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-κB. J. Appl. Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 121.Vita JA, Keaney JF, Jr, Larson MG, Keyes MJ, Massaro JM, Lipinska I, Lehman BT, Fan S, Osypiuk E, Wilson PW, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004;110:3604–3609. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- 122.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-κB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension. 2009;53:973–978. doi: 10.1161/HYPERTENSIONAHA.108.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh N, Prasad S, Singer DR, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci. 2002;102:595–600. [PubMed] [Google Scholar]
- 125.Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- 126.Gendron ME, Thorin E. A change in the redox environment and thromboxane A2 production precede endothelial dysfunction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H2508–H2515. doi: 10.1152/ajpheart.00352.2007. [DOI] [PubMed] [Google Scholar]
- 127.Stewart KG, Zhang Y, Davidge ST. Aging increases PGHS-2-dependent vasoconstriction in rat mesenteric arteries. Hypertension. 2000;35:1242–1247. doi: 10.1161/01.hyp.35.6.1242. [DOI] [PubMed] [Google Scholar]
- 128.Woodman CR, Price EM, Laughlin MH. Selected contribution: aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol. 2003;95:2164–2170. doi: 10.1152/japplphysiol.01073.2002. [DOI] [PubMed] [Google Scholar]
- 129.Challah M, Nadaud S, Philippe M, Battle T, Soubrier F, Corman B, Michel JB. Circulating and cellular markers of endothelial dysfunction with aging in rats. Am J Physiol. 1997;273:H1941–H1948. doi: 10.1152/ajpheart.1997.273.4.H1941. [DOI] [PubMed] [Google Scholar]
- 130.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 131.Rajagopalan S, Brook R, Mehta RH, Supiano M, Pitt B. Effect of losartan in aging-related endothelial impairment. Am J Cardiol. 2002;89:562–566. doi: 10.1016/s0002-9149(01)02297-4. [DOI] [PubMed] [Google Scholar]
- 132.Bruel A, Oxlund H. Changes in biomechanical properties, composition of collagen and elastin, and advanced glycation endproducts of the rat aorta in relation to age. Atherosclerosis. 1996;127:155–165. doi: 10.1016/s0021-9150(96)05947-3. [DOI] [PubMed] [Google Scholar]
- 133.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zieman SJ, Melenovsky V, Clattenburg L, Corretti MC, Capriotti A, Gerstenblith G, Kass DA. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens. 2007;25:577–583. doi: 10.1097/HJH.0b013e328013e7dd. [DOI] [PubMed] [Google Scholar]
- 135.Stefanec T. Endothelial apoptosis: could it have a role in the pathogenesis and treatment of disease? Chest. 2000;117:841–854. doi: 10.1378/chest.117.3.841. [DOI] [PubMed] [Google Scholar]
- 136.Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 137.Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol. 2000;74:337–343. doi: 10.1016/s0960-0760(00)00110-2. [DOI] [PubMed] [Google Scholar]
- 138.Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. 2009;94:3513–3520. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009;587:5541–5549. doi: 10.1113/jphysiol.2009.178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci. 2010;120:13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol. 2007;100:403–408. doi: 10.1007/s00421-007-0447-2. [DOI] [PubMed] [Google Scholar]
- 143.Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2009;106:1925–1934. doi: 10.1152/japplphysiol.91232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol. 2005;98:940–946. doi: 10.1152/japplphysiol.00408.2004. [DOI] [PubMed] [Google Scholar]
- 146.Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens. 2009;22:250–256. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis. 2009;3:347–356. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr. 2009;89:485–490. doi: 10.3945/ajcn.2008.26856. [DOI] [PubMed] [Google Scholar]
- 149.McCall DO, McGartland CP, McKinley MC, Patterson CC, Sharpe P, McCance DR, Young IS, Woodside JV. Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose-dependent manner. Circulation. 2009;119:2153–2160. doi: 10.1161/CIRCULATIONAHA.108.831297. [DOI] [PubMed] [Google Scholar]
- 150.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bechlioulis A, Kalantaridou SN, Naka KK, Chatzikyriakidou A, Calis KA, Makrigiannakis A, Papanikolaou O, Kaponis A, Katsouras C, Georgiou I, et al. Endothelial function, but not carotid intima–media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab. 2010;95:1199–1206. doi: 10.1210/jc.2009-2262. [DOI] [PubMed] [Google Scholar]
- 155.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118:1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pinto S, Virdis A, Ghiadoni L, Bernini G, Lombardo M, Petraglia F, Genazzani AR, Taddei S, Salvetti A. Endogenous estrogen and acetylcholine-induced vasodilation in normotensive women. Hypertension. 1997;29:268–273. doi: 10.1161/01.hyp.29.1.268. [DOI] [PubMed] [Google Scholar]
- 157.Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101:2258–2263. doi: 10.1161/01.cir.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 158.Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P, Yeung AC, Creager MA. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann Intern Med. 1994;121:936–941. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- 159.Saitta A, Altavilla D, Cucinotta D, Morabito N, Frisina N, Corrado F, D’Anna R, Lasco A, Squadrito G, Gaudio A, et al. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol. 2001;21:1512–1519. doi: 10.1161/hq0901.095565. [DOI] [PubMed] [Google Scholar]
- 160.Majmudar NG, Robson SC, Ford GA. Effects of the menopause, gender, and estrogen replacement therapy on vascular nitric oxide activity. J Clin Endocrinol Metab. 2000;85:1577–1583. doi: 10.1210/jcem.85.4.6530. [DOI] [PubMed] [Google Scholar]
- 161.Tagawa H, Shimokawa H, Tagawa T, Kuroiwa-Matsumoto M, Hirooka Y, Takeshita A. Short-term estrogen augments both nitric oxide-mediated and non-nitric oxide-mediated endothelium-dependent forearm vasodilation in postmenopausal women. J Cardiovasc Pharmacol. 1997;30:481–488. doi: 10.1097/00005344-199710000-00012. [DOI] [PubMed] [Google Scholar]
- 162.Teede HJ, Liang YL, Kotsopoulos D, Zoungas S, Craven R, McGrath BP. Placebo-controlled trial of transdermal estrogen therapy alone in postmenopausal women: effects on arterial compliance and endothelial function. Climacteric. 2002;5:160–169. [PubMed] [Google Scholar]
- 163.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO., III Effects of estrogen replacement therapy on peripheral vasomotor function in postmenopausal women. Am J Cardiol. 1995;75:264–268. doi: 10.1016/0002-9149(95)80033-o. [DOI] [PubMed] [Google Scholar]
- 164.Vehkavaara S, Hakala-Ala-Pietila T, Virkamaki A, Bergholm R, Ehnholm C, Hovatta O, Taskinen MR, Yki-Jarvinen H. Differential effects of oral and transdermal estrogen replacement therapy on endothelial function in postmenopausal women. Circulation. 2000;102:2687–2693. doi: 10.1161/01.cir.102.22.2687. [DOI] [PubMed] [Google Scholar]
- 165.Herrington DM, Espeland MA, Crouse JR, III, Robertson J, Riley WA, McBurnie MA, Burke GL. Estrogen replacement and brachial artery flow-mediated vasodilation in older women. Arterioscler Thromb Vasc Biol. 2001;21:1955–1961. doi: 10.1161/hq1201.100241. [DOI] [PubMed] [Google Scholar]
- 166.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17β-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27:1782–1787. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 167.Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Pelliccia F, Volterrani M, Fini M, Collins P, Rosano GM. Time since menopause influences the acute and chronic effect of estrogens on endothelial function. Arterioscler Thromb Vasc Biol. 2008;28:348–352. doi: 10.1161/ATVBAHA.107.158634. [DOI] [PubMed] [Google Scholar]
- 168.Kelemen M, Vaidya D, Waters DD, Howard BV, Cobb F, Younes N, Tripputti M, Ouyang P. Hormone therapy and antioxidant vitamins do not improve endothelial vasodilator function in postmenopausal women with established coronary artery disease: a substudy of the Women’s Angiographic Vitamin and Estrogen (WAVE) trial. Atherosclerosis. 2005;179:193–200. doi: 10.1016/j.atherosclerosis.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 169.Gerhard M, Walsh BW, Tawakol A, Haley EA, Creager SJ, Seely EW, Ganz P, Creager MA. Estradiol therapy combined with progesterone and endothelium-dependent vasodilation in postmenopausal women. Circulation. 1998;98:1158–1163. doi: 10.1161/01.cir.98.12.1158. [DOI] [PubMed] [Google Scholar]
- 170.Sorensen KE, Dorup I, Hermann AP, Mosekilde L. Combined hormone replacement therapy does not protect women against the age-related decline in endothelium-dependent vasomotor function. Circulation. 1998;97:1234–1238. doi: 10.1161/01.cir.97.13.1234. [DOI] [PubMed] [Google Scholar]
- 171.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on endothelium-dependent vasodilation in postmenopausal women receiving estrogen. Circulation. 2001;104:1773–1778. doi: 10.1161/hc4001.097035. [DOI] [PubMed] [Google Scholar]
- 172.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 173.Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- 174.Deng YB, Wang XF, Le GR, Zhang QP, Li CL, Zhang YG. Evaluation of endothelial function in hypertensive elderly patients by high-resolution ultrasonography. Clin Cardiol. 1999;22:705–710. doi: 10.1002/clc.4960221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, Dzau VJ. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation. 2003;107:2185–2189. doi: 10.1161/01.CIR.0000066320.27195.B4. [DOI] [PubMed] [Google Scholar]
- 177.Hamilton SJ, Chew GT, Davis TM, Watts GF. Fenofibrate improves endothelial function in the brachial artery and forearm resistance arterioles of statin-treated Type 2 diabetic patients. Clin Sci. 2010;118:607–615. doi: 10.1042/CS20090568. [DOI] [PubMed] [Google Scholar]
- 178.Hamilton SJ, Chew GT, Davis TM, Watts GF. Niacin improves small artery vasodilatory function and compliance in statin-treated type 2 diabetic patients. Diab Vasc Dis Res. 2010;7:296–299. doi: 10.1177/1479164110376206. [DOI] [PubMed] [Google Scholar]
- 179.Orr JS, Dengo AL, Rivero JM, Davy KP. Arterial destiffening with atorvastatin in overweight and obese middle-aged and older adults. Hypertension. 2009;54:763–768. doi: 10.1161/HYPERTENSIONAHA.109.138248. [DOI] [PubMed] [Google Scholar]
- 180.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, Bosello O, Lechi A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23:936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 182.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 183.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr. 1997;65:855–860. doi: 10.1093/ajcn/65.3.855. [DOI] [PubMed] [Google Scholar]
- 184.Tanko LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 185.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 186.Walker AE, Seibert SM, Donato AJ, Pierce GL, Seals DR. Vascular endothelial function is related to white blood cell count and myeloperoxidase among healthy middle-aged and older adults. Hypertension. 2010;55:363–369. doi: 10.1161/HYPERTENSIONAHA.109.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kunieda T, Minamino T, Miura K, Katsuno T, Tateno K, Miyauchi H, Kaneko S, Bradfield CA, FitzGerald GA, Komuro I. Reduced nitric oxide causes age-associated impairment of circadian rhythmicity. Circ Res. 2008;102:607–614. doi: 10.1161/CIRCRESAHA.107.162230. [DOI] [PubMed] [Google Scholar]
- 188.Singh N, Graves J, Taylor PD, MacAllister RJ, Singer DR. Effects of a ‘healthy’ diet and of acute and long-term vitamin C on vascular function in healthy older subjects. Cardiovasc Res. 2002;56:118–125. doi: 10.1016/s0008-6363(02)00514-x. [DOI] [PubMed] [Google Scholar]